Abstract
Novel approaches towards cancer therapy are urgently needed. One approach might be to target ion channels mediating Ca2+ entry because of the critical roles played by Ca2+ in many cell types, including cancer cells. There are several types of these ion channels, but here we address those formed by assembly of transient receptor potential canonical (TRPC) proteins, particularly those which involve two closely related members of the family: TRPC4 and TRPC5. We focus on these proteins because recent studies point to roles in important aspects of cancer: drug resistance, transmission of drug resistance through extracellular vesicles, tumour vascularisation, and evoked cancer cell death by the TRPC4/5 channel activator (−)-englerin A. We conclude that further research is both justified and necessary before these proteins can be considered as strong targets for anti-cancer cell drug discovery programmes. It is nevertheless already apparent that inhibitors of the channels would be unlikely to cause significant adverse effects, but, rather, have other effects which may be beneficial in the context of cancer and chemotherapy, potentially including suppression of innate fear, visceral pain and pathological cardiac remodelling.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite advancements in prevention strategies, diagnostics and therapeutics, cancer remains a major worldwide health problem. Unacceptably high rates of treatment failure exist, often due to the adaptable nature of tumour cells. In many cases, localised non-metastatic cancers can be treated with surgery alone, but for those that relapse or present with metastatic disease, systemic treatment options are typically required. Chemotherapy is a common approach, but this is associated with only modest benefit in most solid tumours and it is ineffective in others, such as renal cell carcinoma. More recently, a number of small-molecule kinase inhibitors have been introduced, but these agents are invariably associated with either innate or acquired drug resistance. Furthermore, currently used therapies are frequently associated with significant adverse effects. Novel pharmacological targets and approaches for cancer therapy are in high demand, and various ion channels have been suggested as potentially useful targets for therapies; notable amongst them are the channels which are permeable to Ca2+ and which therefore often allow Ca2+ entry into cells (Arcangeli et al. 2009; Monteith et al. 2012; Prevarskaya et al. 2011).
There are many types of Ca2+-permeable channel, but here we focus on those formed by assembly of transient receptor potential canonical (TRPC) proteins and particularly those which involve two closely related members of the family: TRPC4 and TRPC5. We focus on these proteins because recent studies suggest that they have roles in several important aspects of cancer: drug resistance, transmission of drug resistance through extracellular vesicles, tumour vascularisation, and evoked cancer cell death. We describe the experimental evidence and discuss the implications for future potential studies and therapeutic strategies. We address not only the potential direct relevance to cancer cells but also relevance to other aspects of biology which are important for many cancer patients.
Control of cell function by intracellular Ca2+
This review focusses on the Ca2+ permeability of TRPC channels. Ca2+ enters cells through a variety of ion channels and is particularly recognised for its importance as a versatile dynamic intracellular regulator of mammalian cell biology (Berridge et al. 2000; Clapham 2007). Finely tuned control of the free intracellular Ca2+ concentration is fundamental for the survival and death of cells in mammals as well as many other types of animal. Plasma membrane ion channels that are permeable to Ca2+ and allow Ca2+ entry down its steep gradient often have positive, although not necessarily beneficial, effects on cells which include increased proliferation, migration and invasiveness (Clapham 2007; Chen et al. 2013). Excessive elevation of intracellular Ca2+ is conversely associated with cytotoxicity (Clapham 2007; Berridge et al. 2000; Fleckenstein et al. 1974; Orrenius et al. 2003).
Transient receptor potential (TRP) proteins and channels
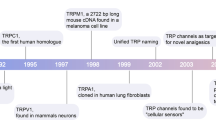
TRPs are membrane proteins which assemble as tetramers around a central ion pore to form non-selective cationic channels, many of which are Ca2+ permeable. There are 28 genes encoding the different TRPs. A greater number of channels exist because of heteromeric assembly involving more than one type of TRP. Although there is differential expression of TRPs in different cell types and tissues, most TRPs are quite broadly expressed. They are expressed in excitable cells, where they contribute positively to electrical excitability alongside voltage-gated Ca2+ channels, and in non-excitable cells, where they promote migration and proliferation and other relatively slow cell changes (Zheng and Phelan 2014; Abramowitz and Birnbaumer 2009; Al-Shawaf et al. 2010; Zeng et al. 2013). The initial discovery of TRP channels arose in photo-transduction studies in Drosophila melanogaster. Mutation in this fly’s TRP gene resulted in a transient, rather than the normally sustained, membrane depolarisation in response to bright light, hence the name transient receptor potential (Minke et al. 1975). Many mammalian homologues were subsequently discovered and cloned, starting with TRP canonical 1 (TRPC1) (Wes et al. 1995; Birnbaumer 2009; Beech 2013; Bon and Beech 2013). The TRP super-family is now categorised into subgroups: TRP canonical (TRPC), TRP vanilloid (TRPV), TRP melastatin (TRPM), TRP ankyrin (TRPA), TRP polycystin (TRPP) and TRP mucolipin (TRPML) (Damann et al. 2008; Birnbaumer 2009).
Voltage-gated channels are considered the proteins most structurally related to the TRPs; akin to the KV1.2 voltage-gated potassium channel, for which there is a crystal structure, all TRPs are suggested to have six membrane-spanning segments and intracellular N- and C-termini. Like KV1 channels, TRP channels may arise from either four identical or four different members of the family (i.e. they may be homotetramers or heterotetramers). Experimental tests of this hypothesis have supported this topology, and cryo-electron microscopy (EM) structural data for two of the TRPs, TRPV1 and TRPA1, have further corroborated it (Liao et al. 2013; Paulsen et al. 2015).
Channels which contain TRPC4 and TRPC5
There are seven TRPC types in mammals, and all are considered to contribute to plasma membrane non-selective cationic channels which confer Na+ as well as Ca2+ permeability. The Na+ entry may contribute functionally by helping to depolarise the membrane potential and elevate intracellular Ca2+ indirectly via Na+–Ca2+ exchange. In humans and the great apes, TRPC2 protein is absent, being encoded by a pseudogene in these species (Vannier et al. 1999; Damann et al. 2008; Abramowitz and Birnbaumer 2009). The TRPCs are notable amongst the TRPs for being likely to exist as heteromers. TRPC1 may not form functional homomeric channels at all, yet there is compelling evidence for its distinctive and important contributions to heteromers with TRPC4 and TRPC5. Although TRPC4 and TRPC5 are capable of forming homomeric channels, TRPC1 is very broadly expressed, and so they probably commonly exist physiologically as heteromers with TRPC1. Further promiscuity has been suggested, even outside the TRPC family, stretching to TRPV4 and TRPP2 (Bai et al. 2008; Ma et al. 2010; Strubing et al. 2001; Sukumar et al. 2012; Xu et al. 2008).
The TRPC channels almost certainly do not have a single physiological activator. As with other TRP channels, there is promiscuity, also called versatility, of activation, which means that multiple activators have been identified and several are often relevant in physiology and patho-physiology, suggesting context-dependent activation. Also consistent with concepts for other TRPs, there are examples where modulators of TRPC channels are not endogenous physiological factors but exogenous chemicals from plants, suggesting that the channels act at least in part to integrate humans and other mammals with the external environment. Modulators of the channels include receptor agonists, hydrogen peroxide, mild acidification, toxic metal ions, oxidised phospholipids, galangin and ω-3 fatty acids (Beech 2013; Tominaga et al. 1998; McKemy 2005; Jordt et al. 2004; Akbulut et al. 2015; Sukumar et al. 2012; Naylor et al. 2015).
Tools for studying roles of TRPC4- and TRPC5-containing channels
Selective and potent pharmacological agents to modulate TRPC4 and TRPC5 channels have been lacking, but this is an active area of investigation and so better tools should be available in the near future for exploring the roles of the channels (Bon and Beech 2013). Some commonly used small-molecule inhibitors such as SKF-93635 and 2-APB are non-specific, and the arising data not of great value. ML204 and clemizole hydrochloride are more recently identified and more specific, inhibiting the channels in the micromolar concentration range (Miller et al. 2011; Richter et al. 2014a). Small-molecule activators of the channels include riluzole and rosiglitazone, but again these are non-specific and lack potency (Jung et al. 2003; Majeed et al. 2011; Richter et al. 2014b; Flemming et al. 2006). Recently we discovered the natural product (−)-englerin A as the first selective and potent small-molecule activator of TRPC4- and TRPC5-containing channels (Akbulut et al. 2015). High-quality small-molecule modulators are especially valuable for understanding the roles of the channels in human tissues and cells obtained from clinical samples.
Short interfering and short hairpin RNAs are used for studying the channels in cells which can be transfected or which are suitable for viral delivery methods (Carson et al. 2015; Ma et al. 2014; Stewart et al. 2015). There are mice available with disrupted TRPC4 or TRPC5 genes (Phelan et al. 2013; Tsvilovskyy et al. 2009). Extracellularly acting inhibitor antibodies have been developed to TRPC1, TRPC4 and TRPC5, which have been useful in revealing roles of the channels (Sukumar et al. 2012; Xu et al. 2005, 2006; Mohl et al. 2011; Akbulut et al. 2015).
Cancer-independent roles of TRPC4- and TRPC5-containing channels
Despite the limitations of the TRPC4 and TRPC5 tools, there is compelling evidence for important roles of TRPC4- and TRPC5-containing channels, especially in animal models of human patho-physiology or in clinical samples. The channels have positive roles in epilepsy, innate fear, pain, adverse cardiac remodelling as well as other aspects of physiology and patho-physiology (Zheng and Phelan 2014; Phelan et al. 2013; Riccio et al. 2014; Westlund et al. 2014; Bon and Beech 2013; Wei et al. 2015; Camacho Londono et al. 2015). TRPC5 knockout mice exhibited reduced innate fear (Riccio et al. 2014), and TRPC4 knockout mice presented with diminished anxiety (Riccio et al. 2014). TRPC1 was up-regulated and had a positive role in neointimal hyperplasia of human saphenous vein, where it may function in partnership with TRPC5 (Kumar et al. 2006; Xu et al. 2006). In vascular smooth muscle cells from human saphenous vein, TRPC5-dependent channels were activated by sphingosine-1-phosphate and helped to drive cell migration (Xu et al. 2006). TRPC5 was also implicated in kidney barrier function, protecting against albuminuria (Schaldecker et al. 2013). TRPC4 was required for the transmission and detection of the colonic visceral pain sensation associated with application of mustard oil. TRPC4−/− mice and mice treated with the TRPC4 inhibitor ML204 showed less lower-body licking and abdominal retractions in response to application of mustard oil (Westlund et al. 2014). Amygdaloid TRPC4 and TRPC5 contributed to maintenance of pain hypersensitivity and neuropathy (Wei et al. 2015).
Direct relevance of TRPC4 and TRPC5 to cancers?
Malignant transformation is associated with diverse molecular changes which include alterations in the expression and activity of membrane channels and transporters (Herve 2015). Although still in its infancy, there is growing interest in understanding these changes within particular cancer types and exploring how modulation of these channels might lead to a novel treatment modality. The clinical relevance of TRP channel gene expression has recently been investigated (Park et al. 2016). Here, we focus on the emerging data related to TRPC4 and TRPC5.
Relationship to vascular endothelial growth factor (VEGF) signalling and angiogenesis
Tumour angiogenesis is a hallmark of cancer and, as such, represents a promising therapeutic target. Currently used VEGF pathway-targeted drugs, such as the VEGF receptor tyrosine kinase inhibitors, are, however, not effective in all cancers. Increased understanding of VEGF signalling and the mechanisms underlying tumour vascularisation is required in order to realise the full potential of this strategy (Vasudev and Reynolds 2014).
Several studies suggest roles of TRPC channels in angiogenesis. Knockdown of TRPC4 and TRPC5 inhibited tube formation in an endothelial cell line (Antigny et al. 2012). Furthermore, a role for TRPC4 in the development of retinal neovascularisation has been suggested. TRPC4 was up-regulated in hypoxia, intravitreal injection of TRPC4 short interfering RNA reduced VEGF-induced retinal neovascularisation in oxygen-induced retinopathy, and TRPC4 short interfering RNA suppressed proliferation and Matrigel-based tube formation of human dermal microvascular endothelial cells (Song et al. 2015). Consistent with these observations and the involvement of heteromeric channels, TRPC1 gene-disrupted zebrafish showed disrupted VEGF-dependent angiogenic sprouting (Yu et al. 2010). TRPC1 knockdown also suppressed migration and proliferation in endothelial progenitor cells (Kuang et al. 2012). However, the TRPC1 inhibitor antibody had relatively little effect against VEGF-evoked Ca2+ entry of some types of endothelial cell, where Orai1 channels instead played roles (Li et al. 2011). Moreover, down-regulation of TRPC4 has been suggested as a trigger for tumour angiogenesis in renal cell carcinoma via a mechanism involving reduced secretion of the angiogenesis inhibitor thrombospondin-1 (Veliceasa et al. 2007). The implications of these channels for tumour angiogenesis require further investigation, and there is a significant possibility of both negative and positive implications depending on the context and contributions of other Ca2+-permeable channels.
Potential role of TRPC5 in chemotherapy resistance
A special role for TRPC5 has been suggested in the development of resistance to cancer chemotherapy. TRPC5 and the multi-drug resistance (MDR) transporter, p-glycoprotein, were found to be up-regulated in the MCF-7 breast cancer cell line following repeated exposure to adriamycin until development of drug resistance (Ma et al. 2012). Inhibition of TRPC5 suppressed the effect on p-glycoprotein expression, leading to the suggestion that up-regulation of p-glycoprotein is downstream of TRPC5 channel activity. The effect was observed not only in vitro but also when MCF-7 cells were used in xenograft studies in mice. A similar effect was observed with the drug paclitaxel.
Subsequent research by the same group found TRPC5 in extracellular vesicles of the MCF-7 cells and suggested that chemotherapy resistance might be transferred to other cancer cells through these vesicles and the introduction of TRPC5 into other cells (Ma et al. 2014). Histological analysis of breast cancer tissue from patients before and after chemotherapy provided support for the idea that a similar phenomenon occurs in tumours of patients. The investigators suggested that identification of tumour-specific TRPC5 in circulating extracellular vesicles might provide a window on the clinical outcome of chemotherapy. In a related study, ATP-binding cassette subfamily B member 1, a member of the MDR family of proteins, was found to be up-regulated because of TRPC5 channel activity in colorectal carcinoma cells (Wang et al. 2015).
Activation of TRPC4/5 channels by (−)-englerin A (EA) and the relationship to renal cell carcinoma cells
Bioactive natural products have proved to be valuable starting points for drug discovery, and screening efforts are continuously on-going to find previously unrecognised natural products with potent and potentially useful effects. The diverse genus Phyllanthus is a known source of biologically active compounds. One of them, Phyllanthus engleri, grows in South East Africa, where the fruit is eaten, tea is made from the leaves as a remedy for common ailments, and burning of the roots generates a toxic smoke. About 7 years ago, EA (Fig. 1a) was isolated from this plant and included in a screen against the NCI-60 cancer cell line panel (Ratnayake et al. 2009). It was found to have a rapid cytotoxic effect on certain types of cancer cell line at nanomolar concentrations. In particular, renal cell carcinoma cell lines were affected as well as a triple-negative breast cancer cell line (Ratnayake et al. 2009). Subsequent studies confirmed the cytotoxic effect on these cell lines (see below). Because of these effects, there were then major medicinal chemistry efforts to devise methods for efficient synthesis of EA (Radtke et al. 2011) and to find its protein target or targets (Sourbier et al. 2013; Akbulut et al. 2015; Carson et al. 2015).
Discovery of (−)-englerin A (EA) as a novel potent and efficacious TRPC4/5 channel activator. a Chemical structure of EA (Akbulut et al. 2015). b–d Measurements of the free intracellular calcium ion (Ca2+) concentration shown as the change (Δ) in fura-2 fluorescence. b Concentration–response data for EA in HEK cells over-expressing TRPC4 (HEK-TRPC4) indicating the 50 % maximum effect (EC50) at 11.2 nM (Akbulut et al. 2015). c As for b except the cells were genetically modified HEK 293 cells induced to over-express TRPC5 (HEK-TRPC5). The fitted curve is a Hill equation indicating an EC50 of 7.59 nM (Akbulut et al. 2015). d Mean responses after 4 min exposure to vehicle, 1 µM EA, or 1 µM EA in the presence of 5 µM ML204 (Akbulut et al. 2015). e Whole-cell current–voltage relationship of membrane current from a single A498 cell during ramp changes in membrane voltage from −100 to +100 mV applied every 10 s. 100 nM EA or its vehicle were bath-applied (Akbulut et al. 2015). f As (e) except with genetically modified HEK 293 cells induced to over-express TRPC4 and transiently express TRPC1 (HEK C4 + C1) (Akbulut et al. 2015)
Sourbier et al. suggested that EA activates protein kinase C θ to starve cells of glucose (Sourbier et al. 2013). However, Akbulut et al. found that this protein kinase C was not expressed in one of the most EA-sensitive renal cancer cell lines (Akbulut et al. 2015). Initial affinity-based chemical proteomics studies yielded no specific target, an explanation for which was considered to be that the target is a low-abundance membrane protein such as a G protein-coupled receptor or ion channel. This led to identification of TRPC4 and TRPC5 channels as targets of EA (Akbulut et al. 2015), and these targets were subsequently confirmed independently by another group (Carson et al. 2015).
EA turned out to be a remarkably efficacious, potent, specific and stereo-selective activator of TRPC4 and TRPC5 channels and TRPC1/TRPC4 and TRPC1/TRPC5 heteromeric channels (Akbulut et al. 2015). As little as 3 nM EA was enough to activate Ca2+ entry through TRPC4 channels or TRPC5 channels over-expressed in human embryonic kidney (HEK) 293 cells. The EC50 values for TRPC4 and TRPC5 were 11.2 and 7.6 nM (Fig. 1b, c). EA activated the channels from the outer face of excised membrane patches in the absence of co-factors, consistent with a direct action on the channels. In the A498 renal cell carcinoma cell line, EA evoked Ca2+ entry through endogenous channels with an EC50 of 9.5 nM, and this response was suppressed by ML204, the small-molecule inhibitor of TRPC4 channels (Fig. 1d). Whole-cell patch-clamp recordings suggested that the channels activated were most probably heteromers of TRPC1 and TRPC4, because the reversal potential and shape of the current–voltage relationship were similar to those of over-expressed TRPC1/TRPC4 channels (Fig. 1e, f). Importantly, the cytotoxic effect of EA could be reconstituted in otherwise resistant cells by over-expressing TRPC4 or TRPC5 and suppressed in cancer cell lines by knockdown of TRPC4 (Akbulut et al. 2015; Carson et al. 2015).
Although the initial study suggested that EA at 5 mg kg−1 lacked toxicity in vivo in mice and was effective against xenograft tumours (Sourbier et al. 2013), we found toxicity at 5 mg kg−1 (but not 2 mg kg−1) (unpublished data) and Carson et al. expressed concern about toxicity in rodents depending on the route of administration and dose (Carson et al. 2015). One challenge has been the formulation for in vivo studies, but a more serious problem has been the metabolic instability of EA, making it difficult to establish a meaningful dosing regimen (Carson et al. 2015). After administration of EA to rats at 5 mg kg−1, EA was detected in the blood at no more than 12 nM (Carson et al. 2015). This concentration is quite low, but it would be expected to activate endogenous TRPC1/TRPC4 channels based on in vitro data for A498 cells (Akbulut et al. 2015). We await studies on more metabolically stable EA derivatives which are active at the channels and information on whether the toxicity is mediated by TRPC4- or TRPC5-containing channels. At this stage, we can say that, in principle, it turns out to be possible to achieve rapid cytotoxicity through a potent and highly efficacious activator of TRPC4/TRPC5-containing channels and that such an agent might have potential as a starting point for a novel anti-cancer drug. There is, nevertheless, more research needed if the in vivo challenges of EA are to be overcome.
The effect of EA was initially emphasised in renal cell carcinoma cells, but it is also active in other but not all cancer cell lines, including cells derived from patients with triple-negative breast cancer and Ewing’s sarcoma (Ratnayake et al. 2009; Carson et al. 2015; Caropreso et al. 2016).
Inhibition of TRPC4-containing channels in cancer cell lines and the EA paradox
In non-small cell lung cancer, the expression of TRPC1 and TRPC4 was suggested to correlate with tumour grade (Jiang et al. 2013) and treatment with short interfering RNA targeted to TRPC1 and TRPC4 or inhibitor antibodies suppressed proliferation of an ovarian carcinoma cell line (Zeng et al. 2013). Conversely, over-expressing TRPC1 or TRPC4 increased proliferation (Zeng et al. 2013). These studies support the idea that TRPC4-containing channels are functionally significant in certain types of cancer cell line. They also reinforce the apparent paradox of the EA findings, where activation of the channels leads to rapid cell death amongst certain cancer cell lines. One explanation could be that it is simply a matter of the bell-shaped relationship between intracellular Ca2+ concentration and cell function, whereby modest elevations of Ca2+ are beneficial for cells, encouraging proliferation and migration, whereas high elevations, for example about 1 µM, are essentially toxic for cells, encouraging apoptosis and necrosis (Orrenius et al. 2003); that is, inhibition of basal activity of the channels might inhibit at least part of the unwanted proliferation and migration in cancer cells, whereas strong activation by a substance like EA might cause rapid cytotoxicity.
Conclusions
There are still few studies of TRPC4 and TRPC5 and TRPC4- and TRPC5-containing channels in cancer cell lines and even fewer on human cancer itself (Table 1). Therefore, any conclusions can only be preliminary. The studies reported so far do, nevertheless, suggest that there might be benefits for some cancer patients in taking a medication that inhibits or, paradoxically, activates these channels. Resistance to chemotherapy, cancer cell proliferation, cancer cell migration and tumour vascularisation might be suppressed, as well as the potential for rapid induction of selective cytotoxicity in certain types of cancer cell, such as those of renal cell carcinoma (Fig. 2). It should be emphasised, however, that most of the data supporting such suggestions have arisen from studies of cancer cell lines, which may often differ substantially from the cancer cells populating the tumours of patients. Moreover, even if suitable small-molecule modulators of the channels can be identified, it needs to be recognised that TRPC4- and TRPC5-containing channels are just one mechanism by which a cancer cell might regulate the intracellular Ca2+ required for Ca2+-dependent cell processes; that is, it is quite conceivable that modulation of the channels could relatively easily be circumvented by an ever-adapting cancer cell.
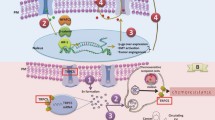
Simplified overview of TRPC4/5 channels as potential therapeutic targets in cancer. a VEGF-activated TRPC1/4/5 channels allow non-selective cation entry. A modest rise in intracellular Ca2+ can lead to an increase in migration, proliferation and tubulogenesis of endothelial cells, leading to angiogenesis. b TRPC5 drives chemoresistance in breast cancer cells as it leads to up-regulation of p-glycoprotein (pgp) which acts to pump drugs from the cell. TRPC5 is expressed in extracellular vesicles (EVs), and a critical role of TRPC5-containing EVs is in the transfer of drug resistance to non-chemoresistant recipient cells. c (−)-Englerin A is a selective TRPC1/4/5 channel activator which causes influx of Ca2+ and Na+ into certain types of cancer cell, which then causes cell death
Despite this recommendation for caution, we conclude that TRPC4 and TRPC5 represent potentially attractive targets for cancer therapeutics. Their diverse role in many of the aspects that drive the metastatic process warrants continued research into their context-dependent function. It is already apparent that inhibitors of the channels would be unlikely to cause significant adverse effects. Instead, it is conceivable that they may be associated with other benefits to patients with advanced malignancy, including suppression of innate fear, pain and pathological cardiac remodelling, where inhibition of these channels may be beneficial (Camacho Londono et al. 2015; Phelan et al. 2013; Zheng and Phelan 2014; Riccio et al. 2014; Wei et al. 2015; Westlund et al. 2014; Bon and Beech 2013).
Abbreviations
- TRPC:
-
Transient receptor potential canonical
- TRPV:
-
Transient receptor potential vanilloid
- TRPM:
-
Transient receptor potential melastatin
- TRPA:
-
Transient receptor potential ankyrin
- TRPP:
-
Transient receptor potential polycystin
- TRPML:
-
Transient receptor potential mucolipin
- 2-APB:
-
2-Aminoethoxydiphenyl borate
- VEGF:
-
Vascular endothelial growth factor
- EA:
-
(−)-Englerin A
- HEK:
-
Human embryonic kidney
- MDR:
-
Multi-drug resistance
- EVs:
-
Extracellular vesicles
References
Abramowitz J, Birnbaumer L (2009) Physiology and pathophysiology of canonical transient receptor potential channels. FASEB J 23:297–328
Akbulut Y, Gaunt HJ, Muraki K, Ludlow MJ, Amer MS, Bruns A, Vasudev NS, Radtke L, Willot M, Hahn S, Seitz T, Ziegler S, Christmann M, Beech DJ, Waldmann H (2015) (-)-Englerin A is a potent and selective activator of TRPC4 and TRPC5 calcium channels. Angew Chem Int Ed Engl 54:3787–3791
Al-Shawaf E, Naylor J, Taylor H, Riches K, Milligan CJ, O’Regan D, Porter KE, Li J, Beech DJ (2010) Short-term stimulation of calcium-permeable transient receptor potential canonical 5-containing channels by oxidized phospholipids. Arterioscler Thromb Vasc Biol 30:1453–1459
Antigny F, Girardin N, Frieden M (2012) Transient receptor potential canonical channels are required for in vitro endothelial tube formation. J Biol Chem 287:5917–5927
Arcangeli A, Crociani O, Lastraioli E, Masi A, Pillozzi S, Becchetti A (2009) Targeting ion channels in cancer: a novel frontier in antineoplastic therapy. Curr Med Chem 16:66–93
Bai CX, Giamarchi A, Rodat-Despoix L, Padilla F, Downs T, Tsiokas L, Delmas P (2008) Formation of a new receptor-operated channel by heteromeric assembly of TRPP2 and TRPC1 subunits. EMBO Rep 9:472–479
Beech DJ (2013) Characteristics of transient receptor potential canonical calcium-permeable channels and their relevance to vascular physiology and disease. Circ J 77:570–579
Berridge MJ, Lipp P, Bootman MD (2000) The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol 1:11–21
Birnbaumer L (2009) The TRPC class of ion channels: a critical review of their roles in slow, sustained increases in intracellular Ca(2+) concentrations. Annu Rev Pharmacol Toxicol 49:395–426
Bon RS, Beech DJ (2013) In pursuit of small molecule chemistry for calcium-permeable non-selective TRPC channels– mirage or pot of gold? Br J Pharmacol 170:459–474
Camacho Londono JE, Tian Q, Hammer K, Schroder l, Camacho Londono J, Reil JC, He T, Oberhofer M, Mannebach S, Mathar I, Philipp SE, Tabellion W, Schweda F, Dietrich A, Kaestner L, Laufs U, Birnbaumer L, Flockerzi V, Freichel M, Lipp P (2015) A background Ca2+ entry pathway mediated by TRPC1/TRPC4 is critical for development of pathological cardiac remodelling. Eur Heart J 36:2257–66
Caropreso V, Darvishi E, Turbyville TJ, Ratnayake R, Grohar PJ, Mcmahon JB, Woldmichael GM (2016) Englerin A inhibits EWS-FLI1 DNA binding in ewing sarcoma cells. J Biol Chem 291:10058–10066
Carson C, Raman P, Tullai J, Xu L, Henault M, Thomas E, Yeola S, Lao J, McPate M, Verkuyl JM, Marsh G, Sarber J, Amaral A, Bailey S, Lubicka D, Pham H, Miranda N, Ding J, Tang HM, Ju H, Tranter P, Ji N, Krastel P, Jain RK, Schumacher AM, Loureiro JJ, George E, Berellini G, Ross NT, Bushell SM, Erdemli G, Solomon JM (2015) Englerin A agonizes the TRPC4/C5 cation channels to inhibit tumor cell line proliferation. PLoS ONE 10:e0127498
Chen YF, Chen YT, Chiu WT, Shen MR (2013) Remodeling of calcium signaling in tumor progression. J Biomed Sci 20:23
Clapham DE (2007) Calcium signaling. Cell 131:1047–1058
Damann N, Voets T, Nilius B (2008) TRPs in our senses. Curr Biol 18:R880–R889
Fleckenstein A, Janke J, Doring HJ, Leder O (1974) Myocardial fiber necrosis due to intracellular Ca overload-a new principle in cardiac pathophysiology. Recent Adv Stud Cardiac Struct Metab 4:563–580
Flemming PK, Dedman AM, Xu SZ, Li J, Zeng F, Naylor J, Benham CD, Bateson AN, Muraki K, Beech DJ (2006) Sensing of lysophospholipids by TRPC5 calcium channel. J Biol Chem 281:4977–4982
Herve JC (2015) Membrane channels and transporters in cancers. Biochim Biophys Acta 1848:2473–2476
Jiang HN, Zeng B, Zhang Y, Daskoulidou N, Fan H, Qu JM, Xu SZ (2013) Involvement of TRPC channels in lung cancer cell differentiation and the correlation analysis in human non-small cell lung cancer. PLoS ONE 8:e67637
Jordt SE, Bautista DM, Chuang HH, McKemy DD, Zygmunt PM, Hogestatt ED, Meng ID, Julius D (2004) Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature 427:260–265
Jung S, Muhle A, Schaefer M, Strotmann R, Schultz G, Plant TD (2003) Lanthanides potentiate TRPC5 currents by an action at extracellular sites close to the pore mouth. J Biol Chem 278:3562–3571
Kuang CY, Yu Y, Wang K, Qian DH, Den MY, Huang L (2012) Knockdown of transient receptor potential canonical-1 reduces the proliferation and migration of endothelial progenitor cells. Stem Cells Dev 21:487–496
Kumar B, Dreja K, Shah SS, Cheong A, Xu SZ, Sukumar P, Naylor J, Forte A, Cipollaro M, McHugh D, Kingston PA, Heagerty AM, Munsch CM, Bergdahl A, Hultgardh-Nilsson A, Gomez MF, Porter KE, Hellstrand P, Beech DJ (2006) Upregulated TRPC1 channel in vascular injury in vivo and its role in human neointimal hyperplasia. Circ Res 98:557–563
Li J, Cubbon RM, Wilson LA, Amer MS, McKeown L, Hou B, Majeed Y, Tumova S, Seymour VAL, Taylor H, Stacey M, O’Regan D, Foster R, Porter KE, Kearney MT, Beech DJ (2011) Orai1 and CRAC channel dependence of VEGF-activated Ca(2+)-entry and endothelial tube formation. Circ Res 108:1190–1198
Liao M, Cao E, Julius D, Cheng Y (2013) Structure of the TRPV1 ion channel determined by electron cryo-microscopy. Nature 504:107–112
Ma X, Qiu S, Luo J, Ma Y, Ngai CY, Shen B, Wong CO, Huang Y, Yao X (2010) Functional role of vanilloid transient receptor potential 4-canonical transient receptor potential 1 complex in flow-induced Ca2+ influx. Arterioscler Thromb Vasc Biol 30:851–858
Ma X, Cai Y, He D, Zou C, Zhang P, Lo CY, Xu Z, Chan FL, Yu S, Chen Y, Zhu R, Lei J, Jin J, Yao X (2012) Transient receptor potential channel TRPC5 is essential for P-glycoprotein induction in drug-resistant cancer cells. Proc Natl Acad Sci U S A 109:16282–16287
Ma X, Chen Z, Hua D, He D, Wang L, Zhang P, Wang J, Cai Y, Gao C, Zhang X, Zhang F, Wang T, Hong T, Jin L, Qi X, Chen S, Gu X, Yang D, Pan Q, Zhu Y, Chen Y, Chen D, Jiang L, Han X, Zhang Y, Jin J, Yao X (2014) Essential role for TrpC5-containing extracellular vesicles in breast cancer with chemotherapeutic resistance. Proc Natl Acad Sci U S A 111:6389–6394
Majeed Y, Bahnasi Y, Seymour VA, Wilson LA, Milligan CJ, Agarwal AK, Sukumar P, Naylor J, Beech DJ (2011) Rapid and contrasting effects of rosiglitazone on transient receptor potential TRPM3 and TRPC5 channels. Mol Pharmacol 79:1023–1030
McKemy DD (2005) How cold is it? TRPM8 and TRPA1 in the molecular logic of cold sensation. Mol Pain 1:16
Miller M, Shi J, Zhu Y, Kustov M, Tian JB, Stevens A, Wu M, Xu J, Long S, Yang P, Zholos AV, Salovich JM, Weaver CD, Hopkins CR, Lindsley CW, McManus O, Li M, Zhu MX (2011) Identification of ML204, a novel potent antagonist that selectively modulates native TRPC4/C5 ion channels. J Biol Chem 286:33436–33446
Minke B, Wu C, Pak WL (1975) Induction of photoreceptor voltage noise in the dark in Drosophila mutant. Nature 258:84–87
Mohl MC, Iismaa SE, Xiao XH, Friedrich O, Wagner S, Nikolova-Krstevski V, Wu J, Yu ZY, Feneley M, Fatkin D, Allen DG, Graham RM (2011) Regulation of murine cardiac contractility by activation of alpha(1A)-adrenergic receptor-operated Ca(2+) entry. Cardiovasc Res 91:310–319
Monteith GR, Davis FM, Roberts-Thomson SJ (2012) Calcium channels and pumps in cancer: changes and consequences. J Biol Chem 287:31666–31673
Naylor J, Minard A, Gaunt HJ, Amer MS, Wilson LA, Migliore M, Cheung SY, Rubaiy HN, Blythe NM, Musialowski KE, Ludlow MJ, Evans WD, Green BL, Yang H, You Y, Li J, Fishwick CWG, Muraki K, Beech DJ, Bon RS (2015) Natural and synthetic flavonoid modulation of TRPC5 channels. Br J Pharmacol 173:562–574
Orrenius S, Zhivotovsky B, Nicotera P (2003) Regulation of cell death: the calcium-apoptosis link. Nat Rev Mol Cell Biol 4:552–565
Park YR, Chun JN, So I, Kim HJ, Baek S, Jeon JH, Shin SY (2016) Data-driven analysis of TRP channels in cancer: linking variation in gene expression to clinical significance. Cancer Genomics Proteomics 13:83–90
Paulsen CE, Armache JP, Gao Y, Cheng Y, Julius D (2015) Structure of the TRPA1 ion channel suggests regulatory mechanisms. Nature 520:511–517
Phelan KD, Shwe UT, Abramowitz J, Wu H, Rhee SW, Howell MD, Gottschall PE, Freichel M, Flockerzi V, Birnbaumer L, Zheng F (2013) Canonical transient receptor channel 5 (TRPC5) and TRPC1/4 contribute to seizure and excitotoxicity by distinct cellular mechanisms. Mol Pharmacol 83:429–438
Prevarskaya N, Skryma R, Shuba Y (2011) Calcium in tumour metastasis: new roles for known actors. Nat Rev Cancer 11:609–618
Radtke L, Willot M, Sun H, Ziegler S, Sauerland S, Strohmann C, Frohlich R, Habenberger P, Waldmann H, Christmann M (2011) Total synthesis and biological evaluation of (−)-englerin A and B: synthesis of analogues with improved activity profile. Angew Chem Int Ed Engl 50:3998–4002
Ratnayake R, Covell D, Ransom TT, Gustafson KR, Beutler JA (2009) Englerin A, a selective inhibitor of renal cancer cell growth, from Phyllanthus engleri. Org Lett 11:57–60
Riccio A, Li Y, Tsvetkov E, Gapon S, Yao GL, Smith KS, Engin E, Rudolph U, Bolshakov VY, Clapham DE (2014) Decreased anxiety-like behavior and Galphaq/11-dependent responses in the amygdala of mice lacking TRPC4 channels. J Neurosci 34:3653–3667
Richter JM, Schaefer M, Hill K (2014a) Clemizole hydrochloride is a novel and potent inhibitor of transient receptor potential channel TRPC5. Mol Pharmacol 86:514–521
Richter JM, Schaefer M, Hill K (2014b) Riluzole activates TRPC5 channels independently of PLC activity. Br J Pharmacol 171:158–170
Schaldecker T, Kim S, Tarabanis C, Tian D, Hakroush S, Castonguay P, Ahn W, Wallentin H, Heid H, Hopkins CR, Lindsley CW, Riccio A, Buvall L, Weins A, Greka A (2013) Inhibition of the TRPC5 ion channel protects the kidney filter. J Clin Invest 123:5298–5309
Song HB, Jun HO, Kim JH, Fruttiger M, Kim JH (2015) Suppression of transient receptor potential canonical channel 4 inhibits vascular endothelial growth factor-induced retinal neovascularization. Cell Calcium 57:101–108
Sourbier C, Scroggins BT, Ratnayake R, Prince TL, Lee S, Lee MJ, Nagy PL, Lee YH, Trepel JB, Beutler JA, Linehan WM, Neckers L (2013) Englerin A stimulates PKCtheta to inhibit insulin signaling and to simultaneously activate HSF1: pharmacologically induced synthetic lethality. Cancer Cell 23:228–237
Stewart TA, Azimi I, Thompson EW, Roberts-Thomson SJ, Monteith GR (2015) A role for calcium in the regulation of ATP-binding cassette, sub-family C, member 3 (ABCC3) gene expression in a model of epidermal growth factor-mediated breast cancer epithelial-mesenchymal transition. Biochem Biophys Res Commun 458:509–514
Strubing C, Krapivinsky G, Krapivinsky L, Clapham DE (2001) TRPC1 and TRPC5 form a novel cation channel in mammalian brain. Neuron 29:645–655
Sukumar P, Sedo A, Li J, Wilson LA, O’Regan D, Lippiat JD, Porter KE, Kearney MT, Ainscough JF, Beech DJ (2012) Constitutively active TRPC channels of adipocytes confer a mechanism for sensing dietary fatty acids and regulating adiponectin. Circ Res 111:191–200
Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D (1998) The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron 21:531–543
Tsvilovskyy VV, Zholos AV, Aberle T, Philipp SE, Dietrich A, Zhu MX, Birnbaumer L, Freichel M, Flockerzi V (2009) Deletion of TRPC4 and TRPC6 in mice impairs smooth muscle contraction and intestinal motility in vivo. Gastroenterology 137:1415–1424
Vannier B, Peyton M, Boulay G, Brown D, Qin N, Jiang M, Zhu X, Birnbaumer L (1999) Mouse trp2, the homologue of the human trpc2 pseudogene, encodes mTrp2, a store depletion-activated capacitative Ca(2+) entry channel. Proc Natl Acad Sci USA 96:2060–2064
Vasudev NS, Reynolds AR (2014) Anti-angiogenic therapy for cancer: current progress, unresolved questions and future directions. Angiogenesis 17:471–494
Veliceasa D, Ivanovic M, Hoepfner FT, Thumbikat P, Volpert OV, Smith ND (2007) Transient potential receptor channel 4 controls thrombospondin-1 secretion and angiogenesis in renal cell carcinoma. FEBS J 274:6365–6377
Wang T, Chen Z, Zhu Y, Pan Q, Liu Y, Qi X, Jin L, Jin J, Ma X, Hua D (2015) Inhibition of transient receptor potential channel 5 reverses 5-fluorouracil resistance in human colorectal cancer cells. J Biol Chem 290:448–456
Wei H, Sagalajev B, Yuzer MA, Koivisto A, Pertovaara A (2015) Regulation of neuropathic pain behavior by amygdaloid TRPC4/C5 channels. Neurosci Lett 608:12–17
Wes PD, Chevesich J, Jeromin A, Rosenberg C, Stetten G, Montell C (1995) TRPC1, a human homolog of a Drosophila store-operated channel. Proc Natl Acad Sci U S A 92:9652–9656
Westlund KN, Zhang LP, Ma F, Nesemeier R, Ruiz JC, Ostertag EM, Crawford JS, Babinski K, Marcinkiewicz MM (2014) A rat knockout model implicates TRPC4 in visceral pain sensation. Neuroscience 262:165–175
Xu SZ, Zeng F, Lei M, Li J, Gao B, Xiong C, Sivaprasadarao A, Beech DJ (2005) Generation of functional ion-channel tools by E3 targeting. Nat Biotechnol 23:1289–1293
Xu SZ, Boulay G, Flemming R, Beech DJ (2006) E3-targeted anti-TRPC5 antibody inhibits store-operated calcium entry in freshly isolated pial arterioles. Am J Physiol Heart Circ Physiol 291:H2653–H2659
Xu SZ, Sukumar P, Zeng F, Li J, Jairaman A, English A, Naylor J, Ciurtin C, Majeed Y, Milligan CJ, Bahnasi YM, Al-Shawaf E, Porter KE, Jiang LH, Emery P, Sivaprasadarao A, Beech DJ (2008) TRPC channel activation by extracellular thioredoxin. Nature 451:69–72
Yu PC, Gu SY, Bu JW, Du JL (2010) TRPC1 is essential for in vivo angiogenesis in zebrafish. Circ Res 106:1221–1232
Zeng B, Yuan C, Yang X, Atkin SL, Xu SZ (2013) TRPC channels and their splice variants are essential for promoting human ovarian cancer cell proliferation and tumorigenesis. Curr Cancer Drug Targets 13:103–116
Zheng F, Phelan KD (2014) The role of canonical transient receptor potential channels in seizure and excitotoxicity. Cells 3:288–303
Author information
Authors and Affiliations
Corresponding authors
Additional information
Special Issue: Ion Channels, Transporters and Cancer.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Gaunt, H.J., Vasudev, N.S. & Beech, D.J. Transient receptor potential canonical 4 and 5 proteins as targets in cancer therapeutics. Eur Biophys J 45, 611–620 (2016). https://doi.org/10.1007/s00249-016-1142-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00249-016-1142-1