Abstract
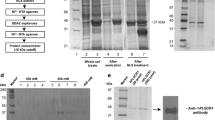
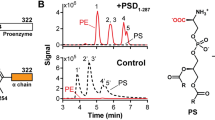
Human phospholipid scramblase (hPLSCR1) is a transmembrane protein involved in rapid bidirectional scrambling of phospholipids across the plasma membrane in response to elevated intracellular calcium (Ca2+) levels. Overexpression of recombinant hPLSCR1 in Escherichia coli BL21 (DE3) leads to its deposition in inclusion bodies (IBs). N-lauroyl sarcosine was used to solubilize IBs and to recover functionally active hPLSCR1 from them. Protein was purified to homogeneity by nickel-nitrilotriacetic acid (Ni2+–NTA) affinity chromatography and was >98% pure. Functional activity of the purified protein was validated by in vitro reconstitution studies, ~18% of 7-nitrobenz-2-oxa-1, 3-diazol-4-yl-phosphatidylcholine (NBD-PC) phospholipids was translocated across the lipid bilayer in the presence of Ca2+ ions. Far ultraviolet circular dichroism (UV-CD) studies reveal that the secondary structure of protein is predominantly an α-helix, and under nondenaturing conditions, the protein exists as a monomer. Here we describe a method to purify recombinant membrane protein with higher yield than previously described methods involving renaturation techniques.





Similar content being viewed by others
References
Basse F, Stout JG, Sims P, Wiedmer T (1996) Isolation of an erythrocyte membrane protein that mediates Ca2+-dependent transbilayer movement of phospholipid. J Biol Chem 271:17205–17210
Bateman A, Finn RD, Sims PJ, Wiedmer T, Biegert A, Soding J (2008) Phospholipid scramblase and Tubby like protein belong to a new super family of membrane tethered transcription factors. Bioinformatics 25:159–162
Callis PR (1997) 1La and 1Lb transitions of tryptophan: applications of theory and experimental observations to fluorescence of proteins. Methods Enzymol 278:113–150
Chang GHF, Barbaro NM, Pieper RO (2000) Phosphatidyl serine dependant phagocytosis of apoptotic glioma cells by normal microglia, astrocytes, and glioma cells. Neuro Oncol 2:174–183
Douette P, Navet R, Bouillenne F, Brans A, Sluse-Goffart C, Matagne A, Sluse EF (2004) Secondary structure characterization by far-UV CD of highly purified uncoupling protein1 expressed in yeast. Biochem J 380:139–145
Drew D, Lerch M, Kunji E, Slotboom DJ, De Gier JW (2006) Optimization of membrane protein over expression and purification using GFP fusions. Nat Methods 3:303–313
Fischer B, Summer I, Goodenough P (1974) Isolation, renaturation and formation of disulfide bonds of eukaryotic proteins expressed in Escherichia coli as inclusion bodies. Biotechnol Bioeng 41:3–13
Gummadi SN, Menon AK (2002) Transbilayer movement of dipalmitoylphosphatidylcholine in proteoliposomesreconstituted from detergent extracts of endoplasmic reticulum: kinetics of transbilayer transport mediated by a single flippase and identification of protein fractions enriched in flippase activity. J Biol Chem 227:25337–25343
Hamada H, Arakawa T, Shiraki K (2009) Effect of additives on protein aggregation. Curr Pharm Biotechnol 10:400–407
Jesevar S, Gaberc-Porekar V, Fonda I, Podobnik B, Grdadolnik J, Menart V (2005) Production of non classical inclusion bodies from which correctly folded proteins can be extracted. Biotechnol Prog 21:632–639
Korepanova A, Moore JD, Nguyen HB, Hua Y, Cross TA, Gao F (2007) Expression of membrane proteins from Mycobacterium tuberculosis in Escherichia coli as fusions with maltose binding protein. Protein Expr Purif 53:24–30
Kurucz I, Titus JA, Jost CR, Seagal DM (1995) Correct disulfide pairing and efficient refolding of detergent- solubilized single chain Fv proteins from bacterial inclusion bodies. Mol Immunol 32:1443–1452
Niegowski D, Hedren M, Nordlund P, Eshaghi S (2006) A simple strategy towards membrane protein purification and crystallization. Int J Biol Macromol 39:83–87
Peternal S, Grdadolink J, Gaberc-Porekar V, Komel R (2008) Engineering inclusion bodies for non denaturing extraction of functional proteins. Microb Cell Fact 7:34
Sahu SK, Gummadi SN, Aradhyam GK (2007) Phospholipid scramblases: an overview. Arch Biochem Biophys 462:103–114
Sahu SK, Gummadi SN, Aradhyam GK (2008) Over-expression of recombinant human phospholipid scramblase 1 in E. coli and its purification from inclusion bodies. Biotech Lett 30:2131–2137
Sahu SK, Archita R, Gummadi SN (2009) GroES and GroEL are essential chaperones for refolding of recombinant human phospholipid scramblase 1 in E.Coli. Biotechnol Lett 31:1745–1752
Stout JG, Zhou Q, Wiedmer T, Sims PJ (1998) Change in conformation of plasma membrane phospholipid scramblase induced by occupancy of its Ca2+ binding site. Biochemistry 37:14860–14866
Tokatlidis K, Dhurjati P, Millet J, Beguin P, Aubert JP (1991) High activity of inclusion bodies formed in E. coli overproducing Clostridium thermocellum endoglucanase. FEBS Lett 282:205–208
Vanhove M, Lejeune A, Pain RH (1998) β-lactamases as models for protein folding studies. Cell Mol Life Sci 54:372–377
Vivian TJ, Calis RP (2000) Mechanism of tryptophan fluorescence shift in proteins. Biophys J 80:2093–2109
Wiedmer T, Zhao J, Nanjundan M, Sims PJ (2003) Palmitoylation of phospholipid scramblase 1 controls its distribution between nucleus and plasma membrane. Biochemistry 42:1227–1233
Williamson P, Bevers ME, Smeets FE, Comfurius P, Schlegel AR, Zwaal AF (1995) Continous analysis of the mechanism of activated transbilayer lipid movement in platelets. Biochemistry 34:10448–10455
Worrall DM, Goss NH (1989) The formation of biologically active beta-galactosidase inclusion bodies in E. coli. Aust J Biotechnol 3:28–32
Zhou Q, Sims PJ, Wiedmer T (1998) Identity of a conserved motif in phospholipid scramblase that is required for Ca2+-accelerated transbilayer movement of membrane phospholipids. Biochemistry 37:2356–2360
Zhou Q, Zhao J, Stout JG, Luhm RA, Wiedmer T, Sims PJ (1997) Molecular cloning of human plasma membrane phospholipid scramblase. J Biol Chem 272:18240–18244
Acknowledgments
This work is supported by a research grant from the Council of Scientific and Industrial Research (CSIR), Government of India, New Delhi. Vincent Gerard Francis acknowledges CSIR for providing fellowship.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Francis, V.G., Majeed, M.A. & Gummadi, S.N. Recovery of functionally active recombinant human phospholipid scramblase 1 from inclusion bodies using N-lauroyl sarcosine. J Ind Microbiol Biotechnol 39, 1041–1048 (2012). https://doi.org/10.1007/s10295-012-1105-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-012-1105-1