Abstract
The mechanisms of Pb(II) toxicity have been studied in human red blood cells using confocal microscopy, immunolabeling, fluorescence-activated cell sorting and atomic force microscopy. The process follows a sequence of events, starting with calcium entry, followed by potassium release, morphological change, generation of ceramide, lipid flip-flop and finally cell lysis. Clotrimazole blocks potassium channels and the whole process is inhibited. Immunolabeling reveals the generation of ceramide-enriched domains linked to a cell morphological change, while the use of a neutral sphingomyelinase inhibitor greatly delays the process after the morphological change, and lipid flip-flop is significantly reduced. These facts point to three major checkpoints in the process: first the upstream exchange of calcium and potassium, then ceramide domain formation, and finally the downstream scramblase activation necessary for cell lysis. In addition, partial non-cytotoxic cholesterol depletion of red blood cells accelerates the process as the morphological change occurs faster. Cholesterol could have a role in modulating the properties of the ceramide-enriched domains. This work is relevant in the context of cell death, heavy metal toxicity and sphingolipid signaling.
Similar content being viewed by others
Introduction
Lead (Pb2+) is one of the most abundant heavy metals on earth. It has been widely used throughout human history, posing a serious health problem to susceptible populations. This metal causes a broad range of biochemical, physiological and behavioural dysfunctions. Pb2+ exposure may affect the central and peripheral nervous system, the hematopoietic and cardiovascular systems, kidney, liver and the reproductive system via physiological, biochemical and behavioural changes1,2. In human blood about 95% lead is accumulated in red blood cells (RBC, erythrocytes)1 suggesting that they could be an important target of lead toxicity in the cardiovascular system3. In erythrocytes lead could suppress hemoglobin synthesis4, induce oxidative stress5, inhibit superoxide dismutase, and lower intracellular glutathione6. Lead is also known to induce shape change in erythrocytes from normal biconcave erythrocyte to spiked echinocyte and even achantocyte7.
Lead has been associated with the induction of apoptosis8. In studies performed on lead-exposed mammal erythrocytes it was found that apoptosis presumably contributes to a decrease in the life-span of erythrocytes and the development of anaemia in cases of lead poisoning7,9,10. Some experiments have disclosed that injured erythrocytes expose phosphatidylserine (PS) at their surface11. Because macrophages are equipped with receptors specific for phosphatidylserine12,13, erythrocytes exposing phosphatidylserine (PS) at their surface will be rapidly recognized, engulfed, and degraded14 thus rapidly eliminated from circulating blood.
It has been reported that micromolar or even submicromolar Pb2+ concentrations cause lipid scrambling and exposure of PS to the outer membrane leaflet in erythrocytes via a scramblase action, as well as calcium entry in proteoliposomes15. However, the cellular mechanism of the process is not yet fully understood and previous reports from our laboratory show that the biophysical properties of RBC membranes are also altered by the effect of lead16. A role of flip-flop inducing lipids, such as long-chain ceramides17,18, has also been considered for the PS exposure mechanism. Ceramides are of particular interest for their bioactive pro-apoptotic signalling role19,20 and their marked effects on membrane biophysical properties with a tendency to form highly-packed segregated domains21, but their role in eryptosis has not been fully characterized yet9,22,23. However, ceramide-enriched domains have been already reported for erythrocytes under a hot-cold hemolytic process24. The fact that erythrocyte membranes have a large amount of cholesterol, circa 45 mol%25, should also be taken into consideration, as it could interfere with ceramide-enriched domains26. The generation of non-purely ceramidic gel domains under saturating conditions is also possible and gel phases containing both ceramide and cholesterol can appear if both lipids are saturating the membrane27,28 which could be the case for RBC membranes under Pb2+ stress. A recent study from our laboratory demonstrated that RBC lipid extracts in the presence of high concentrations of ceramide exhibit lamellar gel domains enriched in both cholesterol and ceramide29.
In this work we provide insights about the lead-induced eryptotic mechanism. A noticeable rise in intracellular calcium via scramblase is initially detected (linked to K+ depletion30, as tested with clotrimazole inhibitor7,31), followed by the generation of ceramide-enriched domains in the RBC membrane and a morphological change to achantocytes over time. This is followed by PS exposure in the outer membrane leaflet. In the next stage spherocytes are formed. Finally, when a critical amount of ceramide is reached, cell lysis occurs. Upon partial cholesterol depletion, the morphological change process became accelerated, suggesting a direct relationship between cholesterol/ceramide-enriched domains in the membrane and the change in morphology, which in turn could govern the kinetics of the eryptotic process.
Results
Lipid scrambling in living cells
To measure the outward movement of lipids, RBC were first incubated with fluorescent lipid probe C6-NBD-PS (1-oleoyl-2-[6-[(7-nitro-2-1,3-benzoxadiazol-4-yl)amino]hexanoyl]-sn-glycero-3-phosphoserine). To ensure that at the start of the floppase activity all the probe was located in the inner leaflet, cells were back-exchanged with BSA. Flopping of NBD lipid was then measured by continuous BSA extraction as described under Materials and Methods. Our results show that exposure to Pb2+, in nanomolar concentrations, significantly increased the translocation of PS, in a dose-dependent manner (Fig. 1A). To test whether cell membrane scrambling was paralleled by loss of cell membrane integrity, release of hemoglobin was determined. Only a negligible fraction of erythrocytes was lysed in the presence of the Pb2+ concentrations used in this experiment, i.e. after 1 h exposure of erythrocytes to 10 nM Pb2+ less than 2% hemoglobin had been released.
Effect of Pb2+ ions on cells. (A) Outward movement of fluorescent lipid analogue C6-NBD-PS in human RBC as a function of Pb2+ concentration. (B) Pb2+ - induced increase in intracellular Ca2+ concentration in human RBC and (C) Pb2+ -induced Ca2+ entrance rate in human RBC. (D) Pb2+ -induced increase in intracellular Ca2+ concentration of CHO cells. Intracellular free calcium was measured with Fura-2AM. Gray columns, scramblase-transfected cells; solid black columns, mock-transfected cells. (E) Flow cytometric analysis of CHO cell staining with annexin V-FITC, the inhibition of Pb2+ -induced phosphatidylserine exposure by channel inhibitor clotrimazole (CLT) can be quantified. (F) Pb2+ -induced increase in intracellular Ca2+ concentration of RBC cells for higher Pb2+ concentrations than in B. Intracellular free calcium was measured with Fura-2AM. Cells were incubated with Pb2+ for 1 h prior to measurements. Intracellular free calcium was measured with Fura-2AM. Mean values of three experiments ± S.D.
Because a calcium-dependent pathway is considered to be a major mechanism for PS exposure in erythrocytes11, additional experiments were performed to elucidate whether intracellular calcium could be increased in erythrocytes after exposure to Pb2+. To this end, Fura2-AM (acetoxymetyl) fluorescence was used to measure changes in intracellular Ca2+ concentration following incubation of erythrocytes in the absence or presence of Pb2+. Intracellular calcium increased by Pb2+ treatment in a dose-dependent way (Fig. 1B). Pb2+ also increased Ca2+ entrance rates (Fig. 1C). Increased intracellular calcium levels can activate scramblase, which in turn causes PS exposure32. These results are in good agreement with those by Kempe, et al.7 and Shin, et al.33.
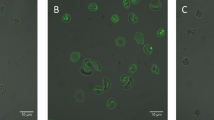
The involvement of a scramblase activity in PS translocation was demonstrated by transfection of a scramblase gene into Chinese hamster ovary (CHO) cells. CHO-K1 cells were chosen as they lack endogenous scramblase (protein scramblase 1, PLSCR1)34. After plasmid transfection and 24 h recovery the cells were treated with Pb2+. Cells were then fixed and stained. At the onset of apoptosis, PS is translocated from the cytoplasmic face of the plasma membrane to the cell surface. Annexin V has a strong affinity for PS and is therefore used as a probe for detecting early events in apoptosis. Pb2+ -stimulated phosphatidylserine exposure in CHO cells was observed using confocal microscopy (Fig. 2). Addition of Pb2+ increased annexin-binding to the cells in a dose dependent manner (Fig. 2). This result was quantified by fluorescence-activated cell sorting (FACS) analysis (Fig. 1E). As illustrated in Table 1, the results show an increase in annexin binding to transfected cells, but not to non-transfected cells. To confirm the involvement of calcium in scramblase activation, intracellular Ca2+ was measured as described under Materials & Methods. The results show an increase in intracellular Ca2+ concentration of transfected CHO cells (Fig. 1D) and in RBC (Fig. 1B), which also happens at higher Pb2+ concentrations in a dose-dependent way (Fig. 1F).
Lead-induced phosphatidylserine translocation in scramblase-transfected CHO cells. Fluorescence imaging of annexin V staining (green) in CHO cells. Annexin V staining was used to detect translocation of phosphatidylserine (PS) from the internal to the external surface of the cell membrane. Control (A), 10 nM Pb2+ (B), and 10 μM Pb2+ (C).
Morphological changes in RBC
Atomic Force Microscopy (AFM) imaging of RBC was achieved under conditions that provided consistent information, without the risk of dragging the cells along with the cantilever tip. Figure 3 shows the time-course of 10 µM Pb2+ effects on RBC morphology. The characteristic biconcave shape is lost in the first minutes, and the cells become almost flat (Fig. 3B). Spikes appear and increase in number in the next ~40 min (echynocytes) (Fig. 3C,D). Then after ~1 h the cells become spherical, and the spikes are lost (spherocytes) (Fig. 3E). At the latter stage cells begin to lyse, and their number under the AFM decreases gradually, as spherocytes are prone to hemolysis35. The data in Table 2 show that after 1 h Pb2+ treatment the diameter of the RBC decreases by about 20% while the maximum thickness increases by ~87%, as expected from a discoidal-to-spheroidal shape change.
Clotrimazole (CLT) is an inhibitor of Ca2+ -sensitive K+ -channels that has been proposed as an antiapoptotic agent36,37. Clotrimazole, which inhibits lead-induced phosphatidylserine flip-flop (Fig. 1E) also hinders the Pb2+ -promoted changes in RBC morphology. CLT-treated cells retain their biconcave form even after overnight treatment with 10 µM Pb2+ (Fig. 3F,G). Cell diameter and maximum thickness are constant for CLT-treated RBC after 20 min 10 µM Pb2+ incubation (Table 2), without any statistically significant difference in diameter (p = 0.26) or thickness at the rim (‘maximum thickness’) (p = 0.86). The fact that clotrimazole inhibited RBC shape change suggests that the latter is dependent on K+ exit.
AFM morphological changes could be confirmed for a large population of cells using flow cytometric measurements of RBC shape38. The results in Fig. 4 indicate for control RBC the bimodal distribution of scattered light typical of the discoidal shape. Treatment with 10 µM Pb2+ gave rise gradually to a more uniform scattering distribution (Fig. 4A,C), associated to sphericity38. In the presence of CLT the discoidal-spherical transition was not detected (Fig. 4C). The different morphologies detected during the Pb2+ incubation process have been quantified and presented in Fig. 5.
Ceramide formation and cell lysis
The mechanism of lead-dependent cell lysis was explored next. In a previous study we had noted the role of ceramide-enriched domains in RBC lysis24. We had also observed that mechanical stress elicited a neutral sphingomyelinase activity in erythrocytes39. We reasoned that Pb2+ -induced morphological changes could perhaps induce a sphingomyelinase (SMase) activity in the cell membrane. In fact, a Pb2+ - induced SM-ase activity was found (Fig. 6A) very similar to the one described by López, et al.39 under mechanical stress conditions. As in the previous case, SMase activity was maximum in the first 6–8 min after the stress onset. SMase activity was confirmed by quantitative mass spectrometry of RBC lipids, measuring the amounts of SM and Cer before and after 20 min treatment with Pb(II). In that period of time SM in RBC membranes slightly decreased from 4.84 ± 0.29 mol% to 4.47 ± 0.25 mol%, while ceramide significantly increased from 0.83 ± 0.06 mol% to 1.02 ± 0.07 mol% (p < 0.01) (average ± S.E.M., n = 9).
Quantitation of sphingomyelin (A) and ceramide (B) over time during Pb(II) incubation. Average of three measurements ± S.E.M. (In some cases the error bars are smaller than the symbol). Representative AFM images of GW4869-treated RBC after 15 h Pb(II) exposure in the absence of lysis (C,D). For experiments in A, SM was quantified using TLC39. For the experiments in B, FACS was used.
It has been observed in many systems, including RBC lipids24,29, that Cer can give rise to rigid domains in the RBC membranes. In order to confirm that this was the case with our samples, RBC were incubated overnight with Pb(II) and the corresponding lipid extracts dispersed in buffer and analysed by differential scanning calorimetry. Endotherms centred at ≈20 and 65 °C, are seen in lipid extracts from Pb(II)-treated, but not from control RBC (Supplementary Fig. 1). This kind of endotherms has been found in other instances of Cer formation in RBC24. Overnight treatment was carried out in order to facilitate the calorimetric observation of Cer. Note that the amount of Cer in overnight-treated cells will be higher than that present after the treatments described in Fig. 6.
The time-course of ceramide formation in the RBC membranes was estimated by labelling the cells with an anti-ceramide antibody and sorting the cells by FACS (Fig. 6B). Control and clotrimazole-treated cells gave no ceramide signal, while those treated with 10 µM Pb2+ indicated a maximum of ceramide contents at 20 min. Cells treated with the neutral SMase inhibitor GW4869 showed much decreased ceramide contents. With respect to cell shapes, flow cytometry confirms that, in the presence and absence of the SMase inhibitor, Pb2+ has identical effects on RBC shapes at least in the first 30 min (Fig. 4A,B). However, AFM images of Pb2+ - and GW4869-treated RBC overnight are equivalent to those found 45–60 min after Pb2+ addition in the absence of GW4869 (Fig. 3D,E and Fig. 6C,D) which reveals that in the presence of the inhibitor no cell lysis occurs. This suggests that SMase activity and ceramide formation are directly related to cell lysis. The involvement of SMase in the process was further tested using glutathione (GSH, 5 mM) as the SMase inhibitor40. Cell lysis was quantified for the three inhibitors (CLT, GSH and GW4869), and all three caused a reduction in cell lysis, their inhibitory efficiency decreasing in the order GSH > CLT > GW4869 (Table 3).
Partial cholesterol removal accelerates morphological change
RBC membranes contain a high amount of cholesterol (~45 mol% lipid, in accordance with Owen, et al.25). Moreover, data from this and other laboratories suggest that a direct cholesterol-ceramide interaction may be taking place at high concentrations of both lipids27,28. Thus an experiment was designed to vary substantially the amount of cholesterol in RBC membranes but without causing major morphological effects per se. Treating the cells with 0.25 mM β-methylcyclodextrin (βMCD) under the conditions described in the Methods section RBC cholesterol (Chol) contents decreases by 31.5 ± 3.5% (n = 3) in agreement with data in the literature41. βMCD treatment preserves the typical doughnut morphology (Fig. 7A), except that the thickness at the rim (‘maximum thickness’) is higher in the βMCD-treated cells (2.1 vs. 1.4 µm, Table 2). The thickness and diameter data suggest that the mode of adhesion of the βMCD-treated cells is different so that they become less flattened in the process. When 10 µM Pb2+ is added the time-course of events is somehow faster, so that spherocytes are predominant after 40 min (Fig. 7B), as compared with 1 h for intact RBC. Pb2+ causes a similar decrease in diameter (21%) as in the native RBC, but the average increase in thickness is only 44%, from 2.1 to 3.1 µm (Table 2). The shorter times required in βMCD-treated cells after Pb2+ addition for spherocyte formation may be caused by a higher free Cer concentration due to lower cholesterol levels. A possible explanation for this result is that, perhaps, under these conditions ceramide-rich domains would be more easily formed. Another possibility is that hypothetical complex gel-like phases enriched in both ceramide and cholesterol become more Cer-enriched, as their properties are modulated by Cer: Chol ratio. This would be in agreement with the results observed in RBC lipid extract experiments29, as RBC lipid extracts in the presence of Cer show Cer-enriched domains which, after Chol depletion, stiffen significantly. Chol – Cer interactions have been further described in a recent review42.
Pb2+ effect on βMCD-treated RBC. RBCs were incubated with 0.25 mM βMCD for 30 minutes at 37 °C for partial cholesterol depletion. AFM imaging of βMCD-treated RBCs reveals that they preserve their typical doughnut morphology (A). When Pb2+ is applied to them at 37 °C, spherocytes can be detected after 40 minutes (B), while without βMCD they took longer (>1 hour, Fig. 3E).
Fluorescence microscopy studies
Confocal fluorescence microscopy studies were carried out with the aim of detecting and localizing ceramide and externalized PS. The former was labeled with an antiCer specific antibody (ALX-804–196 as primary, Alexa Fluor 633 as secondary), while the latter was identified through the ability of PS to bind Annexin V. Ceramide (pseudocolour red) appears in Fig. 8 as localized dots, whose number increases for 20 min after Pb2+ addition, in agreement with the FACS data (Fig. 4B). Ceramide is known to give rise to rigid microdomains as soon as it is formed by hydrolysis of SMase24,43,44. ALX-804–196 specificity against ceramide has been questioned45. However in our hands the antibody binds Cer with much higher affinity than phosphatidylcholine (Supplementary Fig. 2).
Confocal microscopy monitorization of ceramide generation over time during Pb2+ incubation. Exposure times: 0 min (A), 8 min (B), 10 min (C), 20 min (D,E). Images are a combination of transmitted light and fluorescence confocal microscopy, where red dots indicate ceramide-enriched zones detected by the antiCer- antibody (Alexa Fluor 633 as secondary antibody).
Annexin V (pseudocolour green) indicates the presence of PS in the RBC plasma membrane outer monolayer. Figure 9A–C show the expected external location of annexin V in Pb2+ -treated RBC after 20 min. This is particularly clear in Fig. 9B, which shows as well the presence of single “poles” enriched in annexin V in the cells. The meaning of these hot spots of scramblase activity is not clear at present. Figure 9D includes a quantitation of externalized PS using FACS. An overlay of the ceramide and annexin V signals 20 min after Pb2+ addition is shown in Fig. 9E. Not all cells displaying extensive annexin V binding contain also ceramide dots. This can be due to the lack of three-dimensional imaging in these samples.
Confocal microscopy visualization of PS exposure after 20 min Pb2+ incubation. Annexin V was used to detect PS translocation and detected by fluorescence (green). Transmitted light (A), fluorescence (B) and mix of both images (C). Percentage of cells that expose PS using FACS (three different experiments, 10000 cells per experiment) (D). RBC treated with both annexin V and antiCer (Alexa Fluor 633 as secondary antibody) (E). Images in C and E are a combination of transmitted light and fluorescence confocal microscopy, where red dots indicate ceramide-enriched zones detected by the antiCer antibody as in Fig. 8.
Discussion
Phospholipid scramblases (PLSCRs) are a group of single-pass plasma membrane proteins that would carry out Ca2+ -dependent, non-specific and bidirectional movement (scrambling) of phospholipids46,47. This randomization of plasma membrane phospholipids leads to the surface exposure of PS that is an essential requirement for phagocytosis, blood coagulation and activation of anti-inflammatory responses in eukaryotic cells48,49,50,51. In particular, PS translocation from the inner to the outer leaflet of the plasma membrane is critical for recognition and clearance of apoptotic cells by macrophages52,53.
The role of PLSCR in apoptosis remains unclear given the presence of additional enzymes associated with plasma membrane phospholipid translocation such as aminophospholipid translocase32,54,55,56. Moreover, PLSCR1 appears to play important roles at the nuclear level, apparently unrelated to membrane lipid motions57,58. PLSCR family members contain a conserved calcium-binding motif, and Zhou, et al.59 found that mutation of residues in this region of PLSCR1 completely removed enzymatic activity. PLSCR1 is phosphorylated at Thr-161 by PKC-δ, which translocates to the plasma membrane during apoptosis34.
Our results show that Pb2+ increases intracellular Ca2+ (Fig. 1B,C,D). This is in agreement with the observations by Kempe, et al.7. These authors attributed the increased cytosolic Ca2+ concentration to either a Pb2+ -induced cell shrinkage, activation of an undetected Ca2+ -channel, or inhibition of Ca2+ -ATPase. The latter enzyme is known to be inhibited by Pb2+ 60,61,62. Earlier work shows that the cytoplasmic Ca2+ concentration and induction of apoptosis are directly related to each other48,49,50. A change in conformation of PLSCR1 upon Ca2+ binding has been proposed by Stout, et al.63. Other results64 show that the ionic interaction of Ca2+ with PLSCR1 peptides lead to a change in conformation of the protein around the Ca2+ binding motifs. However, free cytosolic Ca2+ may also act as a second messenger and activate many cytosolic proteins involved in apoptotic pathways64. Lang’s group7 has demonstrated that [Pb2+] > 0.1 µM also activates erythrocyte K+ channels, leading to erythrocyte shrinkage, and to activation of the erythrocyte scramblase, causing PS exposure. Pb2+ ions have been demonstrated to directly activate Ca2+ -sensitive Gardos K+ -channels (BK channels) in human erythrocytes65 and similar Ca2+ -sensitive K+ -channels in other cell types66,67. However, the fact that we have found that Pb2+ induced Ca2+ entry and PS exposure in CHO cells only when the latter had been transfected with PLSCR1 gene (Figs 1 and 2, and Table 1) is a direct demonstration that scramblase is involved in Pb2+.-induced PS flopping. In addition, alternative Ca2+ -independent mechanisms of PLSCR1 activation have been suggested under certain conditions68.
Clotrimazole is an inhibitor of BK channels. It has been found to inhibit Pb2+ -induced PS flip-flop motion and annexin V binding (Fig. 1E). The inhibition is largely due to a channel blockade69. BK channels open up when the intracellular Ca2+ concentration increases, and allow the efflux of intracellular K+ to the extracellular medium70. Clotrimazole also inhibits the Pb2+ -induced morphological changes in the RBC, which suggests a role for the perturbation of ionic concentration in the shape changes. Dreher, et al.71 reported that modest increases in intracellular Ca2+ concentrations caused the appearance of pathologic changes in erythrocyte shape and deformability. However these changes were prevented when the cells were suspended in high-K+ buffers, suggesting a role for K+ in the shape changes. Glaser, et al.72 and Bifano, et al.73 have also related shape changes to membrane potential in RBC, thus to K+ concentrations and fluxes. Bifano, et al.73 observed that increased intracellular Ca2+ enhanced K+ conductance and cell membrane hyperpolarization. Under these circumstances these authors observed echinocytosis, just as in our case (Fig. 3B–D). Clotrimazole blocks the K+ channels and no echinocytosis is observed (Fig. 3F,G).
Echinocytosis is very likely to elicit the SMase activity described by López, et al.39, as any other membrane bending process, thus a cause-effect relationship is observed between Pb2+, increase in intracellular Ca2+ 7, K+ outflow71, echinocytosis73, and SMase activation39. SMase activity is found in our cells under conditions causing echinocytosis (Fig. 6A). The generation of ceramide could explain the reported increase in the order of the membranes by the effect of Pb2+ 16, which could act through membrane platforms (Fig. 8). SMase inhibition by GW4869 does not inhibit echinocytosis (Fig. 5C) but CLT does inhibit spike formation, and CLT inhibits both echinocytosis and SMase activity (Figs 3F and 6B). This supports the flow of information summarized in Fig. 10. Cholesterol depletion accelerates the morphological change and this could be related to ceramide-enriched platforms, as less cholesterol promotes their generation and could stiffen the domains. However, recent reports indicate that scramblase 1 could also interact with cholesterol74, and the possibility of cholesterol depletion enhancing scramblase activity cannot be discarded.
The last step in this series of events would be the PS exposure to the outer membrane as a result of flip-flop phospholipid motion. As far as we know, flip-flop can occur either as a result of SMase-dependent ceramide formation17,18, or through the action of a scramblase, or both. Human scramblase 1 has been shown to catalyze phospholipid flip-flop in the absence of ceramide15,47,75,76. In our hands, SMase inhibition by GW4869 partially inhibits PS externalization (Fig. 9D), while inhibition of Ca2+ entry, essential for scramblase activation, by clotrimazole abolishes all downstream changes. These data suggest that scramblase and ceramide act jointly towards the membrane lipid scramblase effect, and subsequent externalization of PS.
Our results also support the idea, proposed by Kempe, et al.7, that scramblase activation is at the origin of the observed anemia after Pb2+ intoxication. Recent findings suggest that PS exposure also occurs under non-apoptotic conditions. Many pathological conditions cause surface exposure of PS, suggesting activation of PLSCRs77. Cytosolic Ca2+ is known to increase in several conditions (e.g. thalassemia, sickle cell disease, glucose-6-phosphate dehydrogenase deficiency) where scramblases need to be activated and the rate of apoptosis is increased78,79. In the light of the above results, lead could be an aggravating factor in those diseases.
In conclusion, in the present work we show evidence of the effects of Pb2+ ions on red blood cells, and we characterize the mechanism of this eryptotic process. Pb2+ causes Ca2+ entry which in turn causes K+ depletion from the cell. This process can be stopped at this point using clotrimazole. After K+ depletion, the red blood cells undergo a morphological change that initially flattens the cell and leads to the generation of spikes (echinocytosis), and finally to spherocytes. Ceramide generation is linked to the morphological change and the process can be stopped at this point using GW4869 neutral sphingomyelinase inhibitor. Finally, the cell activates a scramblase and ‘flip-flop’ lipid motion occurs, exposing PS from the inner leaflet of the cell membrane to the outer monolayer, and leading to hemolysis. This work is relevant in the context of cell death, heavy metal toxicity and sphingolipid signaling.
Materials and Methods
Materials
Lead acetate, Fura-2AM, EMEM (Essential Minimum Eagle Medium), BSA (bovine serum albumin), Clotrimazole and GW4869 were obtained from Sigma (St Louis, MO, USA). Annexin V-fluorescein isothiocyanate conjugate (FITC) Kit was purchased from Molecular Probes (Eugene, OR, USA). Alexa Fluor 633 goat anti-mouse IgM, Lipofectamine 2000 and pc DNA 3.1 were from Invitrogen (Carlsbad, CA, USA). 1-oleoyl-2-[6-[(7-nitro-2-1,3-benzoxadiazol-4-yl)amino]hexanoyl]-sn-glycero-3-phosphoserine (C6-NBD-PS) was obtained from Avanti Polar Lipids Alabaster, AL. ALX-804-196 Ceramide, mAb (MID 15B4) (“antiCer” antibody) was purchased from Enzo Life Sciences, Inc. (Farmingdale, NY, USA). All other reagents and solvents for assay were of analytical reagent grade and were purchased from Sigma-Aldrich.
Isolation of RBC
Whole blood was obtained from informed healthy volunteers by venipuncture. RBC were collected by centrifugation (1700 g, 10 min) and resuspended in RBC buffer (32 mM HEPES, 125 mM NaCl, 1 mM MgSO4, 1 mM CaCl2, 5 mM KCl, 5 mM glucose, pH 7.2). The process was repeated two more times to increase efficiency. The haematocrit level was adjusted to 1% (A412 ≈ 0.6 for a 25 µl -on- 1 ml distilled water dilution).
Labeling of RBC with C6-NBD phospholipids
Washed human RBC were resuspended in RBC buffer to a cell concentration of 5.108 cells/mL and loaded with 2 µM C6-NBD-PS. Translocation of the probe proceeded for 1 h at 37 °C, resulting in 75–95% internalization. To measure outward movement, residual C6-NBD-lipid remaining in the cells outer monolayer was removed by washing for 5 min with ice-cold 0.5% BSA prior to the experiment.
Translocation of RBC membrane lipids from the inner to the outer leaflet
Outward movement of C6-NBD-PS was measured using the BSA back-exchange procedure as described by Connor, et al.80. Labelled RBC were incubated with different Pb2+ concentrations at 37 °C. Briefly, 200 µL aliquots from the cell suspension were removed at the indicated time intervals and placed on ice for 5 min in the presence or absence of 1% BSA. Pellets obtained after 3 min centrifugation at 12000 g were solubilized in 2 mL 1% (w/v) Triton X-100, and the amount of externalised probe was determined by comparing the fluorescence intensity associated with the cells before and after back-exchange. The amount of probe extracted into BSA was related to the control sample incubated without Pb2+, that was established as 100%. The fluorescence assay was measured at 37 °C, using an SLM Aminco 8100 spectrofluorometer, equipped with a circulating water bath. The excitation and emission wavelengths were 480 and 525 nm respectively.
Hemolysis assay
The hemolysis assay was performed in 1-ml test tubes by mixing the erythrocyte suspension (A412 ≈ 0.6) with the required lead concentrations. Inhibitors were applied previously in the corresponding samples and preincubated for 1 hour. The mixtures were incubated at 37 °C for 1 h in the presence of Pb(II) with gentle shaking. After centrifugation at 1700 g for 5 min, hemolytic activity was measured as the increase in A412 (i.e., increase in hemoglobin content) of the supernatant (Equations 1 and 2). Inhibition efficiency was calculated following Equation 3.
Δ%lysisPb stands for the increase in cell lysis for the Pb-containing sample without inhibitor, and Δ%lysisPb+inh stands for the increase in cell lysis for the Pb + inhibitor sample. Complete inhibition of the process (Δ%lysisPb+inh = 0) would render a 100% inhibition efficiency, while the opposite case, a lack of effect of the inhibitor (Δ%lysisPb+inh = Δ%lysisPb), would render a 0% inhibition efficiency.
Measurement of cell [Ca2+]i
Cells were loaded with 4 µM Fura2-AM at 37 °C for 30 min. Excess Fura2-AM was removed by rinsing twice with buffer. Both the cells undergoing Pb2+ treatment and the control group were resuspended in buffer. Fluorescence intensity was monitored at an emission wavelength of 510 nm, by using a pair of excitation wavelengths at 340 nm and 380 nm, and subsequently expressed as the ratio of light excited at 340 nm to that at 380 (FR). Cells were permeabilized with 0.03% Triton X-100 and Fmax was obtained. EGTA (3 mM final concentration) was then added and Fmin was obtained. Sf2/Sb2 is the ratio between the excitation efficiencies of free probe and Ca2+ -bound probe at 380 nm. [Ca2+]i was calculated according to the equation of Grynkiewicz, et al.81 (Equation 4).
Where Kd is 371 ± 71 nM.
Cell culture
Chinese hamster ovary CHO-K1 cells were obtained from the American Type Culture Collection. These cells were maintained in EMEM containing 10% foetal bovine serum and 5000 U/ml penicillin and streptomycin under a humidified atmosphere of 5% CO2 in air.
Transfection of CHO cells
CHO-K1 cells, grown to 70–80% confluence, were transfected with pcDNA-PLSCR1 or pcDNA-3/1 (the latter lacking the scramblase gene) using LIPOFECTAMINETM 2000 reagent according to the manufacturer’s instructions. Transfected cells were grown for 24 h before analysis. 5 h after transfection cells were switched to growth medium containing 350 µg/ml Geneticin antibiotic (G418) and the selection medium was refreshed every 24 h.
Mass spectrometric analysis of RBC sphingolipids
Lipid extraction was performed using a modified MTBE protocol (see Guri, et al.82). Briefly RBC were transferred into a 2 mL Eppendorf tube. Then, 360 μl methanol was added and vortexed. A mixture of lipid standards (see Table 4) was added and the cells were vortexed for 10 min at 4 °C using a Cell Disruptor Genie (Scientific Industries, Inc., Bohemia, NY). MTBE (1.2 mL) was then added and the samples were incubated for 1 h at room temperature with shaking (750 rpm). Phase separation was induced by adding 200 μl H2O. After 10 min incubation at room temperature, the sample was centrifuged at 1000 × g for 10 min. The upper (organic) phase was transferred to a 13 mm screw-cap glass tube and the lower phase was extracted with 400 μl artificial upper phase (MTBE/methanol/water (10:3:1.5, v/v/v)). The two upper phases were combined and the total lipid extract (in a 13 mm glass tube) was dried in a Centrivap at 50 °C or under a nitrogen flow. The aliquot was deacylated to eliminate phospholipids by methylamine treatment (Clarke method). 0.5 mL monomethylamine reagent (MeOH/H2O/n-butanol/Methylamine solution (4:3:1:5 v/v) was added to the dried lipid, followed by sonication (5 min). Samples were then mixed and incubated for 1 h at 53 °C and dried (as above). The monomethylamine-treated lipids were desalted by n-butanol extraction. 300 μl H2O-saturated n-butanol was added to the dried lipids. The sample was vortexed, sonicated for 5 min and 150 μl MS-grade water was added. The mixture was vortexed thoroughly and centrifuged at 3200 × g for 10 min. The upper phase was transferred to a 2 mL amber vial. The lower phase was extracted twice more with 300 μl H2O-saturated n-butanol and the upper phases were combined and dried (as above).
Sphingolipids were detected on a Triple Quadrupole TSQ Vantage mass spectrometer (Thermo Fischer Scientific, Waltham, MA). Sphingolipid aliquots were resuspended in 250 μl chloroform/methanol (1:1 v/v) (LC-MS/HPLC grade) and sonicated for 5 min. The samples were pipetted into a 96-well plate (final volume = 100 μl). The samples were diluted 1:10 in positive mode solvent (Chloroform/Methanol/Water (2:7:1 v/v) + 5 mM ammonium acetate) and infused into the mass spectrometer. Tandem mass spectrometry for the identification and quantification of sphingolipid molecular species was performed using Multiple Reaction Monitoring (MRM) with a TSQ Vantage Triple Stage Quadrupole Mass Spectrometer (Thermo Fisher Scientific) equipped with a robotic nanoflow ion source, Nanomate HD (Advion Biosciences, Ithaca, NY). The collision energy was optimized for each lipid class. The detection conditions for each lipid class are listed below (Table 4). Ceramide species were also quantified with a loss of water in the first quadrupole. Each biological replicate was read in 2 technical replicates (TR). Each TR comprised 3 measurements for each transition. Lipid concentrations were calculated relative to the relevant internal standards and then normalized to the total lipid content of each lipid extract (mol%).
Assays for PS externalization and ceramide domain visualization
Cells grown on glass coverslips were rinsed with PBS and incubated with different concentrations of Pb2+. Annexin-V-FITC staining was performed according to the manufacturer’s instructions. After rinsing coverslips with binding buffer to remove unbound annexin-V, cells were fixed with 3% paraformaldehyde for 15 min and rinsed twice with PBS. As for ceramide domain visualization, cells were incubated with antiCer, centrifuged at 1700 g for 10 min to remove supernatant (cells were resuspended with RBC buffer) and reincubated with Alexa Fluor 633 followed by an additional centrifugation step. Cells were resuspended with RBC buffer, then fixed with 3% paraformaldehyde. Coverslips were mounted on glass slides. Confocal images were acquired with an Olympus Fluoview FV500 confocal microscope.
Fluorescence-activated cell sorting analysis
Fluorescence-activated cell sorting (FACS) analysis was performed as described previously83. After incubation in the presence or absence of Pb2+ cells were stained with Annexin-V-FITC and fluorescence was measured using flow cytometric analysis (FACS Calibur; Becton-Dickinson, Franklin Lakes, NJ). For each sample, the fluorescence from 10,000 events was acquired for data analysis and the results were expressed as the mean fluorescence intensity of cells, selected in a forward- versus side-scatter window. Annexin fluorescence intensity was measured in fluorescence channel FL-1 with λex = 488 nm and λem = 530 nm, while for ceramide detection λex = 625 nm and λem = 660 nm was used. All measurements were performed in triplicate. These experiments were performed in the Flow Cytometry Service of the University of the Basque Country (UPV/EHU).
RBC preparation for atomic force microscopy (AFM) imaging
Red blood cell suspension was prepared by 80-fold dilution of the 1% haematocrit-adjusted suspension in RBC buffer. This diluted suspension was set on an ethanol-washed round glass coverslip and left for 10–15 minutes for adhesion. To both facilitate AFM imaging and stop the Pb(II)-induced cell death process, 0.5% glutaraldehyde was added after the adhesion time.
AFM imaging
Experiments were carried out at room temperature (23 °C) in a JPK Nanowizard II AFM (JPK Instruments, Berlin, Germany). Images were captured at 1 Hz with 512 × 512 pixels. For this purpose, V-shaped MLCT silicon nitride cantilevers were used (Bruker AXS, Karlsruhe, Germany) in contact mode, with enough force to reduce the overestimation of cell diameters at the edges without compromising cell integrity. Images were collected at 512 × 512-pixel resolution at a scanning rate of 1 Hz and line-fitted using the JPK Data Processing software as required. Cell diameters were measured on the images along the Y and X axis for each cell, combining both data (as RBC have almost circular shape when attached to support). Cell thickness was measured by cross-section analysis of the RBC images.
Cholesterol partial depletion
β-methylcyclodextrin (βMCD) was prepared in RBC buffer to a 0.25 mM final concentration. RBC were incubated in this βMCD-containing buffer at 37 °C for 30 minutes to achieve partial cholesterol removal40. Then RBC were washed by centrifugation (1700 g, 10 min) discarding the supernatant and resuspended in βMCD-free RBC buffer. Cholesterol quantification was performed with the cholesterol oxidase/peroxidase Automated Systems Reagent (BioSystems, Barcelona, Spain) kit.
Ethical Aspects
All methods were carried out in accordance with relevant guidelines and regulations. Informed consent was obtained from all subjects (blood donors). This study was approved by the Research Ethics Committee from the University of the Basque Country (Comité de Ética en la Investigación y la Práctica Docente de la Universidad del País Vasco/Euskal Herriko Unibertsitatea, CEID/IIEB)
References
Goyer, R. A. Lead toxicity: current concerns. Environmental Health Perspectives 100, 177 (1993).
Goyer, R. A. & Clarkson, T. W. Toxic effects of metals. Casarett & Doull’s Toxicology. The Basic Science of Poisons, Fifth Edition, Klaassen, CD [Ed]. McGraw-Hill Health Professions Division, ISBN 71054766 (1996).
Battistini, V. et al. Erythrocyte delta‐aminolaevulic acid dehydrase activity in anaemia. British journal of haematology 20, 177–184 (1971).
Monteiro, H. P., Abdalla, D. S., Augusto, O. & Bechara, E. J. Free radical generation during delta-aminolevulinic acid autoxidation: induction by hemoglobin and connections with porphyrinpathies. Arch Biochem Biophys 271, 206–216 (1989).
Mateo, R., Beyer, W. N., Spann, J., Hoffman, D. & Ramis, A. Relationship between oxidative stress, pathology, and behavioral signs of lead poisoning in mallards. Journal of Toxicology and Environmental Health Part A 66, 1371–1389 (2003).
Sugawara, E., Nakamura, K., Miyake, T., Fukumura, A. & Seki, Y. Lipid peroxidation and concentration of glutathione in erythrocytes from workers exposed to lead. British journal of industrial medicine 48, 239–242 (1991).
Kempe, D. S. et al. Stimulation of erythrocyte phosphatidylserine exposure by lead ions. American Journal of Physiology-Cell Physiology 288, C396–C402 (2005).
He, L., Poblenz, A. T., Medrano, C. J. & Fox, D. A. Lead and calcium produce rod photoreceptor cell apoptosis by opening the mitochondrial permeability transition pore. Journal of Biological Chemistry 275, 12175–12184 (2000).
Lang, F., Gulbins, E., Lang, P. A., Zappulla, D. & Foller, M. Ceramide in suicidal death of erythrocytes. Cellular physiology and biochemistry: international journal of experimental cellular physiology, biochemistry, and pharmacology 26, 21–28, https://doi.org/10.1159/000315102 (2010).
Aguilar-Dorado, I. C. et al. Eryptosis in lead-exposed workers. Toxicology and applied pharmacology 281, 195–202, https://doi.org/10.1016/j.taap.2014.10.003 (2014).
Bratosin, D. et al. Programmed cell death in mature erythrocytes: a model for investigating death effector pathways operating in the absence of mitochondria. Cell death and differentiation 8, 1143–1156 (2001).
Fadok, V. A., de Cathelineau, A., Daleke, D. L., Henson, P. M. & Bratton, D. L. Loss of phospholipid asymmetry and surface exposure of phosphatidylserine is required for phagocytosis of apoptotic cells by macrophages and fibroblasts. Journal of Biological Chemistry 276, 1071–1077 (2001).
Henson, P. M., Bratton, D. L. & Fadok, V. A. The phosphatidylserine receptor: a crucial molecular switch? Nature reviews Molecular cell biology 2, 627–633 (2001).
Boas, F. E., Forman, L. & Beutler, E. Phosphatidylserine exposure and red cell viability in red cell aging and in hemolytic anemia. Proceedings of the National Academy of Sciences 95, 3077–3081 (1998).
Shettihalli, A. K. & Gummadi, S. N. Biochemical evidence for lead and mercury induced transbilayer movement of phospholipids mediated by human phospholipid scramblase 1. Chemical research in toxicology 26, 918–925 (2013).
Ahyayauch, H. et al. Effects of chronic and acute lead treatments on the biophysical properties of erythrocyte membranes, and a comparison with model membranes. FEBS open bio 3, 212–217, https://doi.org/10.1016/j.fob.2013.04.001 (2013).
Contreras, F. X., Villar, A. V., Alonso, A., Kolesnick, R. N. & Goñi, F. M. Sphingomyelinase activity causes transbilayer lipid translocation in model and cell membranes. The Journal of biological chemistry 278, 37169–37174, https://doi.org/10.1074/jbc.M303206200 (2003).
Contreras, F. X., Basanez, G., Alonso, A., Herrmann, A. & Goni, F. M. Asymmetric addition of ceramides but not dihydroceramides promotes transbilayer (flip-flop) lipid motion in membranes. Biophysical journal 88, 348–359, https://doi.org/10.1529/biophysj.104.050690 (2005).
Hannun, Y. A. Functions of ceramide in coordinating cellular responses to stress. Science 274, 1855–1859 (1996).
Kolesnick, R. The therapeutic potential of modulating the ceramide/sphingomyelin pathway. The Journal of clinical investigation 110, 3–8, https://doi.org/10.1172/JCI16127 (2002).
Sot, J., Bagatolli, L. A., Goñi, F. M. & Alonso, A. Detergent-resistant, ceramide-enriched domains in sphingomyelin/ceramide bilayers. Biophysical journal 90, 903–914, https://doi.org/10.1529/biophysj.105.067710 (2006).
Lang, F., Lang, K. S., Lang, P. A., Huber, S. M. & Wieder, T. Mechanisms and significance of eryptosis. Antioxidants & redox signaling 8, 1183–1192, https://doi.org/10.1089/ars.2006.8.1183 (2006).
Lang, E., Bissinger, R., Gulbins, E. & Lang, F. Ceramide in the regulation of eryptosis, the suicidal erythrocyte death. Apoptosis: an international journal on programmed cell death 20, 758–767, https://doi.org/10.1007/s10495-015-1094-4 (2015).
Montes, L. R. et al. Ceramide-enriched membrane domains in red blood cells and the mechanism of sphingomyelinase-induced hot-cold hemolysis. Biochemistry 47, 11222–11230, https://doi.org/10.1021/bi801139z (2008).
Owen, J. S., Bruckdorfer, K. R., Day, R. C. & McIntyre, N. Decreased erythrocyte membrane fluidity and altered lipid composition in human liver disease. J Lipid Res 23, 124–132 (1982).
Castro, B. M., Silva, L. C., Fedorov, A., de Almeida, R. F. & Prieto, M. Cholesterol-rich fluid membranes solubilize ceramide domains: implications for the structure and dynamics of mammalian intracellular and plasma membranes. The Journal of biological chemistry 284, 22978–22987, https://doi.org/10.1074/jbc.M109.026567 (2009).
Busto, J. V. et al. Lamellar gel (Lβ) phases of ternary lipid composition containing ceramide and cholesterol. Biophysical journal 106, 621–630, https://doi.org/10.1016/j.bpj.2013.12.021 (2014).
García-Arribas, A. B., Busto, J. V., Alonso, A. & Goñi, F. M. Atomic force microscopy characterization of palmitoylceramide and cholesterol effects on phospholipid bilayers: a topographic and nanomechanical study. Langmuir 31, 3135–3145, https://doi.org/10.1021/la504047n (2015).
García-Arribas, A. B. et al. Ceramide-induced lamellar gel phases in fluid cell lipid extracts. Langmuir 32, 9053–9063 (2016).
Lang, P. A. et al. Role of Ca2+ -activated K+ channels in human erythrocyte apoptosis. American journal of physiology. Cell physiology 285, C1553–1560, https://doi.org/10.1152/ajpcell.00186.2003 (2003).
Brugnara, C., de Franceschi, L. & Alper, S. L. Inhibition of Ca(2+)-dependent K+ transport and cell dehydration in sickle erythrocytes by clotrimazole and other imidazole derivatives. The Journal of clinical investigation 92, 520–526, https://doi.org/10.1172/JCI116597 (1993).
Contreras, F., Sánchez-Magraner, L., Alonso, A. & Goñi, F. M. Transbilayer (flip-flop) lipid motion and lipid scrambling in membranes. FEBS letters 584, 1779–1786 (2010).
Shin, J. H. et al. Lead-induced procoagulant activation of erythrocytes through phosphatidylserine exposure may lead to thrombotic diseases. Chem Res Toxicol 20, 38–43, https://doi.org/10.1021/tx060114+ (2007).
Frasch, S. C. et al. Regulation of phospholipid scramblase activity during apoptosis and cell activation by protein kinase Cδ. Journal of Biological Chemistry 275, 23065–23073 (2000).
Bratosin, D., Estaquier, J., Ameisen, J. C. & Montreuil, J. Molecular and cellular mechanisms of erythrocyte programmed cell death: impact on blood transfusion. Vox sanguinis 83(Suppl 1), 307–310 (2002).
Iannelli, A. et al. Anti-apoptotic pro-survival effect of clotrimazole in a normothermic ischemia reperfusion injury animal model. Journal of Surgical Research 171, 101–107 (2011).
Mandal, S. et al. S-allyl cysteine in combination with clotrimazole downregulates Fas induced apoptotic events in erythrocytes of mice exposed to lead. Biochimica et Biophysica Acta (BBA)-General Subjects 1820, 9–23 (2012).
Piagnerelli, M. et al. Effects of sample temperature on red blood cell shape in septic patients. Clinical hemorheology and microcirculation 30, 463–466 (2004).
López, D. J. et al. Accumulated bending energy elicits neutral sphingomyelinase activity in human red blood cells. Biophysical journal 102, 2077–2085 (2012).
Liu, B. & Hannun, Y. A. Inhibition of the neutral magnesium-dependent sphingomyelinase by glutathione. Journal of Biological Chemistry 272, 16281–16287 (1997).
D’Auria, L. et al. Micrometric segregation of fluorescent membrane lipids: relevance for endogenous lipids and biogenesis in erythrocytes. J Lipid Res 54, 1066–1076 (2013).
García-Arribas, A. B., Alonso, A. & Goñi, F. M. Cholesterol interactions with ceramide and sphingomyelin. Chemistry and physics of lipids 199, 26–34 (2016).
Ruiz-Arguello, M. B., Veiga, M. P., Arrondo, J. L., Goñi, F. M. & Alonso, A. Sphingomyelinase cleavage of sphingomyelin in pure and mixed lipid membranes. Influence of the physical state of the sphingolipid. Chem Phys Lipids 114, 11–20 (2002).
Hartel, S., Fanani, M. L. & Maggio, B. Shape transitions and lattice structuring of ceramide-enriched domains generated by sphingomyelinase in lipid monolayers. Biophysical journal 88, 287–304, https://doi.org/10.1529/biophysj.104.048959 (2005).
Cowart, L. A., Szulc, Z., Bielawska, A. & Hannun, Y. A. Structural determinants of sphingolipid recognition by commercially available anti-ceramide antibodies. Journal of lipid research 43, 2042–2048 (2002).
Wiedmer, T., Zhou, Q., Kwoh, D. Y. & Sims, P. J. Identification of three new members of the phospholipid scramblase gene family. Biochimica et Biophysica Acta (BBA)-Biomembranes 1467, 244–253 (2000).
Basse, F., Stout, J. G., Sims, P. J. & Wiedmer, T. Isolation of an erythrocyte membrane protein that mediates Ca2+ -dependent transbilayer movement of phospholipid. The Journal of biological chemistry 271, 17205–17210 (1996).
Verhoven, B., Schlegel, R. & Williamson, P. Mechanisms of phosphatidylserine exposure, a phagocyte recognition signal, on apoptotic T lymphocytes. The Journal of experimental medicine 182, 1597–1601 (1995).
Bevers, E. M., Comfurius, P. & Zwaal, R. Platelet procoagulant activity: physiological significance and mechanisms of exposure. Blood reviews 5, 146–154 (1991).
Fadok, V. A. et al. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. The Journal of Immunology 148, 2207–2216 (1992).
Gaipl, U. S. et al. Exposure of anionic phospholipids serves as anti-inflammatory and immunosuppressive signal–implications for antiphospholipid syndrome and systemic lupus erythematosus. Immunobiology 207, 73–81 (2003).
Fadok, V. A., Bratton, D. L. & Henson, P. M. Phagocyte receptors for apoptotic cells: recognition, uptake, and consequences. The Journal of clinical investigation 108, 957–962 (2001).
Fadok, V. A. & Chimini, G. In Seminars in immunology. 13, 365–372 (2001).
Bevers, E. M., Comfurius, P., Dekkers, D. W. & Zwaal, R. F. Lipid translocation across the plasma membrane of mammalian cells. Biochimica et Biophysica Acta (BBA)-Molecular and Cell Biology of Lipids 1439, 317–330 (1999).
Zhao, J., Zhou, Q., Wiedmer, T. & Sims, P. J. Level of expression of phospholipid scramblase regulates induced movement of phosphatidylserine to the cell surface. Journal of Biological Chemistry 273, 6603–6606 (1998).
Williamson, P. et al. Phospholipid scramblase activation pathways in lymphocytes. Biochemistry 40, 8065–8072 (2001).
Bateman, A. et al. Phospholipid scramblases and Tubby-like proteins belong to a new superfamily of membrane tethered transcription factors. Bioinformatics 25, 159–162 (2009).
Chen, C.-W., Sowden, M., Zhao, Q., Wiedmer, T. & Sims, P. J. Nuclear phospholipid scramblase 1 prolongs the mitotic expansion of granulocyte precursors during G-CSF-induced granulopoiesis. Journal of leukocyte biology 90, 221–233 (2011).
Zhou, Q., Sims, P. J. & Wiedmer, T. Identity of a conserved motif in phospholipid scramblase that is required for Ca2+ -accelerated transbilayer movement of membrane phospholipids. Biochemistry 37, 2356–2360 (1998).
Mas-Oliva, J. Effect of lead on the erythrocyte (Ca2+, Mg2+)-ATPase activity Calmodulin involvement. Molecular and cellular biochemistry 89, 87–93 (1989).
Hechtenberg, S. & Beyersmann, D. Inhibition of sarcoplasmic reticulum Ca(2+)-ATPase activity by cadmium, lead and mercury. Enzyme 45, 109–115 (1991).
Bettaiya, R., Yallapragada, P. R., Hall, E. & Rajanna, S. In vitro effect of lead on Ca(2+)-ATPase in synaptic plasma membranes and microsomes of rat cerebral cortex and cerebellum. Ecotoxicology and environmental safety 33, 157–162, https://doi.org/10.1006/eesa.1996.0020 (1996).
Stout, J. G., Zhou, Q., Wiedmer, T. & Sims, P. J. Change in conformation of plasma membrane phospholipid scramblase induced by occupancy of its Ca2+ binding site. Biochemistry 37, 14860–14866 (1998).
Sahu, S. K., Aradhyam, G. K. & Gummadi, S. N. Calcium binding studies of peptides of human phospholipid scramblases 1 to 4 suggest that scramblases are new class of calcium binding proteins in the cell. Biochimica et biophysica acta 1790, 1274–1281, https://doi.org/10.1016/j.bbagen.2009.06.008 (2009).
Simons, T. Influence of lead ions on cation permeability in human red cell ghosts. The Journal of membrane biology 84, 61–71 (1985).
Cao, Y.-J. & Houamed, K. M. Activation of recombinant human SK4 channels by metal cations. FEBS letters 446, 137–141 (1999).
Nishizaki, Y. et al. PbCl2‐induced hyperpolarization of rat thymocytes: Involvement of charybdotoxin‐sensitive K+ channels. Environmental toxicology 18, 321–326 (2003).
Francis, V. G. & Gummadi, S. N. Biochemical evidence for Ca2+ -independent functional activation of hPLSCR1 at low pH. Cellular & molecular biology letters 20, 177–195, https://doi.org/10.1515/cmble-2015-0017 (2015).
Wu, S.-N., Li, H.-F., Jan, C.-R. & Shen, A.-Y. Inhibition of Ca2+ -activated K+ current by clotrimazole in rat anterior pituitary GH 3 cells. Neuropharmacology 38, 979–989 (1999).
Yuan, P., Leonetti, M. D., Pico, A. R., Hsiung, Y. & MacKinnon, R. Structure of the human BK channel Ca2+ -activation apparatus at 3.0 Å resolution. science 329, 182–186 (2010).
Dreher, K. L. et al. Retention of water and potassium by erythrocytes prevents calcium-induced membrane rigidity. The American journal of pathology 92, 215 (1978).
Glaser, R., Gengnagel, C. & Donath, J. The influence of valinomycin induced membrane potential on erythrocyte shape. Biomedica biochimica acta 50, 869–877 (1990).
Bifano, E. M., Novak, T. S. & Freedman, J. C. Relationship between the shape and the membrane potential of human red blood cells. The Journal of membrane biology 82, 1–13 (1984).
Posada, I. M. et al. A cholesterol recognition motif in human phospholipid scramblase 1. Biophysical journal 107, 1383–1392 (2014).
Sánchez-Magraner, L. et al. The C-terminal transmembrane domain of human phospholipid scramblase 1 is essential for the protein flip-flop activity and Ca2+ -binding. The Journal of membrane biology 247, 155–165 (2014).
Francis, V. G., Mohammed, A. M., Aradhyam, G. K. & Gummadi, S. N. The single C‐terminal helix of human phospholipid scramblase 1 is required for membrane insertion and scrambling activity. FEBS journal 280, 2855–2869 (2013).
Smrž, D., Lebduška, P., Dráberová, Lu, Korb, J. & Dráber, P. Engagement of Phospholipid Scramblase 1 in Activated Cells. Journal of Biological Chemistry 283, 10904–10918 (2008).
Bevers, E. M., Comfurius, P., Dekkers, D., Harmsma, M. & Zwaal, R. Transmembrane phospholipid distribution in blood cells: control mechanisms and pathophysiological significance. Biological chemistry 379, 973–986 (1997).
Yabu, T., Imamura, S., Yamashita, M. & Okazaki, T. Identification of Mg2+ -dependent neutral sphingomyelinase 1 as a mediator of heat stress-induced ceramide generation and apoptosis. Journal of Biological Chemistry 283, 29971–29982 (2008).
Connor, J., Bucana, C., Fidler, I. J. & Schroit, A. J. Differentiation-dependent expression of phosphatidylserine in mammalian plasma membranes: quantitative assessment of outer-leaflet lipid by prothrombinase complex formation. Proceedings of the National Academy of Sciences of the United States of America 86, 3184–3188 (1989).
Grynkiewicz, G., Poenie, M. & Tsien, R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. Journal of Biological Chemistry 260, 3440–3450 (1985).
Guri, Y. et al. mTORC2 Promotes Tumorigenesis via Lipid Synthesis. Cancer cell 32, 807–823. e812 (2017).
Andree, H. et al. Binding of vascular anticoagulant alpha (VAC alpha) to planar phospholipid bilayers. Journal of Biological Chemistry 265, 4923–4928 (1990).
Acknowledgements
AGA was a predoctoral student supported by the Basque Government and later by the University of the Basque Country (UPV/EHU). This work was also supported in part by grants from the Spanish Government (FEDER/MINECO BFU 2015-66306-P to F.M.G. and A.A.) and the Basque Government (IT849-13 to F.M.G. and IT838-13 to A.A.), and by the Swiss National Science Foundation.
Author information
Authors and Affiliations
Contributions
H.A. and A.G.A. contributed equally to this work and planned, performed, and analyzed most of the experiments. J.V.B. and E.G.R. gave advice and performed confocal microscopy experiments. F.X.C. performed the scramblase transfections and helped with CHO cell management. A.R. provided Pb(II) and advice on its adequate use, and helped with RBC management, including haemolytic controls. B.G.M. performed the cholesterol quantitation assays. N.J.R. carried out the lipidomics experiments. C.M. provided FURA-2 for calcium quantification. J.S. performed the calorimetry measurements, supervised most of the experimental part and helped in the analysis of results. A.A. and F.M.G. supervised and designed the overall research and wrote the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ahyayauch, H., García-Arribas, A.B., Sot, J. et al. Pb(II) Induces Scramblase Activation and Ceramide-Domain Generation in Red Blood Cells. Sci Rep 8, 7456 (2018). https://doi.org/10.1038/s41598-018-25905-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-25905-8
- Springer Nature Limited
This article is cited by
-
Phospholipid scramblase 3: a latent mediator connecting mitochondria and heavy metal apoptosis
Cell Biochemistry and Biophysics (2023)
-
Plasma membrane effects of sphingolipid-synthesis inhibition by myriocin in CHO cells: a biophysical and lipidomic study
Scientific Reports (2022)
-
Whole blood transcriptomic analysis reveals PLSCR4 as a potential marker for vaso-occlusive crises in sickle cell disease
Scientific Reports (2021)
-
Are cysteine residues of human phospholipid scramblase 1 essential for Pb2+ and Hg2+ binding-induced scrambling of phospholipids?
European Biophysics Journal (2021)
-
Relationship between gestational diabetes and serum trace element levels in pregnant women from Eastern Iran: a multivariate approach
Environmental Science and Pollution Research (2021)