Abstract
Transient receptor potential (TRP) channels are sensors for a variety of cellular and environmental signals. Mammals express a total of 28 different TRP channel proteins, which can be divided into seven subfamilies based on amino acid sequence homology: TRPA (Ankyrin), TRPC (Canonical), TRPM (Melastatin), TRPML (Mucolipin), TRPN (NO-mechano-potential, NOMP), TRPP (Polycystin), TRPV (Vanilloid). They are a class of ion channels found in numerous tissues and cell types and are permeable to a wide range of cations such as Ca2+, Mg2+, Na+, K+, and others. TRP channels are responsible for various sensory responses including heat, cold, pain, stress, vision and taste and can be activated by a number of stimuli. Their predominantly location on the cell surface, their interaction with numerous physiological signaling pathways, and the unique crystal structure of TRP channels make TRPs attractive drug targets and implicate them in the treatment of a wide range of diseases. Here, we review the history of TRP channel discovery, summarize the structures and functions of the TRP ion channel family, and highlight the current understanding of the role of TRP channels in the pathogenesis of human disease. Most importantly, we describe TRP channel-related drug discovery, therapeutic interventions for diseases and the limitations of targeting TRP channels in potential clinical applications.
Similar content being viewed by others
Introduction
Transient receptor potential (TRP) channels, which were first discovered in 1969,1 they are multimodal ion channels that act as sensors of chemically toxic and physical stimuli.2 These channels are widely distributed in various tissues and play a variety of roles.3 Despite having been studied for a long time, the significance of TRP channels remains unclear. In addition, changes in TRP channels have been observed in a variety of diseases.4 More importantly, the regulation of TRP channels can be exploited to prevent or treat diseases. In this review, we present a historical perspective on TRP channels. We then discuss the structure and function of TRP channels and summarize their different types, focusing on their key roles in various physiological and pathological conditions, particularly in pain, inflammatory bowel disease (IBD) and its complications, respiratory diseases, neurological disorders, and cardiovascular diseases (CVDs). We also highlight therapeutic interventions, including physical and pharmacological ones, regarding TRP channels. Finally, we conclude this review with a summary of frequent adverse events that occur during treatments.
History of TRP channels
TRP channels were first proposed over 50 years ago (Fig. 1). In 1969, Cosens et al. identified a visual mutant in Drosophila.1 The mutant showed a transient rather than a continuous response to bright light stimuli. Minke et al. were the first to name the mutant “transient receptor potential” (trp) in 1975 based on its electrophysiological phenotype.5 In 1989, Montell et al.6 and Wong et al.7 successively cloned the trp gene and recognized it as a transmembrane protein. Since then, researchers have focused on expanding the understanding of trp.
Minke’s group was the first to establish a link between trp mutants and Ca2+.8 Shortly thereafter, Minke and Selinger confirmed that the trp protein is a plasma membrane component (or part of one) that oscillates between Ca2+-transporting and non-transporting states.9 A strong compelling evidence demonstrated that TRP is a light-activated and Ca2+, permeable channel, as discovery by Hardie and Minke.10 Meanwhile, Kelly and colleagues observed that TRP-like proteins are typical of voltage-gated channels.11 They also identified the ankyrin repeats in the known amino acid (aa) sequence of TRP. After the 1990s, the presence of TRPs was confirmed in various types of cell lines.12,13 These reports provided a glimpse into the biological functions of TRPs. In addition, TRPs are widely present in healthy human tissues.
In 2002, Montell et al.14 and Clapham et al.15 undertook work to unify the TRP nomenclature. The 28 channel subunit genes were subdivided into seven subfamilies, namely, TRPA (ankyrin), TRPC (canonical), TRPM (melastatin), TRPML (mucolipin), TRPN (NO-mechano-potential, NOMP), TRPP (polycystin) and TRPV (vanilloid), based on their gene sequences. Mammals are represented in all subfamilies.16 Research on diseases based on TRPs has received a great deal of attention in the last two decades. In particular, the development of molecular biology technologies has improved the understanding of TRPs.
TRP ion channel family
Classifications of TRP
Based on sequence and topological differences,6 mammalian TRPs can be categorized into the following seven subfamilies: group 1 with five subfamily members (TRPC, TRPV, TRPM, TRPN, and TRPA), and group 2 with two subfamilies (TRPP and TRPML) (Table 1 and Fig. 2).17 An additional subfamily, TRPY, consists of yeast TRPs, which are distantly related to group 1 and group 2 TRPs.18 The first and second groups both have six transmembrane structural domains and are permeable to cations. TRPA, TRPV, and TRPC channels contain ankyrin repeat sequences in their intracellular N-terminal structural domains, whereas TRPC and TRPM subfamilies possess a proline-rich “TRP structural domain” in the C-terminal region near the presumed transmembrane segment.19 Group 2 channels also have high sequence homology in their transmembrane structural domains and are only distally related to the genes of group 1 channels because they contain a large lumenal/extracellular domain between transmembrane helix 1 (S1) and S2.20
TRPC
TRPC channels were the first identified members of the TRP family.21 TRPC channels are nonselective cation channels.22 They range from a few tens to a few hundred kilodaltons.23 In 1995, the full sequence of the first human homolog (TRPC1) was reported.24 Since then, seven mammalian TRPC proteins (TRPC1–7) have been described.14 Based on aa similarities, mammalian TRPCs fall into four subsets: TRPC1, TRPC2, TRPC3/6/7, and TRPC4/5.25 TRPC channel proteins are expressed in excitable and non-excitable cells.26 TRPCs have a broad tissue-specific distribution and are therefore involved in various pathophysiological functions.27
TRPV
TRPV channels are a part of the TRP channel superfamily and named for their sensitivity to vanilloid and capsaicin.28,29 TRPVs are divided into two types.30 TRPV1–4 can be thermally activated and are therefore called thermal TRP channels.31 One of the first clues toward understanding the functional diversity of TRPV channels came from the structural studies of the N-terminal ankyrin repeat domain.32 In most tissues, these domains serve as sensors for different pain stimuli (heat, pressure, and pH) and contribute to the homeostasis of electrolytes, the maintenance of barrier functions, and the development of macrophages.33,34 Given fundamental role in a multitude of physiological and pathophysiological processes, TRPV channels are promising targets for drug development.
TRPA
In 1999, the first human TRPA protein, TRPA1, was discovered during a screening of downregulated genes following the oncogenic transformation of fibroblasts.35 TRPA1 is the only TRPA protein present in humans.36,37 TRPA1 was previously called ANKTM1 because the protein consists of numerous N-terminal ankyrin repeats.38 TRPA1 is a sensor for diverse noxious external stimuli such as intense cold, irritating compounds, mechanical stimuli, reactive chemicals, and endogenous signals associated with cellular damage.39 This diversity of functions and expression in nociceptive nerve fibers, epithelial cells, and various other cells make this channel relevant to a wide range of diseases and an attractive therapeutic target.40
TRPM
Since the first cloning of TRPM1 in 1998, tremendous progress has been made in the identification of novel members of the TRPM subfamily and their functions.41 The TRPM subfamily consists of eight members; TRPM1–TRPM8.42 TRPMs have been involved in several physiological and pathological processes, including cellular proliferation,43 temperature sensing,44 vascular development,45 cancer progression,46 neurological diseases,47 endothelial dysfunction,48 inflammation,49 type II diabetes,50 and many other processes. TRPM channels possess a large cytosolic domain consisting of 732 and 1611 aa for each subunit, making them the largest members of the TRP superfamily.51
TRPN
The TRPN subfamily was named based on the founding member, Drosophila NOMPC.52 Mammals do not encode any TRPN members.53 TRPNs are found in worms, flies, and zebrafish.54 Genetic screening, calcium imaging, and electrophysiological analysis have identified TRPN as a mechanically gated channel in eukaryotes.55 A TRPN channel protein is essential for sensory transduction in insect mechanosensory neurons and in vertebrate hair cells.56 However, without a molecular marker for the protein, its exact location and role in transduction are uncertain.
TRPP and TRPML
TRPP and TRPML belong to group 2 of TRP channels and have limited homology similarity to group 1. Furthermore, the large loop between transmembrane domains (TMD) 1 and 2, which discriminates them from group 1.25 Invertebrate TRPPs have been described in C. elegans,57 Drosophila (AMO),58 and the sea urchin (suPC2).59 The three human genes encoding for the TRPP protein family are polycystic kidney disease 2 (PKD2 and TRPP2), PKD2-like 1 (PKD2L1 and TRPP3), and PKD2-like 2 (PKD2L2 and TRPP5).17 TRPPs may comprise the most primitive TRP subfamily given that as microbial homologs of TRPP2 are found in yeast.60 In addition to functioning as a cation influx channel, TRPP2 is also localized in the endoplasmic reticulum (ER) membrane where it is proposed to serve as a new type of Ca2+-release channel.61
The mucolipin family of the ion channel TRP superfamily (TRPML) includes three members: TRPML1, TRPML2, and TRPML3.62 The molecular weights of TRPMLs are around 65 kDa.63 TRPML1, was cloned during the search for the genetic determinants of the lysosomal storage disease MCOLN.64 Mutations of this protein are characterized by severe neurodegeneration. Defects in TRPML function are predicted to have important effects on organelle acidification, vesicle fusion, endosome maturation, and signaling; thus, suggesting that this protein family plays a key role in normal and pathological conditions.65,66
Structures of TRP channels
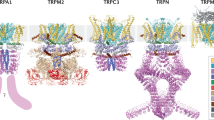
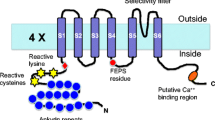
Since the discovery of TRPs, researchers have systematically analyzed their structure TRPs through technical means (Table 2 and Fig. 3). The progressive development of sequencing and analytical techniques has revealed several key structural features of TRPs. TRP channels have six transmembrane spanning domains (S1–S6), with a pore-forming loop between S5 and S6, and both the C and N termini are located intracellularly.67 The cytoplasmic end of the S6 helix forms to form the lower gate, which opens and closes to regulate cation entry into the channel. The S1–S4 domains may gate the pore in response to ligand binding, but the paucity of positively charged arginine in the S4 helix indicates the weak voltage sensitivity of TRP channels. All elements outside the S5–S6 region provide means of either subunit association. They also act as a linker for the elements that control the gating. Cryo-electron microscopy (cryo-EM) confirmed that TRPs have a structure similar to that of voltage-gated ion channels.68 Most TRPs form functional channels as homotetramers, but heteromultimerization is frequently observed.69 This condition creates a potential problem for drug discovery efforts because heteromultimers (which are difficult to recreate in heterologous expression systems) may have distinct pharmacological properties.
Structure of TRP channels. Representative structures for each TRP subfamily. In each small figure, the top one is the side view and the bottom one is the top view. a TRPA1 (PDB ID: 6PQP); b TRPV1 (PDB ID: 7LP9); c TRPM8 (PDB ID: 7WRB); d TRPC3 (PDB ID: 6CUD); e TRPML1 (PDB ID: 7SQ8); f TRPN (PDB ID: 5VKQ); g TRPP2 (PDB ID: 5T4D)
TRPVs
The earliest structural information obtained for the TRPV subfamily was the X-ray crystal structures of the isolated cytoplasmic ankyrin repeat domain from TRPV2 in 2006, immediately followed by similar structures for other TRPV channels.70,71 As is true for all TRP channels, TRPV channels are tetrameric and each monomer features the classic six transmembrane helix architecture of voltage-gated ion channels in its TMD.72 The overall sequence similarity of TRPVs is more than 40%. TRPV protein has an ~400–450-residue N-terminal domain with 3–6 intracellular ankyrin repeats, which are essential for channel function.70,73 An ~150-residue C-terminus that acts as a platform for interactions with other proteins and ligands.74
Liao et al. used advances in EM to determine the structure of the mammalian TRPV1 channel at a resolution of 3.4 Å.75 S1–S4 form a bundle similar to the voltage-sensing domain in voltage-sensitive ion channels.72 S5 and S6 extend from the S1–S4 bundle, and the conserved “TRP structural domain” interacts with the S4–S5 linker.72,76 TRPV1 has a wide extracellular “mouth” with short selective filtering.75 This domain-swapped pore domain consists of S5 and S6, which form the central pore and lower gate, whereas a short loop and helix between S5 and S6, termed the pore helix (PH), forms the upper gate or selectivity filter.72 The dynamic communication between the upper and lower gates may be the basis for the integration of various physiological signals.76 Notably, the motion of S6 in the TRPV1 structure can be roughly described as small, medium, or large. When the motion is small, the π-helix is retained, and the channel gate is formed by I679.77 In the intermediate category, the π-helix transitions to the α-helix, and the gate is formed by M682.77 When S5/S6 motion is large, the π-helix conformation is observed, and the open gate (9.8 Å) is formed by I679.77 However, transitions between π and α have also been observed in TRPV2 and V3 structures, but the functional consequences of such transitions remain to be fully understood.78,79
TRPCs
Given the great advancements in single-particle cryo-EM technology, high-resolution structures of TRPCs became available in 2018.80 TRPC2 is thought to be a pseudogene in humans.81 The overall architecture of the homologous TRPC3, TRPC4, TRPC5, and TRPC6 is consistent with that of TRP.82,83,84 The structure of TRPCs is similar to the voltage sensor structural domains of voltage-gated K+, Na+ and Ca2+ channels, with S5–S6 forming a structurally conserved ion conductance or pore domain common to all TRP channels, voltage-gated channels, inwardly-rectifying K channels, and bacterial K and Na channels.85,86 All TRPC structures contain a three-helix region designated as the pre-S1 elbow and pre-S1 helix before the transmembrane S1 helix.87,88 This region is halfway embedded in the membrane, with its N-terminal exposed to the cytoplasmic side to connect with a long stretch of tightly folded linker helices located at the proximal N-terminus of each protomer.89 Immediately before the linker helices are the four ankyrin-like repeats that form the outskirt of the cytoplasmic architecture, which completely surrounds the four helical bundles composed of the second C-terminal helix.90
Currently, the resolved region of the cytoplasmic structural domain of TRPC4 reaches approximately 80 Å, which is relatively short compared with that of other TRP channels.91 Helices S5 and S6 are exchanged with the respective helix of the adjacent protomer and form a pore at the center of the quaternary channel with a negatively charged extracellular opening; residue E555 at the top end of the pore tower is conserved in TRPC1, TRPC4 and TRPC5 and forms a salt bridge with R556 of the adjacent protomer.91 Fan et al.92 proposed that TRPC3 is in a lipid-occupied closed state with a structure of 3.3 Å. TRPC3 has four elbow-like membrane reflux helices before the first transmembrane helix.92 The third transmembrane helix, S3, is very long, forms a unique transmembrane structural domain and constitutes an extracellular structural domain that may act as a sensor for external stimuli.92
TRPA
The TRPA1 gene encodes a large protein consisting of about 1100 aa (1119 aa in humans, 1125 aa in rats, 1115 aa in mice, 1120 aa in zebrafish, 1197 aa in Drosophila, and 1193 aa in C. elegans), with an estimated molecular mass between 120 and 130 kDa.93 TRPA1 has distinctively large intracellular NH2 and COOH termini, which together account for about 80% of its molecular mass.94 Each subunit of TRPA1 consists of six transmembrane alpha sheets (S1–S6) plus a re-entrant pore loop between S5 and S6; homotetramers are formed by “domain exchange” interactions and establish a conserved transmembrane core.95,96 The distinguishing feature of TRPA1 is its long N-terminus with 14–18 predicted ankyrin repeats, each consisting of 33 aa.97,98 A structural domain containing a sequence of five ankyrin repeats surrounds the coiled coil and connects to another extended feature, which forms a crescent-shaped element.95 TRPA1 also possesses a distinct tetrameric coiled coil at the center of the channel, beneath the permeation pore. This stalk-like structure is stabilized by the interaction of positively charged residues on the exterior surface of the coiled coil with inositol polyphosphates.94 These interactions are essential for TRPA1 channel activity.94,99,100
TRPMs
In late 2017, several TRPM structures were solved using single-particle cryo-EM.101 TRPMs have four shared homologous regions: a TMD consisting of six transmembrane helices, a TRP helix, and C-terminal domains containing the conserved TRP domain followed by a coiled-coil region that differs among members.16,102 Despite their common structural features, the members of the TRPM subfamily are less conserved than those of other subfamilies. Several TRPM channels, such as TRPM2, uniquely contain a functional nucleoside diphosphate linked to another moiety/ADP ribose domain and a kinase domain that resembles to protein kinase A to a certain extent.103,104 TRPM2 is structurally characterized by its C-terminal NUDT9-H structural domain, a homologue of the human ADP-ribose pyrophosphatase NUDT912.105 In other words, these TRPMs combine features of ion channels and enzymes and are thus referred to by some as “chanzymes”.106,107,108 The ion conductance pore of TRPM2 is restricted at two locations, a selective filter located close to the extracellular entrance, which is formed by a hinge connecting the PH and the P ring, and a gate lined by S6 near the inner end of the cell.105
TRPM4 consists of three layers, namely, the N-terminal nucleotide-binding domain, ankyrin repeat domain, and the C-terminal coiled-coil helix, which form the base layer of TRPM4.109 The middle layer consists of a linking helix structure containing 12 helices and forming a scaffold for multiple interactions between structures within the subunit.109 The S1–S6 and TRP structural domains embedded in lipid form the top layer of the channel.109 The C-end coiled spiral structure forms a parallel disc-shaped coil.110 The discoid coil is surrounded by a large, intertwined cytoplasmic structural domain consisting of four N-terminal TRPM homology regions (MHR1–4), which are highly conserved in the TRPM subfamily.95,102,111 Of these, MHR1–2 contain an eight-stranded β-sheet surrounded by eight α-sheets.110 By contrast, MHR3–4 are composed of stacked α-sheets and connected to the transmembrane structural domain via MHR4, which clasps the TRP structural domain; as a result, an interaction occurs between the cytoplasmic structural domain and the transmembrane core.109,112 In 2018, Autzen et al.112 compared the structures of Ca2+-free and Ca2+-bound TRPM4 and observed an additional density in the hydrophilic pocket on the cytoplasmic side of the S1–S4 structural domain (this density represents a true bound Ca2+).
The mouse TRPM8 channel is a three-layer homologous structure. The top transmembrane channel region consists of the pre-S1 structural domain, TMD, and TRP structural domain.113 The TMD consists of a voltage-sensor-like structure (VSLD) consisting of transmembrane helices S1 to S4, and a pore domain formed by S5 and S6 helices,113,114 similar to the previously identified TRPV structure.75,79 The TMD of TRPM8 also has several features that distinguish it from the structure of other TRP ion channels. First, relative to the structure of apo TRPV1, the PH of TRPM8FA is positioned 12 Å away from the central axis, tilted by 8°, and shifted by 5 Å toward the outside of the cell.101 Second, no non-α helical elements (e.g., 310 or π helices) can be found in the TMs of TRPM8, which in other TRP channels are proposed to provide helical bending points for channel gating.115 Finally, although TRPV channels have a pre-cellular S1 helix, TRPM8FA contains three additional helices between S1 and the presumed pre-cellular S1 helix in the membrane region.101 Diver et al.116 observed a substantial rearrangement in the S4–S5 linker in the TRPM8 structure, and it repositioned the S1–S4 and pore domains relative to the TRP helix.
TRPPs
The TRPP subfamily has three members (TRPP2, TRPP3, and TRPP5).117 The PKD1 and PKD2 genes encoding polycystin-1 and polycystin-2 proteins, respectively, were originally named TRPP1 and TRPP2.118,119,120 However, members of the PKD1 family do not belong to the TRP family. PKD proteins are divided into two groups based on sequence homology: typical isoforms with 6 TMs (PKD2 subfamily), such as TRPP2, TRPP3, and TRPP5; and another group with 11 TMs and a short intracellular C-terminal tail (PKD1 subfamily), represented by PKD1, PKD1L1, PKD1L2, PKD1L3 and PKD-REJ7.121 The TRPP protein can form homotetramers and heterotetramers,122,123 such as the PKD1/TRPP2 and PKD1L3/TRPP3 complexes, with members of the PKD1 family.124,125,126
PKD1 is a 4,303-aa, 465-kDa integral membrane protein containing a large N-terminal extracellular structural domain and an intracellular C-terminal structural domain.127 Unlike PKD1, TRPP2 is a 968-aa, 110-kDa protein with 6 TMs and a pore-forming loop,128 and has intracellular amino and carboxyl termini.129 Shen et al.130 showed that TRPP2 is a Na/K conducting channel with low permeability and small single-channel conductance to Ca2+. The complexes of PKD1 and TRPP2 exhibit a 1:3 structural ratio.131 TRPP2 and PKD1 bind directly through their C-termini to form a complex containing three TRPP2 and one PKD1.132 This association involves a coiled-coil structure at the C-terminus of both proteins.125 R807X, E837X, and R872X of TRPP2 and R4227X and Y4236X of PKD1 can lead to deletion of the coiled-coil structural domain, which results in the mutation of ADPKD.133,134
TRPP3 (polycystin-2 like 1 (PKD2L1)) and TRPP2 share high sequence similarity (79% homology and 62% identity).135 Unlike TRPP2, the PH and S6 of TRPP3 are involved in the opening of the upper and lower gates to adopt an open conformation.136 Su et al.136 suggested that the simultaneous occurrence of gate opening and voltage-sensing domain (VSD) conformational changes in TRPP3 is caused by coupling between the VSD and pore domains. PKD1L3/TRPP3 complexes form calcium-permeable, non-selective cation channels.137,138 The structure of PKD1L3/TRPP3 in the apo and Ca2+-loaded states shows two Ca2+ binding sites at selectivity filter and VSDIII respectively.139 It also has a 1:3 stoichiometry TRP structure protected by Lys2069 of PKD1L3 and Asp523 from the three subunits of TRPP3.139
TRPN and TRPMLs
Drosophila NOMPC protein is the first member of the TRPN subfamily.140 The Drosophila NOMPC protein shares significant homology with the TRP superfamily.52 The N-terminus of NOMPC contains 29 ankyrin repeats, the largest number among TRP channels.141 Unlike other TRP family proteins, the ankyrin repeat structure of NOMPC is a quadruple structure, with each structural domain acting like a helical spring.142 The TMD of each NOMPC subunit consists of six transmembrane α-helices (S1–S6) and a re-entrant pore loop located between S5 and S6.142 The TRP structural domain is sandwiched between the linker helix and the S4–S5 linker. The short helix following the TRP structural domain is wrapped around the elbow in front of S2 and S1.142 However, the C-terminus is mostly unstructured, with a short helix fragment interacting with the linker domain.142 No TRPN has been found in mammals.
The TRPML subfamily is defined by a human protein, mucolipin 1.143 Mucolipin 1 (TRPML1) is a 580 aa-long protein probably restricted to intracellular vesicles.144 TRPML1 contains two proline-rich regions, namely, a lipase serine active site in the N-terminus and a dileucine motif suggestive of lysosomal targeting in the C-terminus.64 TRPML1 is characterized by its longer S2 helix than that of PKD2 and protrudes from the membrane bilayer-embedded portion of the cell membrane, with an extension of the N-terminal S1 helix (pre-S1) that includes two small alpha helices (α1 and α2) rich in many basic aa.145 Several aromatic and hydrophobic residues in the PH 1, S5, and S6 helices of TRPML1 and the S6 helix of the adjacent subunit form a hydrophobic cavity to accommodate the agonist.145 Phosphatidylinositol-3,5-bisphosphate binds to the N-terminal end of TRPML1, that is away from the pore, and a helix-turn-helix extension between the S2 and S3 segments may link ligand binding to pore opening.146 Two other members of the TRPML subfamily, namely, TRPML2 and TRPML3, are encoded by MCOLN2 and MCOLN3 genes, respectively.147 Similar to TRPML1, they are active in late endosomes and lysosomes.148 TRPML3 is divided into an extracellular structural domain (ECD), a TMD, and a cytosolic structural domain.115 The ECD consists of two β-sheets and two extracellular helices that form a ring that caps the extracellular side of the channel and is structurally similar to the ECD of TRPML1 and PKD2.115,130,149,150 Unlike PKD2, in which the ECD interacts extensively with the TMD, in TRPML3, minimal interaction exists between the ECD and the TMD.115,151
Agonists and antagonists
Various stimuli, including noxious and innocuous heat or cold stimuli, changes in osmolarity, proton gradients, and irritant compounds, have the potential to activate TRP channels.152 The activity of TRP channels is also modulated by several natural products, herbs, and compounds of plant origin. For stimulation by temperature, each temperature-sensing TRP channel has a specific activation threshold. Notably, numerous TRP agonists and antagonists are not specific; cross-reactivity can be observed between different TRP channels.
Several potent small-molecule agonists and inhibitors of TRPV1, TRPV4, and TRPA1 have entered clinical trials for the treatment of inflammatory, neuropathic, and visceral pain; however, the therapeutic mechanism of action of these compounds is unclear.153 The TRPA1 agonist cinnamaldehyde improves the capability of glucose metabolism by slowing down the gastric emptying rate and food intake via the TRPA1-ghrelin pathway.154 In addition, oral administration of HC-030031, an inhibitor of TRPA1, significantly reversed mechanical hypersensitivity in a spinal nerve ligation model of complete Freund’s adjuvant (CFA)-induced inflammatory pain and neuropathic pain in rats.155 However, the mechanism by which it regulates TRPA1 is unclear.
Capsaicin is the most classic TRPV1 agonist, and it has a role in enhancing pain.156 Capsaicin has a “tail up, head down” conformation. Molecular dynamic simulations revealed that the capsaicin molecule flips from extracellular to intracellular and can subsequently enter the intracellular TRPV1 binding site.157 Oral administration of GSK1016790A activates TRPV4 and reduces atherosclerotic plaque formation.158
Menthol is known to be an agonist of TRPM8. However, the mechanism by which TRPM8 channels respond to this drug is not well understood. Mutations in residues within the transmembrane and TRP structural domains impair the efficacy of menthol, such as Y745, R842, and L1009 (all residue numbers belong to mouse TRPM8), impair the efficacy of menthol, which suggests that this structural domain affects initial binding downstream.159 Later, in a cryo-EM of the structure of full-length avian TRPM8, the menthol binding site was located within a voltage-sensor-like structural domain.101 The cavity near Y745 and R842 was identified as the binding pocket for menthol. Recently, Xu et al. proposed that menthol, with its hydroxyl group as a hand, specifically grasps R842, and with its isopropyl group as a leg, it stands on I846 and L843, combining TRPM8 by a “grasp and stand” mechanism.160 Thus, numerous agonists and antagonists of TRPs have been reported (Table 3).
Signaling pathways affected by TRP channels
TRP channels exhibit ion selectivity. For example, TRPV5 and TRPV6 are selective toward Ca2+ and have a selective filter similar to that of voltage-gated potassium channels.161 Monovalent, divalent, and large organic cations pass through TRPV1, V2, V3, and TRPA1 and may allow dynamic ion selectivity.77 TRPM3 is found to be a constitutive Ca2+ and Mn2+ permeable channel, as shown by in vitro studies.162 TRPM4 and TRPM5 are voltage-regulated and conduct monovalent cations, such as Na+ or K+, without significant Ca2+ penetration.163 The activity of TRPM7 is regulated by intracellular Ca2+ and Mg2+ and is permeable to almost all physiological divalent cations and trace metals such as Ni2+.164 TRPM6 is also permeable to trace metals, including Mg2+ and Ca2+, and is inhibited by intracellular Mg2+.165 Activation of TRP channels causes ionic changes inside and outside the cell to trigger downstream pathways. Multiple signaling pathways are affected by TRPs, and these pathways include the mitogen-activated protein kinase (MAPK) pathway, transforming growth factor (TGF)-β signaling pathway, nuclear factor kappa-B (NF-κB) pathway, and AMP-activated protein kinase (AMPK) pathway.
MAPK pathway
The MAPK pathway includes extracellular signal-regulated kinases (ERK MAPK), c-Jun N-terminal kinases (JNK) or stress-activated protein kinases, and p38 MAPK.166 MAPK pathways regulate processes ranging from proliferation and differentiation to apoptosis.167 They affect gene expression, metabolism, cell division, cell morphology, and cell survival.168
In most cases, inflammatory stimulation of cells causes an inward flow of calcium.169 Several members of TRP channels act as calcium-permeable channels, and their activation induces calcium influx, which is involved in the regulation of the MAPK signaling pathway.170 In human corneal epithelial cells, the activation of TRPV1 receptor was followed by an increase in Ca2+ influx, which led to MAPK activation, followed by the increased release of interleukin (IL)-6 and IL-8, inducing an inflammatory response.171 Upregulated TRPV5 expression was observed in chondrocytes from rats with osteoarthritis, which regulated a Ca2+ influx to activate the phosphorylation of calmodulin-dependent kinase II (CaMKII).172 Activated p-CaMKII causes extracellular signaling-regulating phosphorylation of ERK 1/2, JNK, and p38 and plays a key role in chondrocyte apoptosis.172 Furthermore, activation of TRPM8 in human bronchial epithelial cells promotes Ca2+ influx, which subsequently leads to increased expressions of IL-6, IL-8, and tumor necrosis factor (TNF)-α via the upregulation of p-ERK and activation of NF-κB, which amplify inflammation.173
Activation of the ERK1/2 pathway in neurons may be involved in acute visceral pain.174 This activation is induced by TRPA1.175 Experimental results by Kondo et al. suggested that mechanical stimulation by TRPA1 may generate action potentials that lead to phosphorylation of ERK1/2 and p38 in dorsal root ganglion (DRG) neurons, which are further involved in the development of visceral pain.176 Activation of TRPV4 regulates the MAPK and phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt) signaling pathways, which regulate cell death and survival.177 When TRPV4 channels are activated, the protein levels of p-p38 MAPK are significantly increase, which induces the apoptosis, which is associated with cerebral ischemic injury.178
TRP channel regulation of the MAPK signaling pathway not only affects pain and inflammation but may also be involved in the fibrotic process. Inhibition of TRPM7 expression reduces liver fibrosis by suppressing the activation and proliferation of hepatic stellate cells, a process that possibly involves the MAPK signaling pathway.179 In addition, TRPV4-mediated Ca2+ influx is involved in regulating the differentiation of human ventricular cardiac fibroblasts to myofibroblasts via the MAPK/ERK pathway.180
TGF-β signaling pathway
The TGF-β superfamily consists of TGF-β1-3, activins and inhibins, growth differentiation factors, myostatin, and BMPs.181 Dysregulation of TGF-β family signaling can lead to a plethora of developmental disorders and diseases, including cancer, immune dysfunction, and fibrosis.182,183
TGF-β signaling underlies cardiac fibrosis.184 Activation of TRPV3 was observed in stress-overloaded rats, and it upregulated protein expressions of collagen I, collagen III, TGF-β1, cyclin E, and cell cycle protein-dependent kinase 2 (CDK2).185 Meanwhile, blocking the TGF-β1 pathway partially reversed the effect of TRPV3 activation.185 This finding suggests that TRPV3 activation exacerbated fibrosis in pressure-overloaded rat hearts by promoting the proliferation of cardiac fibroblasts through the TGF-β1/CDK2/cyclin E pathway.185
Changes in TRPC3 expression and atrial fibrosis are closely related.186 Angiotensin (Ang) II induces migration and proliferation of atrial fibroblasts and upregulates the expression levels of TRPC3 and fibrosis biomarkers.187 The TRPC3-selective blocker Pyr3 significantly attenuated Ang II-induced upregulation of TRPC3, collagen I, collagen III, and TGF-β1 through the molecular mechanism of the TGF-β/Smad2/3 signaling pathway and attenuates neonatal rat atrial fibroblast migration and proliferation.187
TRPV1 exerts protective effect against cardiac and renal fibrosis, mainly through the TGF-β signaling pathway.188 TRPV1 was significantly downregulated in human liver fibrotic tissues.189 By contrast, knockdown of TRPV1 resulted in a significant increase in the expression of various liver fibrosis markers, and enhanced the promotion of TGF-β in hematopoietic stem cell proliferation, cell cycle, apoptosis and extracellular matrix expression.189 Selective activation of TRPV1 in mice with renal fibrosis inhibited α-smooth muscle actin (SMA) but promoted E-cadherin expression in human proximal tubular epithelial cells primarily through the inhibition of TGF-β1-Smad2/3 signaling.190 These data suggest that TRPV1 activation attenuates the progression of cardiac and renal fibrosis by inhibiting TGF-β and its downstream regulatory signaling pathways,191 and exerts cardioprotective and renoprotective effects.
Canonical NF-κB pathway
The canonical NF-κB is activated by various stimuli to transduce rapid but transient transcriptional activity to regulate the expressions of various proinflammatory genes and also serves as a critical mediator of the inflammatory response.192
Kang et al.193 observed that by blocking TRPA1 induced a decrease in IL-6 and IL-13 levels were induced. Furthermore, blockade of NF-κB inhibited TRPA1 expression, whereas blockade of TRPA1 had no significant effect on NF-κB activation.193 However, several researchers have come to the opposite conclusion. Lee et al.194 concluded that inhibition of TRPA1 channel activity inhibited the production of pro-inflammatory cytokines and the activity of NF-κB.
In a model of cisplatin-induced nephrotoxicity, blockade of TRPA1 alleviated apoptosis, reduced the levels of IL-1β, IL-6, TNF-α, and interferon (IFN)-γ, decreased the levels of cleaved caspase-3, cleaved poly (ADP-ribose) polymerase PARP, and inducible nitric oxide synthase (iNOS), reduced the expression of p-IKKβ, p-JNK, p-ERK and p-p38, and enhanced the expression of IκBα.195 TRPA1 expression was increased in renal tubular epithelial cells from patients with acute kidney injury. Activation of TRPA1 increased intracellular Ca2+ levels, increased NADPH oxidase activity, activated MAPK/NF-κB signaling, and increased IL-8 levels.196 In conclusion, TRPA1 regulates inflammation through the MAPK/NF-κB signaling pathway.
TRPV4 is involved in the molecular mechanisms of inflammation mainly through NF-κB. In a mouse model of temporal lobe epilepsy, activation of TRPV4 increased Toll-like receptor 4 (TLR4) expression, which led to the phosphorylation of IκK and IκBα; as a result, NF-κB activation and nuclear translocation occurred, with a resulting increase in the levels of pro-inflammatory cytokines.197 Thus, blocking TRPV4 downregulates high mobility group box 1 (HMGB1)/TLR4/IκK/κBα/NF-κB signaling, which produces an anti-inflammatory response and neuroprotective capacity.197
Scholars have proposed the involvement of TRPM8 channels in the DRG, through the regulation of NF-κB signaling, in the pathogenesis of hyperalgesia in neuropathic pain rats.198 Activation of TRPM8, which inhibits nuclear localization of NF-κB, led to the repression of TNF (encoding TNF-α) gene transcription.199
TRPC6 is beneficial in alleviating inflammation and apoptosis. Activated TRPC6 reduces Ca2+ entry into cells, which inhibits nuclear translocation and phosphorylation of NF-κB and reduces apoptosis, cytotoxicity, and inflammatory responses.200 By contrast, TRPC1 channels cause a Ca2+ influx downstream of Ca2+/calmodulin-dependent protein kinase β (CaMKKβ), which promotes NF-κB activation.201
AMPK pathway
The AMPK pathway promotes mitochondrial health and homeostasis and plays a major role in the regulation of cellular energy homeostasis.202 Inhibition of TRPV1 attenuates rutaecarpine-induced intracellular Ca2+ levels and phosphorylation of CaMKII, CaMKKβ, AMPK, and endothelial NO synthase.203 In addition, increased expression of TRPV1 in patients with blistering skin injury led to an upregulation of intracellular Ca2+ levels and CaMKKβ, AMPK, unc-51-like kinase 1 and mammalian target of rapamycin (mTOR) activities, which cause skin toxicity through excessive activation of autophagy.204
Upregulation of TRPC5 protein expression levels mediates cell survival autophagy via the CaMKKβ/AMPKα/mTOR pathway.205 Notably, not all TRP channels regulate the AMPK signaling pathway. By contrast, activation of AMPK normalizes TRPC6 expression.206
Altogether, these results suggest that TRPs can perform important biological functions by regulating downstream signaling pathways to influence disease development. Targeting key molecules and pathways that mediate abnormal states may be critical for the management of TRP channel-related diseases.
Physiological functions of TRP channels
TRPs have long been an important topic in the scientific community, with a growing number of publications suggesting their involvement in various of biological processes. TRPs are distributed in a variety of tissues, and have a wide range of physiological functions.207 Thus, the biological functions of TRPs must be explored to further understand the development and progression of diseases.
Pain
Pain is the primary reason why people seek medical care.208 Until the 1960s, pain was considered an inevitable sensory response to tissue damage.209 Pain can be categorized as nociceptive (from tissue injury), neuropathic (from nerve injury), or nociplastic (from a sensitized nervous system), all of which affect work-up and treatment decisions at every level.210 Opioids are effective pain relievers, but acting on the brain, they can become addictive.211 Pain exerts an enormous personal and economic burden.208,212 A rational strategy to avoid the side effects of opioid is to target the starting point of the pain pathway: the neuroreceptors that produce pain.213 Certain members of the TRP family (TRPV, TRPA, and TRPM) constitute key detectors and transducers of nociceptive stimuli.214 Therefore, researchers have expressed great interest in the analysis of TRP channels.
Activation of TRPA1 induces pain
TRPA1 plays a role in various forms of pain, including neuropathic cold pain, inflammatory pain, and hereditary episodic pain syndromes.215,216 As shown by dermal experiments, the activation of TRPA1 by certain compounds triggers pain and heat sensations.215,216,217 Sustained activation of TRPA1 by endogenous substances induces chronic pain involved in various disorders,218 including fibromyalgia, osteoarthritis, inflammation, diabetes, obesity, migraine, neuropathy, bronchitis, and emphysema.219
Chembridge-5861528 blocks TRPA1 and relieves mechanical nociception after nerve injury.220 Koh et al.221 observed that inhibition of TRPA1 expression reduced the development of mechanical, thermal, and cold heterosensitivity. The experimental results of Sagalajev et al.222 showed that injury-induced oxidative stress in the amygdala stimulated TRPA1 to promote neuropathic pain behavior. This pain perception involved the inhibition of medullary serotonergic feedback inhibition acting on spinal 5-HT1A receptors, which was reversed by TRPA1 antagonists.223,224 In Schwann cells, activation of TRPA1 induces NADPH oxidase 1-dependent H2O2 release, maintains macrophage infiltration of injured nerves, and sends paracrine signals to activate TRPA1 in phagocytosed nociceptors to maintain mechanical nociception (Fig. 4).225
TRPA1 and TRPV1 in pain regulation. Sensory nervous system injury, heat, or agonist activation of TRPV1, leading to Ca2+ influx and release of SP and CGRP. At this point, the nerve fibers release a variety of trophic factors (NGF, and glial cell-derived neurotrophic factor (GDNF)) and PKC to reduce the sensitivity of TRPV1. This condition puts the nerve fibers and neurons in an excitable state, which causes ectopic discharges and leading to pain. Following tissue injury, pro-inflammatory substances are released, including cytokines, chemokines and more, which causes the recruitment of activated macrophages to the lesion site. Macrophage-dependent ROS and NO evoke Ca2+/NADPH oxidase 1 (NOX1), respectively, and subsequently activate TRPA1 in phagocytosed neuroreceptors to maintain mechanical nociception. On the other hand, CGRP activates CGPR receptors on Schwann cells. CGPR receptors are transported to the endosomes and sustained signaling leads to the release of nitric oxide. Nitric oxide targets TRPA1 in Schwann cells, which excites neurons and transmits pain signals. CGRP calcitonin gene-related peptide, SP substance P, NGF nerve growth factor, GDNF glial cell-derived neurotrophic factor, PKC protein kinase C, ROS reactive oxygen species, NO nitric oxide, NOX1 NADPH oxidase 1
Activation of TRPA1 channels leads to an influx of sodium and calcium ions, which induces the depolarization of nociceptive nerve ending that is needed to generate the centrally propagating nociceptive signal.226 TRPA1 is expressed not only on peripheral but also on central terminals of nociceptive primary afferent nerve fibers.227 On central terminals located within the spinal dorsal horn, TRPA1 amplifies glutamate release and thereby transmission of nociceptive signals to spinal interneurons and projection neurons.228,229
Topical administration of selective TRPA1 agonists in vivo induced nociceptive behavior in experimental animals and pain sensation in human beings.216,230 Bautista et al.231 used TRPA1-deficient mice to better demonstrate that environmental stimulants and endogenous analgesics depolarize nociceptive receptors via TRPA1 to elicit inflammatory pain. TRPA1 antagonists showed no effect on all pain. Intrathecal application of a low dose of TRPA1 antagonist attenuated formalin-induced tactile allodynia but had no effect on acute pain behavior.232 In addition, systemic administration of HC-030031 (TRPA1 antagonist) showed to have anti-isokinetic and inflammatory pain efficacy in a CFA model.155 Interestingly, low doses of intrathecal HC-030031 can also able to attenuate pain.233 By contrast, a similar dose of a TRPA1 antagonist had no effect on healthy rats.232,234
TRPVs in pain
TRPV1 is a heat-activated cation channel modulated by inflammatory mediators and contributes to acute and chronic pain.235 About 30% of the TRPA1-expressing neurons co-express TRPV1.93 TRPV1, which is widely expressed in bipolar neurons, transmits sensory information from the periphery to the somatosensory cortex via the spinal cord.236,237 Stimulation of TRPV1 evokes a burning sensation, which reflects the central role of the channel in pain.238
Trpv1 knockout mice in an inflammatory model exhibited attenuated thermal nociception, which sparked great interest among researchers to develop TRPV1 antagonists with analgesic properties.239,240 TRPV1 is involved in toxic chemicals and heat-induced pain and promotes peripheral sensitization.241 Analgesics may enhance nociceptive receptor activity by increasing TRPV1 expression.242
In the case of cutaneous and visceral pain, the downregulation of TRPV1 activity produces antinociceptive effects.243,244 TRPV1 in skin non-neuronal cells may release pro-inflammatory agents acting on nociceptive endings, which sensitizes neuron-expressed TRPV1 channels and triggers pain signals.245,246
TRPV4 primarily mediates inflammatory pain and neuropathic pain. Colonic mechanical pain and nociception were significantly relieved in Trpv4 knockout mice.247 In mice, topical treatment with a TRPV4-selective inhibitor decreased UVB-evoked pain behavior, epidermal tissue damage, and endothelin-1 expression.248 In sunburned humans, elevated expressions of epidermal TRPV4 and endothelin-1 were observed.249 Activation of TRPV4 induced the transcriptional regulation and release of cytokines and tachykinins (substance P (SP) and calcitonin gene-related peptides (CGRP)),247 which ultimately led to chronic pain.250 Elevated prostaglandin E2 (PGE2) is also thought to contribute to TRPV4-mediated nociceptive behavior,251 which is important in pathological pain states associated with multiple diseases.252 In addition, TRPV4 plays a role in chronic DRG compression-induced neuropathic mechanical pain via the p38 MAPK pathway.253
Although studies on TRPVs related to pain have mainly focused on TRPV1 and TRPV4. TRPV2 and TRPV3 are also promising targets. TRPV2 is widely expressed in neuronal and non-neuronal cells.254,255 Increased expression of TRPV2 is associated with thermal hyperalgesia and mechanical thermal after oral mucosal incision.256 However, the role of TRPV2 in sensory neurons is currently unknown, and a limited number of studies have been conducted in relation to pain.
By contrast, TRPV3 does not act as extensively as other channels, and its studies have focused on skin diseases.257 TRPV3 has been suggested as a candidate transducer contributing to pain hypersensitivity associated with inflammatory states.258,259 Analgesic effects were observed in a model of pruritus treated with selective and potent inhibitors of the TRPV3 channel by Han et al.260. TRPVs are a promising therapeutic target in pain research.
TRPMs in pain
Numerous studies have linked the TRPM8 channel to pain. The channel is mainly expressed on peripheral sensory neurons and is known as a cold temperature sensor. However, it is also expressed in deep viscera. The administration of TRPM8 antagonists, knockdown of the TRPM8 gene, or ablation of primary afferent neurons expressing TRPM8 can be effectively relieve the cold pain associated with tissue and nerve damage.261,262 The experimental results of Zuo et al.263 demonstrated that TRPM8 played a role in cold soreness and hyperalgesia following chronic trigeminal nerve injury. Activation of TRPM8 effectively reduces the painful behavior caused by chemical irritation, toxic heat, and inflammation.264,265 TRPM8 can also amplify the analgesic effects of their agonists by forming complexes with 5-HT-1B receptors.266 In addition, when TRPM8 od TRPA1 and TRPV1 are co-activated, an analgesic effect is produced.267
In addition to TRPM8, significant advances have been made in the role of TRPM2 in pain. Exposure of TRPM2 to H2O2 significantly increased mRNA and protein levels of pro-inflammatory cytokines (IL-1β, IL-6, and TNF-α) and a chemokine (CXCL2).268,269 Remarkably, the inhibition of TRPM2 expression can affect the elevated levels of cytokines and chemokines.269 TRPM2 may play a role in inflammatory pain. In animal experiments, TRPM2-deficient mice were unresponsive to mechanical and thermal nociception in a model of keratin-induced inflammation and exhibited reduced edema.270 Therefore, TRPM2 contributes to inflammatory pain by promoting peripheral immune functions.
TRPM2 and TRPM8 are the most relevant members of the family of TRPMs regarding pain, but the role of other members of the family in pain should not be underestimated. Pharmacological and genetic studies have shown that TRPM3 is associated with various pain patterns. Su et al.271 concluded that TRPM3 plays a role in thermal hyperalgesia and spontaneous pain but not in cold and mechanical hyperalgesia. The heat-activated TRPM3 ion channel is a potential target for new analgesics, but its mechanism in relation to pain is unknown and needs to be explored.
TRPs are arguably the most widely studied analgesic targets today. TRPV1 has been validated as a target for analgesic drugs in preclinical studies,272 and its side effects should not be underestimated. Whether other pain-related TRP channels act as the sole mediators of nociception, and the mechanisms of analgesia remains to be determined.
IBD and complications
IBD, which is one of the global disorders of the 21st century,273 is a chronic recurrent disease of the gastrointestinal (GI) tract characterized by an inflammatory process that requires lifelong medication.274 Over the past decades, IBD has been regarded as a worldwide public healthcare challenge,275 and it can usually be classified into two major forms: Crohn’s disease (CD) and ulcerative colitis (UC). IBD was prevalent in Western countries in the past,276 but a significantly increasing incidence has been observed in developing countries more recently.277
The pathogenesis of IBD is complicated. Multiple factors constitute a confluence of risk factors for IBD, and they may include genetic and environmental changes that cause dysregulation of the intestinal barrier, immune responses, and microbiota.278 It not only affects the GI tract but is also a major causative factor of a number of complications, such as nonalcoholic fatty liver disease (NAFLD) and psoriasis.279,280 NAFLD in particular has been considered a leading cause of liver disease in IBD patients,281 with a prevalence of 8–40%.282 Meta-analyses have shown that patients with psoriasis are 1.70 times more likely to have CD and 1.75 times more likely to have UC.283 Therefore, among the numerous complications of IBD, NAFLD, and psoriasis negatively affect the health of patients and are difficult to cure. Thus, they bring a heavy burden to patients and families.
Alleviating IBD symptoms: emerging TRP targets
Traditional strategies for the treatment of IBD have relied on the frequent administration of high doses of drugs, including antibiotics, anti-inflammatory agents (such as 5-aminosalicylic acid and corticosteroids), and biological and immunosuppressive agents (azathioprine, 6-mercaptopurine, methionine, cyclosporin-A, and tacrolimus),284 to reduce inflammation.285 The emergence of monoclonal antibodies as biological therapies has significantly increased the treatment options for IBD.286 Numeros TNF-α antibodies, such as adalimumab or certolizumab, are used in the clinical treatment of IBD.287 Antibodies targeting IL-12 and IL-23, such as ustekinumab and natalizumab, have also been proposed for clinical approval.288 These drugs can effectively relieve early inflammatory symptoms, but their long-term use can cause toxicity and damage organs. These problems may be effectively avoided when certain TRP modulators are used.
TRPVs in IBD
Activation of TRPV4 receptors in the GI tract can trigger inflammation.289 TRPV4 is expressed and functional in human colon samples, human intestinal epithelial cell lines (Caco-2 and T84), and inflamed colons of mice; 4α-phorbol-12,13-didecanoate (4αPDD) activated TRPV4 in the GI tract and increased the intracellular calcium ion concentration, which ultimately leads to chemokine release and colitis.290 Cenac et al.291 demonstrated that 4αPDD selectively activated TRPV4 in colonic sensory neurons, which affected visceral nociception and hypersensitivity. Fichna et al.292 first observed the significantly higher expression of TRPV4 mRNA in the tissues of IBD patients compared with those of non-IBD patients. Activation of TRPV4 receptors in the GI tract has a pro-inflammatory effect, and selective blockade of TRPV4 in an animal model of IBD alleviated colitis and the pain associated with intestinal inflammation. De Petrocellis et al.293 showed that activation and desensitization of TRPV channels mediated intracellular Ca2+ concentrations. Interference with TRPV1–4 channel activity and expression may have multiple potential therapeutic applications for IBD.
Increased TRPV1 nerve fibers in patients with IBD correlated with abdominal pain severity.294 Upregulation of TRPV1 protein expression in IBD patients affected nerve endings and immune cells, which suggests the importance of TRPV1 in colonic immune regulation of the inflammatory response and in the pathogenesis of IBD.295 Over 20% of patients with IBD have significantly elevated TRPV1 expression in the rectum, which is a key point in mediating inflammatory pain.296 The process of producing neuropathic pain in patients with IBD is associated with the activation of TRPV1 because it promotes the release of neuropeptides, such as SP and CGRPs from peripheral terminals (Fig. 5).297 Moreover, increased levels of TRPV1 were observed in patients with acute exacerbations of IBD and in experimental models of acute colitis.298 Pretreatment of the IBD model with the TRPV1 antagonist JYL1421 reduced visceral hypersensitivity, inhibited colorectal dilation, and improved microscopic colonic inflammation.299 All these findings support the idea that TRPVs at least partially participate in the regulation of IBD.
TRP channels in IBD. TRP channels can be stimulated by a variety of factors in the intestinal lumen, including temperature, chemoregulators, and inflammation, and act as auxiliary transducers for GPCR. Interestingly, TLRs play a major role in the regulation of TRPV1 signaling. Stimulation of TRP channels on EC triggers the release of the neuropeptides CGRP and SP, which activate surrounding immune cells and release more inflammatory factors such as TNF-α, IL-1β, histamine, and IFN-γ. These inflammatory factors accumulate at the GPCR of primary afferent sensory neurons. Subsequent activation of TRP channels on neurons triggers the release of neurokinin A, CGRP, and SP, which promote pain perception and inflammatory responses. Created with BioRender.com
Activation of TRPM8 for the treatment of IBD
TRPM8 mRNA is highly expressed in colonic DRG neurons, and TRPM8 protein is distributed throughout the nerve fibers of the colon wall. Harrington et al.300 suggested that TRPM8, which is present in colonic sensory neurons, couples to TRPV1 and TRPA1 and inhibits their downstream chemosensory and mechanosensory effects. In animal models of post-inflammatory colonic allergy, increased TRPM8 expression significantly attenuated hypersensitivity to mechanical stimuli and reduced the symptoms of colonic allergy.301
Inflammation in mouse colitis models can be alleviated by the activation of TRPM8. Selective activation of TRPM8 reduces the release of inflammatory neuropeptides, inhibits the release of pro-inflammatory cytokines and chemokines, and prevents the accumulation of leukocytes in the colon.302 Inhibitors of phospholipase A2 (PLA2) reduce the response of TRPM8 to cold and agonists, and lysophospholipids as products of PLA2 have the opposite effect on TRPM8.303 Notably, PLA2 levels are elevated in patients with CD and UC,304 whereas levels of lysophospholipids are clearly decreased.305 These changes may also have implications for the function of TRPM8 during colitis.
TRPA1 agonists
Ample evidence indicates that TRPA1 plays a major role in the development of IBD.4 2,4,6-Trinitrobenzene sulfonic acid (TNBS) induces colitis with colonic dilation and increases sensitivity to chemical stimuli; TRPA1 expression is upregulated in the colonic afferent DRG neurons.306 Intrathecal administration of the TRPA1 antisense oligodeoxynucleotide inhibitor resulted in the downregulation of TRPA1 expression in the DRG and inhibited IBD-induced nociceptive hypersensitivity, colonic dilation, and allyl intracolonic isothiocyanate.307 Dinitrobenzene sulfonic acid (DNBS) binds to the intracytoplasmic N-terminal cysteine residue of the TRPA1 protein; thus, effective treatment of DNBS-induced colitis is achieved by antagonizing TRPA1 or genetic deletion of TRPA1, and therefore, TRPA1 is considered a direct target of DNBS-induced IBD.308
Application of TRPA1 agonists alleviated DNBS-induced colitis inflammation in mice. Activation of TRPA1 restored the integrity of the colonic epithelial mucosa, inhibited the production of nitric oxide, IL-10, and IFN-γ, and significantly reduced nitrite levels.309 These data suggest the significant role of TRPA1 in restoring intestinal mucosal integrity and reducing inflammatory factors after IBD. It may also be used to provide a new therapeutic strategy for the relief of IBD.
Complications of IBD: NAFLD
NAFLD is one of the most prevalent complications of IBD,310 and it may occur at any stage of the natural course of the disease.311 The prevalence of NAFLD ranges from 1.5% to 55% in UC and from 1.5% to 39.5% in CD (with an overall mean prevalence of 23%).312 Patients with IBD with NAFLD tend to have stable liver disease that is stable for 4–6 years.313 NAFLD is also the most common liver disease, with a prevalence of up to 25% worldwide314; thus, it imposes a significant economic burden on patients and leads to a health-related decline in quality of life.315 It is characterized by fibrosis, inflammation, fatty deformation, and lipid deposition in hepatic parenchymal cells.
TRPV as a therapeutic target in NAFLD
Activation of TRPV1 increases energy metabolism and prevents the accumulation of visceral fat.316 Monoacylglycerols (MGs) with unsaturated long-chain fatty acids are new TRPV1 agonists that along with capsaicin, are found in food and capsaicin to prevent the accumulation of visceral fat. Iwasaki et al.317 tested a diet of MGs that activated TRPV1 and found a reduction in the weight of white adipose tissue and lower levels of serum glucose, total cholesterol, and free fatty acids. Activation of TRPV1 also enhanced energy expenditure and increased thermogenesis, which further reduced visceral fat accumulation in mice and prevented NAFLD.318 Therefore, activation of TRPV1 is considered an important candidate for reducing the risk of diseases, such as NAFLD (Fig. 6).
Effect of TRP channels on NAFLD. HC-067047 (HC) inhibits TRPV4 activity, increases CYP2E1 activity, and upregulates IL-1β, IFN-γ, HMGB1, and MCP-1 expressions, which lead to NAFLD. Activation of TRPV1 increases ATP consumption and cholesterol metabolism, reduces thermogenesis and calcium ion concentrations, decreases ROS levels, visceral fat accumulation, and toxicity and ultimately inhibits NAFLD development. Conversely, inhibition of TRPV1 activity is followed by a decrease in serum glucose and insulin, which can exacerbate the disease. Interestingly, curcumin counteracts the development of NAFLD by inhibiting TRPM2, which results in lower calcium ion concentrations and ROS levels and inhibits hepatocyte damage and death. Created with BioRender.com
Long-term activation of TRPV1 in tissues causes upregulation of hepatic uncoupling protein 2 (UCP2), which effectively prevented high-fat diet-induced NAFLD in mice.319 Previous studies have shown that TRPV1 activation by dietary capsaicin resulted in the upregulation of UCP2 expression in a wild-type (WT) mouse liver model but not in Trpv1 knockout mice. These results suggest that the mechanism by which TRPV1 is activated to prevent exacerbation of fatty liver may be associated with increased UCP2-mediated hepatic β-oxidation.320 Thus, dietary capsaicin may serve as a promising intervention for people at high risk of fatty liver disease. However, whether TRPV1 is involved in the regulation of lipid accumulation and the underlying mechanisms involved in this process are currently unclear.
Song et al.321 observed that ruthenium red or a synthetic siRNA against TRPV4 inhibited the proliferation of HSC-T6 cells, which caused downregulation of myofibroblast markers, including α-SMA and Col1α1. Seth et al.322 reported that TRPV4 responded to the cytotoxic environment of the liver and negatively regulated cytochrome p450 enzymes (CYP2E1). Activation of TRPV4, which releases NO to act on neighboring hepatocytes, inactivates CYP2E1-induced redox toxicity (Fig. 6). TRPV4 acts as a cellular stress sensor in diseased tissues of patients with NAFLD, and thus, it constitutes an endogenous defense molecule that may act as a novel regulator in a protective manner for hepatocytes.
TRPM and TRPC channel involvement in NAFLD
Assessment of acetaminophen-induced liver injury based on blood liver enzyme concentrations and liver histology revealed significantly less severe liver injury in Trpm2 knockout mice than in WT mice.323 TRPM2 channels are integral in the mechanism of acetaminophen-induced hepatocellular death. Thus, TRPM2-mediated cell death is an important mechanism in the pathogenesis of NAFLD-induced liver injury (Fig. 6).324 However, blockade of TRPM7 inhibits the activation and proliferation of primary hematopoietic stem cells and induces apoptosis in activated cells; ER stress was identified as a possible underlying molecular basis.325 TRPM2 and TRPM7 are valuable future therapeutic targets for NAFLD and serve as basis for discoveries related to in this disease.
Arterial flow-regulated diastolic function, which leads to atherosclerosis, is lower in patients with NAFLD than in the healthy population. TRPC3-related endothelial mechanisms that promote NAFLD drive the progression of atherosclerosis. In vitro, TRPC3 is inhibited in hepatic sinusoidal endothelial cells, which makes them less sensitive to ER stress-induced apoptosis; therefore, the pro-apoptotic influence of TRPC3 may increase other fibrotic factors in vivo.326 A positive correlation was found among enhanced expression of endothelial TRPC channels, development of early steatohepatitis, and atherosclerotic load in a mouse model of nonalcoholic fatty liver hyperlipidemia fed with a traditional Western-style diet.
Nishiyama et al.327 speculated that TRPC3 or TRPC6 may be involved in the fibrotic process of NAFLD. However, systemic deletion of either TRPC3 or TRPC6 does not attenuate liver fibrosis, inflammation, nor steatosis. These findings suggest that TRPC3 and TRPC6 are unlikely to be implicated in liver dysfunction and fibrosis in NAFLD mouse models.328 No effective therapy is available for NAFLD, and its exact pathogenesis remains to be elucidated. All these data indicate that TRP channels may be an interesting target for drug development for the treatment of NAFLD.
Extraintestinal complications of IBD: psoriasis
A distinct bidirectional association exists between psoriasis and IBD.329 At the epidemiological level, patients with psoriasis are at increased risk of developing IBD, and vice versa.330 On the skin, TRPs are interesting pharmacological targets because they allow for topical treatment and may reduce the risk of severe harmful effects.331 TRP channels are widely expressed in various skin cell types, including keratinocytes,332 melanocytes,333 nerve endings, and immune cells.245 Their functions involve hair growth,334 wound healing,335 skin cell proliferation and differentiation, immunity,336 skin barrier function,337 and skin inflammation and pruritus.338,339 IBD and psoriasis are relatively common diseases that require long-term treatment. Immunosuppressive drugs are currently the most commonly used treatment in clinical practice and a significant overlap has been observed between effective drugs for the treatment of IBD and psoriasis. The development of potent targeted therapies for TRPs is changing the outlook for chronic inflammatory diseases such as IBD and psoriasis.
Remitting effect of inhibition of TRPVs on psoriasis
TRP channels contribute differently to various psoriasis symptoms. Activation of TRPV1 and TRPC4 was found to be detrimental to psoriasis, whereas TRPA1 was found beneficial in preclinical studies.340 Lee et al.341 reported that the inhibition of TRPV1 activity, which activates ALX/FPR2 receptors, coupled with the control over CGRP release, significantly alleviated psoriasis-like pruritus and inflammation in an imiquimod (IMQ) animal model of psoriasis.
The TRPV1 and NaV1.8 neuroreceptors control the cutaneous immune response by interacting with dermal dendritic cells to inhibit the production of several key effector cytokines in psoriatic dermatitis and regulate the IL-23/IL-17 pathway.342 Neurons expressing TRPV1 can mediate itch signaling.343 However, intrathecal injection of capsaicin (10 μg) disrupts the central terminals of TRPV1-expressing neurons.344 Therefore, we hypothesize that TRPVs are considered novel targets for the treatment of psoriasis by suppressing itching to relieve psoriasis symptoms.
Modulation of TRPM and TRPC channels
Cold activation of TRPM8 reduces pruritus caused by psoriasis.345 This mechanism possibly occurs through blocking of histaminergic and non-histaminergic pruritic pathways by pharmacological hypothermic cooling.346 Activation of TRPM8 also reduces inflammation-induced psoriasis due to its capability to strongly improve edema, inhibit mechanical nociception, and decrease levels of inflammatory cytokines (IL-1β, TNF-α, and IL-6); as a result, the inflammatory response is reduced.347 In conclusion, these studies provide strong evidence that TRPM8 is a major cold sensor for amelioration of pruritus and inflammation in various dermatological conditions; it enables the use of TRP channel modulators in the treatment of psoriasis.348
In the IMQ-induced psoriasis mouse model, activation of TRPM8 channels stimulated Ca2+ responses in cervical DRG neurons, reduced scratching behavior in IMQ mice,349 attenuated IMQ-induced morphological changes in lesions, and controlled the infiltration of dermal neutrophils, dendritic cells, and Th17 cells.350 Activation of TRPM8 channels also significantly inhibited the generation of iNOS, cyclooxygenase (COX)-2, TNF-α, and IL-6, and prevented the phosphorylation of IκBα, NF-κB p65, ERK, JNK, and p38 MAPKs.351 Thus, activation of TRPM8 channels is thought to be effective in ameliorating the symptoms of IMQ-induced pruritus and psoriasis-like inflammation, which make it a promising strategy for the treatment of psoriasis.
The level of 5-HT is higher in the skin of patients with psoriasis than that in healthy individuals.352 Specific inhibition of TRPC4 immediately controlled the neuronal activity induced by α-methyl-5HT in dermal nerve preparations.353 Interestingly, visual signs of skin inflammation, such as erythema and desquamation, were significantly improved after TRPC4 inhibition and were accompanied by a reduction in epidermal thickness and inflammatory cell infiltration. This effect was achieved by silencing TRPC4 in the DRG and showed an effective reduction in psoriatic pruritus even after repeated applications of IMQ.354 Therefore, TRPC4 antagonists may soon be used clinically for the treatment of acute pruritus and psoriasis.
Respiratory system
Various TRP channels are widely distributed in the lungs and are involved in pathophysiological processes.355 A growing body of research suggests that TRP channels may become targets for drug use in the treatment of respiratory diseases.
2019 coronavirus disease (COVID 19)
The COVID-19 pandemic, which is caused by severe acute respiratory disease coronavirus 2 (SARS-CoV-2), has resulted in hundreds of millions of confirmed cases worldwide.356 Calcium channel blockers are protective against death from SARS-CoV-2 infection and inhibit the infectivity of SARS-CoV-2 in epithelial lung cells.357 Given that coronaviruses require Ca2+ ions to enter host cells, Ca2+ are considered essential for viral entry into host cells.357 Interestingly, most TRP channels are highly permeable to Ca2+.358,359 Therefore, the researchers hypothesized that TRP channels are involved in certain processes in COVID-19.
The report by Leidinger et al. points to the idea that TRPC6 channels are involved in physiology of normal human lungs and in COVID-19 pneumonia.360 They suggested that TRPC6 may exacerbate SARS-2-induced inflammation, but the molecular mechanism remains to be elucidated. Several patients with COVID-19 will develop acute respiratory distress syndrome (ARDS).361,362 Khodadadi et al.363 found increased expression of TRP cation channels after the treatment of ARDS. Drugs may exert their protective effect against ARDS through TRPs.
Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) has also been defected in some COVID-19 patients.364,365,366 Clinical studies have shown that TRPM3 channel activity is impaired in patients with post-COVID-19 conditions, which suggests impaired ion mobilization; such condition may impede cellular function and lead to chronic post-infection symptoms such as ME/CFS.367
Symptoms of COVID-19 associated with TRPA1/TRPV1 include the following: cough,368 smell, and taste disturbances,369,370,371,372 loss of appetite,373 nausea,374 vomiting,375 inflammatory responses, and pain.40 However, several clinical studies targeted TRP channels for the treatment of COVID-19. TRPA1 and TRPV1 may provide a breakthrough in the treatment of symptoms of COVID-19.
Given that a common denominator of COVID-19-related symptoms is impaired redox homeostasis, which is responsible for reactive oxygen species (ROS) accumulation, several TRP channels are also involved in oxidative stress. Bousquet et al.376 hypothesized the involvement of TRPA1 and TRPV1 in COVID-19. In another direction of thought, harmful stimuli activate TRPV1 and increase the release of SP and pro-inflammatory cytokines,377 which exacerbate the symptoms of patients infected with COVID-19.
TRPV4 channels are involved in the recruitment of neutrophils and macrophages during lung injury.378 In addition, TRPV4 has been associated with pulmonary edema and pulmonary fibrosis.379 Therefore, TRPV4 is a novel target for COVID-19 treatment.380 TRPs are involved in pathophysiological processes that largely overlap with the symptoms of COVID-19. Published papers on COVID-19 have proliferated, but the mechanisms underlying the disease and TRPs remain inconclusive. TRP channels represent promising targets for the relief of multiple symptoms of COVID-19.
Cough
Cough is a reflex action of the respiratory tract for clearing the upper airways.381 It is globally prevalent across all age groups. Stimulation of bronchopulmonary vagal C-fibers by inflammatory mediators can also cause cough.382,383 TRPV1 is expressed in the C-fiber sensory nerves of the vagus in the lungs and is involved in the afferent sensory loop of the cough reflex.384 Trevisani et al.385 noted the pathogenic role of TRPV1 in cough in animal experiments. Iodo-resiniferatoxin (a potent TRPV1 inhibitor) significantly reduced cough induced by inhalation of capsaicin or citric acid in conscious guinea pigs.386 Therefore, inhibition of TRPV1 expression is a potential target for cough treatment.
TRPV4 plays a role in increasing sensitivity in the afferent arm the cough reflex.387 Selective activation of TRPV4 caused depolarization of airway sensory nerves and cough symptoms in experimental animals. Conversely, TRPV4 antagonists can reverse this phenomenon.388,389 In addition, the selective P2X3 antagonist AF-353 inhibits TRPV4-induced neural depolarization and coughing in animals and humans, which suggests that TRPV4 can cause ATP release and relieve cough.388 Thus, activation of TRPV4, through the release of ATP is likely to be a pathological cause of the cough.
The TRPA1 receptor (not activated by capsaicin) has become a topic of interest in the field of cough treatment because it is known to be activated by ligands such as acrid smoke.390 Birrell et al.391 found in normal humans and guinea pigs that inhalation of cinnamaldehyde (a TRPA1 agonist) produced a dose-related increase in cough. Glenmark’s compound GRC 17536, a potent and specific TRPA1 antagonist, effectively suppressed the cough induced by a citric acid challenge in guinea pigs.392 A clinical study was subsequently conducted but was terminated due to the lack of clinical efficacy.
TRPM2 may participate in oxidative stress at the cough center by regulating cough.393 In the case of TRPM8, menthol prevents coughing by activating TRPM8.394 Neurophysiological, immunohistochemical and molecular analyses suggest that the cough suppressant effect of peppermint occurs via a reflex initiated from nasal TRPM8.395,396 Based on the properties of TRPs, TRPM8 is undoubtedly one of the targets of cough. Most clinical studies targeting TRPs for the treatment of cough have ended with no drug efficacy. Future research should focus on new strategies to targeting this channel.
Chronic obstructive pulmonary disease (COPD) and asthma
Asthma and COPD both cause airway obstruction and are associated with chronic inflammation of the airways;397 they are also very common respiratory diseases.398,399 A key risk factor for developing respiratory disease is smoking.400,401 The use of TRPV1 and TRPV4 antagonists inhibits cigarette smoke-induced ATP release from human bronchial epithelial cells and may be associated with COPD-influenced increases in pulmonary ATP.402 Activation of TRPV4 and opening of pannexin 1 channels leads to ATP release, which contributes to the pathogenesis of COPD.403 Baxter et al. also noted that TRPV4 gene expression was increased in the lung tissue of COPD patients.402 At the genetic level, 7 of the 20 single-nucleotide polymorphisms of TRPV4 tested have been associated with COPD, which suggests a potential relationship between TRPV4 protein structure and disease pathogenesis.404
Contraction of airway smooth muscle in human and guinea pig tissues was observed in vitro with TRPV4 agonists, and this condition may exacerbate asthma.405 McAlexander et al. suggested that activation of TRPV4 leads to airway constriction in humans and is entirely dependent on the production of cysteine leukotrienes.406 Therefore, activation of TRPV4 may exacerbate symptoms in patients with asthma.
Several endogenous TRPV1 agonists are present in diseased lungs, such as those affected by COPD and asthma. The products of lipoxygenase metabolism of arachidonic acid, such as 12-(S)- and 15-(S)-hydroperoxyeicosatetraenoic acid, are released during asthma attacks and have a stimulatory effect on TRPV1.407 Another endogenous agonist of TRPV1, anandamide, is also synthesized in the lungs.408 The increased sensitivity of patients with COPD or asthma to inhaled capsaicin may explain the involvement of TRPV1 in the exacerbation of symptoms of these diseases.409
In contrast to TRPV1 and TRPV4, little is known about TRPV2.410 TRPV2 acts in the early stages of receptor-mediated phagocytosis by macrophages.411 Its relevance to alterations in macrophage responsiveness and host defense in COPD still needs to be determined.
Andre et al.412 demonstrated that bronchial ring constriction produced by cigarette smoke extracts and acrolein or crotonaldehyde was inhibited by TRPA1 inhibitors. Furthermore, TRPA1 activation and increased Ca2+ influx promoted neuropeptide release from the isolated guinea pig airway tissue. These data suggest that airway neurogenic inflammation is mediated via TRPA1 stimulation, and suggest that TRPA1 may play a role in the pathogenesis of COPD.
Notably, TRPC6 mRNA expression significantly increases in alveolar macrophages from COPD patients, whereas there was no difference has been observed in the expressions of other TRPC subfamily members.413,414 TRPC6 is a potential candidate target for COPD, but its role in the regulation of macrophage function under physiological and pathophysiological conditions remains to be determined.
A novel truncated functional TRPM8 variant activated by cold and menthol has been identified in human bronchial epithelial cells.415 Whether TRPM8 expression is altered in the airway nerves or airway epithelium of individuals with cold-induced asthma and disease exacerbation remains to be elucidated.416
Other respiratory diseases
Idiopathic pulmonary fibrosis (IPF) is a prototype of chronic, progressive, and fibrotic lung disease.417 Rahaman et al.418 determined that TRPV4 activity was upregulated in lung fibroblasts from IPF patients, and mediated the onset of lung fibrosis. TRPV4 also plays an important role in the formation of pulmonary edema and acute lung injury.378,419 TRPV4 agonists caused pulmonary endothelial permeability, which was inhibited by the non-selective TRP blocker ruthenium red and was absent in Trpv4 knockout mice.420 Evidence showing that activation of TRPV4 may be involved in disrupting the integrity of the epithelial and endothelial barriers suggests the possible role of TRPV4 in edema formation. Studies also reported that TRPC6 is involved in pulmonary edema formation.421 Selective TRPC6 blockers were found to inhibit pulmonary ischemia-reperfusion-induced edema.422
A number of TRP channel modulators have entered clinical trials and significant progress has been made in describing the physiopathological function of TRP channels in the respiratory system.
Nervous system
Neurological disorders are the leading cause of disability and the second leading cause of death worldwide.423 TRP channels are widely distributed in neurons and the brain,424,425 and are thought to be associated with neurological disorders. They are involved in neurological disorders such as epilepsy, stroke, dementia, anxiety, neurodegenerative diseases, and depression.
TRPC
TRPC is involved in chemokine ligand 2-mediated neuroprotection. Specific blockade of TRPC1 and TRPC5 inhibited ERK/cAMP response element binding protein activation and provided neuroprotection.426 TRPCs have also been linked to Parkinson’s disease (PD) and Alzheimer’s disease (AD). TRPC1 overexpression may exert PD protection by mediating an inward calcium flow, inhibiting cytochrome c release in mitochondria,427 and reducing MPP-induced neurotoxicity and apoptosis.428 In AD, each member of the TRPC channel performs a different role. TRPC1, TRPC3, and TRPC4 are found to be involved in neuroprotection,429,430 TRPC3 may be involved in the dysregulation of tau proteins in AD, and TRPC5 possibly participates is involved in neurodegeneration.431
In addition to these disorders, the TRPC channels are involved in neurological disorders, such as autism, bipolar disorder (BD), and mental retardation.432 Griesi-Oliveira et al.433 found that the sequence of the TRPC6 gene on chromosome 11 was disrupted in autistic patients, resulting in haploinsufficient function. Later, they studied the effects of TRPC6 disruption on pluripotent stem cell-derived cortical neuronal cells. They observed that alterations in neurons of autistic patients were associated with disrupted expression of TRPC6.432 A behavioral study of Trpc6 knockout mice by Griesi et al. found no alterations in social or repetitive behavior, but a reduction in exploratory behavior may be associated with clinical signs of autism.433
Evidence from functional studies points to the involvement of TRPC3 and TRPC7 in BD.434 Clinical results demonstrated reduced TRPC7 expression in a subgroup of BD patients.435 Interestingly, this reduction was inversely correlated with the basal level of Ca2+ in B lymphocyte lines (BLCLs) from BD patients. Andreopoulos et al.436 treated BD rats with chronic lithium and TRPC3 protein levels were significantly reduced, whereas mRNA levels were not.437 In a study by Roedding et al. investigated whether TRPC3 expression and function were affected by oxidative stress in BD and control BLCLs; chronic mitochondrial-generated oxidative stress reduced TRPC3 protein levels and Ca2+ influx through these channels, but no significant difference was observed between the two groups.438 Thus, TRPC3 is probably not an ideal target for BD.
Depression is a common psychological disorder in the society and is also a neurological disorder.439 Hypericin acts as a non-selective neurotransmitter reuptake inhibitor and has antidepressant effects.440 It produces a partial antidepressant effect by activating TRPC6 channels, increasing sodium permeability in the presynaptic membrane, reducing the sodium gradient driving neurotransmitter reuptake through the transporter, and increasing the levels of monoamine neurotransmitters in the synaptic cleft.441 To a certain extent, depression is also associated with brain-derived neurotrophic factors (BDNF).442 TRPC channel-induced calcium transients regulate the density and morphology of dendritic spines in BDNF, to produce antidepressant effects.441,443
TRPC channels play an important role in vasospasm in hemorrhagic stroke, neuronal death and survival in ischemic stroke, and thrombin-induced pathological changes in astrocytes, and triggering of stroke by affecting blood pressure and atherosclerosis.444 In Ca2+ imaging experiments, Ca2+ influx through TRPC6 activated MAPK signaling and promoted the neuroprotective chemokine CXCL1,445 which protects nerves following stroke. The unique channel characteristics and downstream pathways of TRPCs render them potential new targets for the treatment of neurological diseases.
TRPVs
PD.
To address the accumulation of fibrillar alpha-synuclein in PD patients.446 Yuan et al.447 found that regulating the opening of TRPV1 channels on the surface of microglia enhanced autophagy, phagocytosis and degradation of α-syn in microglia, which improved the status of PD. TRPV4 contributed to the loss of dopamine neurons and motor deficits in the substantia nigra of PD mice by mediating ER stress and inflammatory pathways.448 Furthermore, activation of TRPV1 channels on astrocytes activated endogenous neuroprotective mechanisms in vivo, which prevented active degeneration of dopamine neurons; this condition led to behavioral recovery via CNTF receptor alpha in nigrostriatal dopamine neurons.449 These studies provide a new perspective on future molecular targets and gene therapies for the treatment of PD.
AD.
One of the features of AD mentioned earlier is the deposition of amyloid in the brain.450,451,452,453 Metabolic facilitation with TRPV1 agonists reduced amyloid lesions and reversed memory impairment in a mouse model of AD.454 In addition, the activation of TRPV1 inhibited endocytosis of the α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor of GluA2 and improved cognition and synaptic function in the AD mouse model.455 Furthermore, HC067047 (a TRPV4 inhibitor) enhanced the expression of the neurogenic marker DCX in cognitively impaired mice and increased the levels of the mature neuronal marker NeuN, which indicated the neuroprotective potential for the treatment of dementia with learning and memory loss.456
Stroke.
Activation of TRPV2 induces the release of nerve growth factor (NGF) and regulated blood-brain barrier (BBB) function, which may be associated with ischemic stroke.457 TRPV4 alleviates ischemic injury by increasing glutamate and overloading Ca2+, thereby reducing neurotoxicity.458 Preservation of microcirculation and BBB function shortly after ischemic stroke is a key neuroprotective effect of TRPV4 inhibition, which suggests a contribution of TRPV4 to postischemic brain injury.459
TRPA1
The toxic effects of amyloid-β on astrocytes triggered by TRPA1 channel activation are key to the progression of AD.460 Furthermore, mitochondria have an important role in the pathogenesis of AD.461 TRPA1 channels reduces the intracellular Ca2+ concentration, apoptosis and intracellular ROS, and this may have a role in the treatment of AD.462 TRPA1 activity, if inhibited, will exacerbate astrocytic lesions, inhibit the production of pro-inflammatory cytokines as well as the activity of astrocytes and the transcription factors NF-κB and nuclear factor of calcineurin-activated T cells (NFAT), and play a key role in the pathogenesis of AD.194
Prolonged activation of TRPA1 by trans-cinnamaldehyde leads to increased epileptic activity.463 In contrast, HC030031 (a TRPA1 antagonist) promotes anticonvulsant effects, and preventes TRPA1 upregulation during seizures.464
Increased NADPH oxidase and superoxide dismutase activities and TRPA1 endogenous agonist levels (hydrogen peroxide and 4-hydroxynonenal) were reported in a mouse model of progressive multiple sclerosis-experimental autoimmune encephalomyelitis.465 Thus, TRPA1 may be involved in depression and anxiety-like behaviors. TRPA1 also exerts tonic control and promotes anxiety and depression.466 Antagonists of TRPA1 are a potential drug category for the treatment of anxiety and mood disorders.
In hypoxia, endothelial TRPA1 channels cause vasodilation, which reduces ischemic damage, a set of phenomena thought to be associated with ischemic stroke.467 In combination with these findings, TRPA1 can be included as a candidate for therapeutic targets in neurological diseases.
TRPM
TRPM2-mediated cellular and molecular crosstalk with amyloid β peptide or oxidative damage results in the formation of AD lesions.468 TRPM2 plays an important role in mediating cell death induced by various oxidative stress-induced pathological factors, including ischemia and reperfusion, the neurotoxic amyloid β-peptide, and MPTP/MPP+, which lead to neuronal death in the brain.324,469 Activation of TRPM2 alters intracellular ion homeostasis, which leads to abnormal initiation of various cell death pathways, making it an important mechanism in the pathogenesis of ischemic stroke and neurodegenerative diseases.470
Hazalin et al.471 found that inhibition of TRPM4 expression at the mRNA and protein levels improved cognitive deficits in chronically underperfused rats, but did not affect their motor function. It has also been suggested that TRPM4 plays a key role in the treatment of ischemic stroke.472 Chen et al.473 examined the link between TRPM4 and ischemic stroke through in vivo assessment and observed that inhibition of TRPM4 improved BBB integrity after reperfusion in ischemic stroke.
The TRP channel may represent a promising therapeutic strategy for neurological disorders. Future research and development should focus on the TRPC channel because it is more widely distributed in the nervous system and existing studies are not sufficiently advanced.
Cardiovascular system
Given the distribution and function of TRP channels in excitatory and non-excitatory cells of the cardiovascular system, they can be a potential therapeutic target for CVD.474 Different TRP family members have various potential functions in CVD, and are mainly involved in hypertrophy, heart failure, arrhythmias, and vascular regulation.16
Cardiac hypertrophy and heart failure
Cardiac hypertrophy and heart failure are closely related. Ang II-induced cardiac hypertrophy is the key to the pathological process of heart failure.475,476 Using siRNA, Onohara et al.477 demonstrated that TRPC3 and TRPC6 were required for Ang II-induced NFAT translocation in cultured rat myocytes, which is a key step in cardiac hypertrophy.478 Furthermore, Ang II stimulation resulted in a significant increase in apoptosis in cultured neonatal rat cardiomyocytes transfected with TRPC7, accompanied by a decrease in atrial natriuretic factor expression and destruction of actin fibers.479 Ang II/phenylephrine-infused TRPC3 transgenic mice showed increased activation of NFAT in vivo, cardiomyopathy, and increased hypertrophy following stimulation with neuroendocrine agonists or pressure overload.480 Mice lacking TRPC1 channels maintained cardiac function, which prevented cardiac hypertrophy, when subjected to hemodynamic stress and neurohormonal overload.481 TRPV3 expression also increased in Ang II-induced cardiomyocyte hypertrophy in vitro, accompanied by an increased intracellular calcium ion concentration, which promoted the expression of calcineurin and phosphorylated CaMKII protein and enhanced nuclear translocation of NFATc3.482
Feng et al.483 observed clinically that TRPM4 currents were more than twofold larger in fibroblasts from heart failure patients than from controls, and this finding implies the possible role of TRPM4 in cardiac fibrosis in heart failure. Moreover, dietary activation of TRPV1 by capsaicin may prevent the cardiac hypertrophy caused by a high-salt diet by improving cardiac mitochondrial dysfunction.484
High blood pressure
High blood pressure is associated with vasoconstriction.485,486 Evidence showing the involvement of TRP channels in the regulation of vasoconstriction is lacking. Furthermore, impaired TRPV1 signaling may contribute to the pathogenesis of hypertension.487 Anandamide activates TRPV1 on perivascular sensory nerves, which leads to the release of CGRP to reduce vasoconstriction.488 Anandamide also inhibits vasoconstriction by activating the TRPV1 receptor in the endothelium, which causes the acute release of NO.489
TRPV4 and TRPM4 also have a regulatory effects on systemic blood pressure. Plasma adrenaline concentrations and urinary excretion of catecholamine metabolites, which may reduce hypertension, greatly increased in Trpm4 knockout mice.490 Furthermore, in Trpv4 knockout mice, the odds of hypertension induced by inhibition of NOS were greater, which was mainly related to the involvement of TRPV4 in vasodilation,491 this finding suggests the vasoprotective effect of endothelial TRPV4.
Atherosclerosis
Atherosclerosis is the process of plaque formation that leads to the development of a number of CVDs.492,493 In addition, atherosclerosis may be associated with the apoptosis of macrophages.494 Tano et al. discovered that Trpc3 knockout mice exhibited reduced macrophage apoptosis and necrosis and increased collagen content.495 Specific inhibition of TRPA1 exacerbated oxidized low-density lipoprotein-induced lipid accumulation in macrophages, and atherosclerotic lesions and hyperlipidemia aggravated.496 Upregulation of TRPM2 enhanced 5-HT vascular reactivity during the development of atherosclerosis.497
Vascular complications
Autosomal dominant PKD is caused by mutations in PKD1 and PKD2 and often associated with vascular abnormalities.498,499 PKD1 and PKD2 encode polycystins TRPP1 and TRPP2, respectively.119 Abnormal intracellular Ca2+ regulation associated with PKD2 haploinsufficiency reduces vascular smooth muscle cell contractions.500 This condition is essential for maintaining the structural and functional integrity of blood vessels.501 Overall, these observations suggest that TRP channels may be an avenue for mitigating CVD.
Endocrine system and metabolism
TRP channels also have a wide range of physiological functions in the endocrine system and metabolism, including in diseases such as cancer, obesity, kidney disease, metabolic syndrome, and diabetes.181,502,503
Cancer
TRP channels play a key role in cancer progression.504 Changes in the expression of TRP channels have been found in a number of cancers, including prostate,505 breast,506 colorectal,507 pancreatic,508 liver,509 and gastric cancers.510 Such finding was mainly due to the inhibition of cell migration and invasion,511 and tumor cell growth and proliferation caused by TRP channels.512 Researchers need to further elucidate the functional role of TRP channels in cancer and their changes.
Obesity
Obesity is characterized by excessive fat accumulation in the body.181 Notably, TRP channels are expressed in adipose tissue.513 In response to exogenous stimulation, TRP channel receptors activate the sympathetic nervous system norepinephrine brown adipose tissue axis to enhance thermogenesis and thereby suppress obesity.514 Oral capsaicin reduces abdominal fat515 and enhances brown fat thermogenesis, white fat browning,516 fatty acid β-oxidation,517 and energy expenditure in obese patients.518 The function of these TRP channels identifies them as potential targets for obesity.
Kidney disease
TRPC6 is expressed in glomerular podocytes. Increased expression levels of TRPC6 were observed in patients with focal segmental glomerulosclerosis (FSGS),519 membranous nephropathies,520 minimal change nephrosis,521 and diabetic nephropathy.522 TRPC6 mutations are involved in FSGS at the genetic level.523 TRPC 6 plays a cytoprotective role in membranous nephropathy.524 Aberrant expression of TRPC6 channels may be an important causative factor in kidney disease, but its clinical application needs further study.
Metabolic syndrome
Metabolic syndrome is a common metabolic disorder that results from the increased prevalence of obesity.525 As mentioned earlier, TRP channels are involved in CVD and obesity. The dysfunction of TRP channels is likely to contribute to increased cardiovascular risk in patients with metabolic syndrome.526 Although no experimental evidence indicates that TRP channels directly affect metabolic syndrome, TRP channels can be investigated as a medicinal target because of their role in related disorders.
Diabetes
TRP channels are related to several aspects of the pathology of diabetes, including insulin release from pancreatic beta cells and secondary diseases such as diabetic neuropathy, nephropathy, and vasculopathy.527 Chung et al.528 observed that the gene expression of TRPC4 was upregulated by 22%, and the protein expressions of TRPC1 and TRPC6 were downregulated by 50% in blood vessels of diabetic patients compared with non-diabetic controls. They suggested that diabetes affected capacitive calcium entry by regulating TRPCs.528 In addition, activation of TRPV4 affects insulin secretion and influences the level of insulin mRNA in cells.529 TRP channels have distinct clinical potential for the treatment or prevention of diabetes and its complications. However, given that TRP channels have a number of conflicting indications, clinical drug development will face a number of challenges.
Urogenital system
Preclinical studies suggested that modulators of TRPs improve genitourinary disorders,530,531 including neurogenic bladder,532 interstitial cystitis,533 and overactive bladder. Extracellular ATP-gated P2X receptors are trimeric non-selective cation channels that are important for nerve conduction.534 After posterior tibial nerve stimulation of neurogenic bladder in rats, inflammatory cell infiltration in bladder tissue was significantly reduced, and TRP and P2X expressions in the bladder and DRG were partially restored.535 Clinical studies have revealed significantly increased levels of TRPM8 mRNA and protein expression in bladder tissue from patients with interstitial cystitis/bladder pain syndrome.536 In addition, the secretion of inflammatory mediators and oxidative stress by TRPA1 into the periphery may influence the pathogenesis of interstitial cystitis/bladder pain syndrome.537
TRPV1 plays a role in the pathophysiology and treatment of overactivity of the detrusor muscle.538 TRPV1 expression was significantly elevated in the urothelium of patients with overactive bladder disease.539 NGF-induced bladder overactivity depends on the interaction of NGF with TRPV1.540 In addition, selective antagonism of TRPM8 inhibits the exaggerated activity of mechanosensitive bladder C-fibers and reduces bladder overactivity.541 These studies suggest that TRP channel modulators may be of value in urogenital disorders.
Immune regulation
Growing evidence demonstrates functional TRP channel expression in immune cells with important implications for immunology. TRP channel activation induces immunomodulatory effects on multiple levels.542
IL-31 is a key neuroimmune link between TH2 cells and sensory nerves in the generation of T cell-mediated pruritus.543 IL-31-induced pruritus was significantly reduced in Trpv1 and Trpa1 knockout mice.544 This finding suggests that TRPV1 and TRPA1 are involved in immune response.
To date, the expression of TRPA1 in immune cells has been poorly studied. Expression of TRPA1 was upregulated in atherosclerotic macrophages from mice deficient in lipoprotein E (Apoe−/−).496 Selective inhibition of TRPA1 increased the levels of serum triglycerides, high-density lipoprotein, total cholesterol, pro-inflammatory cytokines, and macrophage inflammatory protein-2 (MIP-2) in Apoe−/− mice.496 This result suggests that TRPA1 performs a regulatory function in macrophage foam cell cholesterol metabolism. Activation of TRPA1 also stimulated the release of TNF-α and IL-8 from the human macrophage line U937.545
In RAW 264.7 macrophages, the TRPM8 agonist icilin activated TRPM8-like channels.546 In vitro, anti-inflammatory effects were produced in mouse peritoneal macrophages by activation of TRPM8.547 Activation of TRPM8 also inhibited the production of pro-inflammatory cytokines in human monocytes and lymphocytes in vitro.548
TRPV1 is activated in bone marrow-derived macrophages, which attenuates lipid accumulation and reduces the production of monocyte chemoattractant protein (MCP-1), MIP-2 and IL-6.549 Activation of TRPV1 in RAW264.7 cells in an inflammatory state inhibited the release of iNOS and NO, COX-2 and PGE2.550 Acharya et al.551 observed that activation of TRPV1, which induces anandamide production by myeloid cells acting on CB2 receptors, increased the number and immunosuppressive function of regulatory CX3CR1hi macrophages.
TRPV4 is expressed and functions in immune cells, including macrophages, monocytes, neutrophils and T cells.552 In alveolar macrophages of WT mice, activation of TRPV4 caused calcium influx and superoxide and nitric oxide production, but this phenomenon was absent in Trpv4 knockout mice.419 Majhi et al.553 revealed that in vitro activation of TRPV4 in T cells led to T cell proliferation and production of pro-inflammatory cytokines IFN-γ, TNF-α and IL-2. These results suggest that TRP channels are involved in the coordination of immune response in immune cells.
TRP channels are also involved in the pathogenesis of immune-mediated chronic inflammatory diseases.554 They regulate macrophage polarization,555 chemokine and cytokine production,556 phagocytosis,411 and proliferation and survival.557 In the future, the expressions of TRP channels in immune cells and the signaling pathways for TRP channel activation in different immune-mediated diseases must be clarified to identify new molecular mechanisms for the treatment of these diseases.
Therapeutic interventions
TRP channels have important functions in diseases. Thus, the use of TRPs as a drug target has therapeutic potential.
Pharmaceutical interventions
In recent years, several modulators of TRPs of chemical, biological, and natural origin have entered clinical trials and they have been used to treat inflammatory, neuropathic, and visceral pain although the therapeutic mechanism of action of these compounds is unclear.153
TRPV1 agonists and antagonists
Various TRPV1 antagonists have been evaluated at various stages of clinical trials for different indications. TRPV1 antagonists show promise for pain treatment. ABT-102, a structurally novel TRPV1 antagonist, has been tested in phase I clinical trials for pain associated with inflammation, tissue damage, and ischemia.558 ABT-102 was clinically well tolerated by repeated dosage but caused a short-term increase in the mean core body temperature in healthy volunteers.559 Phase II clinical trials have been completed for the use of AZD1386, also an antagonist of TRPV1, for neuropathic pain, dental pain, nonerosive reflux disease, and osteoarthritis.560 However, in patients with osteoarthritis and nonerosive reflux disease, the clinical trial was terminated because there was no significant reduction in pain was observed.561,562
Capsaicin in chili peppers bestows the sensation of spiciness and is a common component found in the fruits of Capsicum.157,563 It is a classical TRPV1 agonist. Studies have reported the beneficial effects of capsaicin acting on TRPV1 in obesity, cardiovascular and GI, various cancers, neurogenic bladder, and skin diseases.564
A randomized trial assessing the effects of TRPV1 antagonist SB705498 on pruritus induced by histamine and cowhage in healthy volunteers has progressed to phase I.565 However, the results of SB705498 on atopic dermatitis showed that it is not clinically relevant for pruritus due to dermatitis. SB705498 has also been investigated for the treatment of migraine (ClinicalTrials.gov: NCT00269022), toothache (ClinicalTrials.gov: NCT00281684) and rectal pain (ClinicalTrials.gov: NCT00461682), all of which have now completed Phase II clinical trials.566 The results of these clinical studies have not been published to date.
Phase II clinical trials of XEND0501 for the treatment of chronic cough and COPD (ClinicalTrials.gov: NCT022233699 and NCT022233686) have been completed. Although XEND0501 was well tolerated, it was not clinically meaningful for patients with refractory cough.567
PAC-14028 completed a Phase II clinical trial for the topical treatment of pruritus, rosacea, and atopic dermatitis (ClinicalTrials.gov: NCT02052531, NCT02052999 and NCT02583022, respectively).568 In 2019, the results of the clinical Phase IIb trial of PAC-14028 cream met safety and efficacy metrics.569 In addition to the TRPV1 antagonists mentioned above, numerous chemical, biological and natural agents, including NEO-6860, JNJ-39439335 and others, have been introduced in clinical trials.
TRPA1 antagonists
Given its involvement in pain, itching, and respiratory syndromes, TRPA1 has been pursued as a promising drug target.570 GDC-0334 is a chemical-derived drug that reduced TRPA1 agonist-induced skin blood flow, pain and pruritus in a phase I clinical trial (ClinicalTrials.gov: NCT03381144).571 GDC-0334 provides a therapeutic basis for assessing TRPA1 inhibition as a clinical treatment for asthma.
In 2012, Glenmark selected GRC 17536 for clinical development as a TRPA1 antagonist. In September 2014, Glenmark announced positive results obtained in the Phase II clinical trial.392 GRC 17536 had statistically significant and clinically relevant response in patients with moderate to severe painful diabetic neuropathy.572 GRC 17536 remains the only TRPA1 antagonist to have completed a Phase IIa study to date.573
In 2015, Orion announced that the TRPA1 antagonist ODM108 (acetylene-containing compound) entered phase I clinical trials for neuropathic pain.570 However, complex non-linear pharmacokinetics emerged and the clinical trial was discontinued in 2016. A key point of progress in TRPA1 research is the efficient oral utilization of the substance. A problem with all TRPA1 antagonists developed to date is that they are highly lipophilic and relatively insoluble. Addressing this issue is necessary for the drug development involving TRPA1.
Regulation of TRPM8
The TRPM8 channel is responsible for the sensation of cold environmental temperatures, it is also clinically associated with pain, itching, and obesity. PF-05105679 is a moderately potent TRPM8 blocker that has been evaluated for the treatment of cold pain sensitivity.574 Winchester et al.575 observed in clinical trials of PF-05105679 that the compound showed significant pain inhibition in the cold press test and no effect on core body temperature was observed. These results suggest that TRPM8 plays a role in acute cold pain signaling, and its dose does not result in a lower than normal body temperature.
Menthol is a natural source of TRPM8 agonists. Topical menthol activates nociceptors on the skin and then desensitizes them.576 When topically applied, menthol can also act as a vasorelaxant, but the mechanism is unclear.577 Menthol is also used clinically for migraines, neuropathy, and muscle pain. It provides a cooling effect, leaving human subjects with a cool sensation on the skin and significantly less discomfort than ice.578
Another TRPM8-related compound, isopulegol (Phase II, ClinicalTrials.gov: NCT02193438), had no positive biomechanical or neurophysiological effects on patients with oral dysphagia due to stroke or neurodegenerative disease no significant adverse events were observed.579
Peppermint oil is another TRPM8 agonist of a natural origin. Winchester et al.575 investigated the role of peppermint oil in a mouse model of colitis. The results showed that peppermint oil, activated TRPM8 and the treated mice showed improvements in macroscopic and microscopic parameters and reduced weight loss during colitis.575 In addition, Peiris et al. observed that the use of TRPM8 agonist icilin for irritable bowel syndrome resulted in reduced release of inflammatory cytokines IL-1β, IL-6, and TNF-α in patient biopsies.580 These results suggest that specific activation of TRPM8 may alleviate experimental colitis and irritable bowel syndrome and may be a promising additional therapy for the treatment of IBD.
Other medicinal TRP uses
TRPV1, TRPA1, and TRPM8 are currently being studied in clinical trials, but clinical studies of other channels should not be overlooked. A TRPV4 inhibitor, GSK2798745 was launched by GlaxoSmithKline as a clinical candidate.581 In 2019, GSK2798745 underwent its first clinical study (ClinicalTrials.gov: NCT02119260). Positive results were obtained; GSK2798745 was well tolerated in healthy volunteers and in patients with stable heart failure, which allowed further evaluation in long-term clinical studies of heart failure and in other indications.582
Preclinical studies have provided a certain rationale to support the development of TRPV3 antagonists for the treatment of inflammatory skin diseases, pruritus and pain.583 However, to date, only one TRPV3 inhibitor (GRC15300) has entered clinical trial targeting osteoarthritis and neuropathic pain.584 Moreover, by the end of 2013, these trials were discontinued. The Sanofi–Glimark agreement was terminated in 2014, and no further developments have been reported since then.584 Although numerous TRP channel drugs have entered clinical trials, only a few have been approved by the US Food and Drug Administration (FDA) (Table 4). In summary, researchers have made progress in understanding the role of TRPs in health and diseases. TRPs show promise as therapeutic targets for the treatment of inflammation, pain, itching, and other conditions.
Physical interference methods
Numerous methods of physical interference are used for TRP channels, and they include moxibustion, acupuncture, photothermal therapy, and electroacupuncture. Moxibustion, acupuncture, and electro-acupuncture are all traditional Chinese medicine treatments.585 These methods are applied to the body surface to avoid the adverse events associated with medication.586 They are mainly used clinically for the relief of pain and inflammation.586,587
The effectiveness of moxibustion therapy is reportedly determined by the activation of TRPV1–4.588 In one study, moxibustion treatment of Trpv1 knockout mice did not produce the significant increase in thermal pain thresholds exhibited by normal mice.589 This result suggested that TRPV1 was involved in the process of increasing the thermal pain threshold in the moxibustion-stimulated mouse spinal cord at L5 and L6.589 In visceral nociceptive rats, the expression of TRPV1 and heat shock protein 70 was increased. This increase was reversed by moxibustion treatment, which also significantly reduced abdominal withdrawal reflex scores and increased the pain threshold pressure.590 Pain treatment of moxibustion may be accomplished by downregulating TRPV1 expression.
Acupuncture significantly increases TRPV1 expression in subcutaneous nerve fibers and modulates TRPV channels in the brain and spinal cord.591 Acupuncture also activates the expression of TRPV1 and TRPV4 in the anatomical layers of the Zusanli acupoint (ST36), including the muscle and epithelial layers.592 Given that the components of calcium wave propagation, which occurs during acupuncture are also expressed in the TRPV1-rich ST36 anatomical surface, TRPV1 may be involved in the analgesic effects associated with acupuncture.592 The researchers used knockout mice to further validate the relationship between TRPV channels and electroacupuncture treatment. Huang et al.593 noticed that in Trpv2 knockout male mice, acupuncture-induced analgesia was suppressed, and activation of mast cells in stimulated acupoints was reduced.
Analgesic effects similar to those observed in acupuncture treatment were also detected in electroacupuncture treatment and mainly related to TRPV channel modulation.594,595,596 Electroacupuncture may improve acid–base-induced chronic pain through its action on TRPV1 and related molecular pathways.597 In addition, inhibition of TRPV1 expression by electroacupuncture plays a role in the neuroinflammatory regulation of PD, and downregulation of cytokine levels.598 For the pain treatment of cancer patients, electroacupuncture treatment downregulated the overexpression of TLR4, myeloid differentiation primary response 88, and TRPV1 in the DRG.599 This condition reduced the activation of spinal glial cells and thus relieved pain. Electroacupuncture modulation of TRPV1 for CVD has also been reported. Electroacupuncture pretreatment enhanced TRPV1 and CGRP signaling, downregulated NF-κB p65 protein expression, reduced the myocardial inflammatory response status, improved acute myocardial ischemic injury and reduced myocardial infarct size.600
With the development of scientific research technology, nanoparticles are becoming more widely used. Gold nanorods are pseudo one-dimensional rod-shaped nanoparticles that can be used for thermal therapy when exposed to near-infrared radiation.601 Zhou et al.602 showed that gold nanorods activated TRPV1 channels through photothermal conversion during exposure to near-infrared irradiation. This nano-loaded anesthetic can produced selective, long-lasting regional anesthetic effects in a rat model of sciatic nerve block. In addition, semi-conducting photothermal nano loads of capsaicin were followed by a photothermal trigger to induce release. The results showed high local concentrations of TRPV1 agonist at the tumor site.603
Current physical interventions in TRP channels focus on electroacupuncture and acupuncture for the treatment of diseases. Based on several properties of the TRP channels, we believe that influencing the TRP channels through cold and heat compresses to relieve pain and inflammation is a promising direction to be confirmed by researchers.
Therapeutic application limitations
The clinical development of TRP channel modulators has provided a scientific basis and new insights into the further use of TRP channels as biomarkers for disease treatment. Despite the appeal of TRP channels in the clinical treatment of pain and inflammation, a number of challenges remain.
In clinical studies, heat and pain are the most common adverse effects of treatment targeting TRP channels. In a study of the effect of inhibition of TRPM8 channels on human pain, the effect on core body temperature and experimental cold pain was assessed by the cold pressor test after PF-05105679 administration.575 A total of 23% and 36% of volunteers (at doses of 600 and 900 mg, respectively) reported hot sensations mainly in the perioral region; two volunteers showed intolerance towards the treatment.575
Capsaicin has long been considered clinically effective for the treatment of bladder disorders; however, capsaicin in the bladder is unacceptable for a number of patients because it causes burning pain.604 Capsaicin also causes profuse sweating and a burning sensation on the mucous membranes of the tongue and mouth, which dissipates after repeated challenges (“desensitization”).605 This desensitization is also known as a persistent refractory state following a painful reaction,532 which can last for up to several months.606
Most antagonists of the TRPV1 channel elevate body temperature. Most patients had a slight increase in body temperature after oral administration of AZD1386 (mean value of 0.4 °C), and only one exceeded 38 °C.607 In addition, Amgen’s Phase 1b clinical trial using AMG 517 in dental pain elicited a prolonged (1–4 days) and significant febrile response (up to 40.2 °C) in human volunteers.608
TRPV1 channel antagonists can elevate the threshold of toxic heat sensation and lead to burns. Previously, Merck/Neurogenics developed MK-2295 for the treatment of pain and cough.609 This compound significantly increased the threshold for toxic heat pain in humans, and several volunteer subjects even perceived scalding water as cold.610 Similar results were observed by AstraZeneca and GlaxoSmithKline on a TRPV1 channel antagonist (SB-705498).607 In addition, mild burns on the mouth (gums) and skin were noted in patients using the TRPV1 channel antagonist JNJ-39439335.611
As TRP channels are expressed in various tissues, systemic administration in the clinical setting may cause varied adverse effects. Among these effects, dizziness, hypertension, heat tremors, headache, nausea, chills, decreased body temperature and paresthesia are more common and should be the focus of researchers (Table 5). Volunteers treated with ticlopidine, clopidogrel and prasugrel (TRPA1 agonists) often complained of GI discomfort such as nausea, vomiting, and diarrhea.612 Activation of TRPV1 by RTX has antiemetic and hypothermic effects, but it can also cause hypertension.613 The most frequently reported events in subjects taking NEO6860 (TRPV1 antagonist) orally were feeling of hotness, headache, paresthesia, nausea and dizziness.614 Therefore, current clinical research can focus on the topical delivery of TRP channel modulators, including topical patches, mucosal sprays, and nano-delivery systems.
The specificity of TRP channel agonists and antagonists needs to be enhanced. As some TRP modulators, such as hydroxy-α-sanshool, activate not only TRPA1, but also TRPV1.615 Voacangine is an antagonist of TRPV1 and TRPM8, but is also an agonist of TRPA1.616
Efficient and specific modulation of TRP channels is a promising to be a new approach to the treatment of various diseases in the future. The above-mentioned adverse events should be considered in clinical studies.
Conclusions and perspectives
With rapid advances in the field of TRPs, numerous publications have demonstrated that TRPs function as important channels intracellularly, and their abnormalities serve as a pathological triggers for diseases. Herein, we summarized all types of TRPs and demonstrated their functions in multiple biological processes. Furthermore, this review summarized the structures and therapeutic interventions and limitations of TRPs from a historical perspective. However, a number of adverse events remain to be addressed in this area, such as fever, pain, nausea, and even lack of therapeutic efficacy in clinical trials of some studies. The cause of these adverse events may be due to the wide distribution of TRP channels in the body, and modulation of TRP channels can cause systemic reactions. In addition, the specificity of existing drugs for TRP channels needs to be enhanced. To overcome some of these side effects, low doses, topical treatment and precise targeted drug delivery can be used.
To address the issue of precise targeted drug delivery, scholars must ensure the specificity of developed drugs for TRP channels, which will reduce cross-infection caused by TRP channels and reduce damage to other tissues. Second, there is a need to clarify the mechanisms by which drugs modulate TRP channels must be clarified. Finally, new and more effective compounds that can reduce the amount of drugs used must be developed.
Improving the targeting of existing drugs, nano-delivery systems are an interesting topic. In future research, on the one hand, the modification of nanoparticles with TRP channel modulators can be explored in order to exert targeted effects. On the other hand, given that graphene can be combined with metal nanostructures, it can the ability to absorb light by relying on the friction of carbon atoms to generate heat and thus modulate thermally activated TRP channels. Both directions are yet to be examined.
In clinical practice, a mixture of modalities is commonly used to treat diseases. Therefore, a combination of physical interventions and drugs can be proposed to exert a synergistic or amplifying effect on the treatment of diseases associated with TRPs. Multiple treatment combinations can be used with a combination of Western and Chinese medicine. Chemical drugs and physical stimulation methods, such as acupuncture and electroacupuncture are used together. We predict that new therapeutic modalities such as hyperthermia, cold compresses, drug delivery, targeted, Synergistic treatment with multiple modalities, and gene based therapies will be increasingly explored in the future for the treatment of human TRP channel-related diseases.
References
Cosens, D. J. & Manning, A. Abnormal electroretinogram from a Drosophila mutant. Nature 224, 285–287 (1969).
Chen, Y. et al. Transient receptor potential channels and inflammatory bowel disease. Front. Immunol. 11, 180 (2020).
Hof, T. et al. Transient receptor potential channels in cardiac health and disease. Nat. Rev. Cardiol. 16, 344–360 (2019).
Kaneko, Y. & Szallasi, A. Transient receptor potential (TRP) channels: a clinical perspective. Br. J. Pharm. 171, 2474–2507 (2014).
Minke, B., Wu, C. & Pak, W. L. Induction of photoreceptor voltage noise in the dark in Drosophila mutant. Nature 258, 84–87 (1975).
Montell, C. & Rubin, G. M. Molecular characterization of the Drosophila trp locus: a putative integral membrane protein required for phototransduction. Neuron 2, 1313–1323 (1989).
Wong, F. et al. Proper function of the Drosophila trp gene product during pupal development is important for normal visual transduction in the adult. Neuron 3, 81–94 (1989).
Suss-Toby, E., Selinger, Z. & Minke, B. Lanthanum reduces the excitation efficiency in fly photoreceptors. J. Gen. Physiol. 98, 849–868 (1991).
Minke, B. & Selinger, Z. The inositol-lipid pathway is necessary for light excitation in fly photoreceptors. Soc. Gen. Physiol. Ser. 47, 201–217 (1992).
Hardie, R. C. & Minke, B. The trp gene is essential for a light-activated Ca2+ channel in Drosophila photoreceptors. Neuron 8, 643–651 (1992).
Phillips, A. M., Bull, A. & Kelly, L. E. Identification of a Drosophila gene encoding a calmodulin-binding protein with homology to the trp phototransduction gene. Neuron 8, 631–642 (1992).
Xu, X. Z., Li, H. S., Guggino, W. B. & Montell, C. Coassembly of TRP and TRPL produces a distinct store-operated conductance. Cell 89, 1155–1164 (1997).
Gillo, B. et al. Coexpression of Drosophila TRP and TRP-like proteins in Xenopus oocytes reconstitutes capacitative Ca2+ entry. Proc. Natl Acad. Sci. USA 93, 14146–14151 (1996).
Montell, C. et al. A unified nomenclature for the superfamily of TRP cation channels. Mol. Cell 9, 229–231 (2002).
Clapham, D. E. et al. International Union of Pharmacology. XLIII. Compendium of voltage-gated ion channels: transient receptor potential channels. Pharm. Rev. 55, 591–596 (2003).
Clapham, D. E. TRP channels as cellular sensors. Nature 426, 517–524 (2003).
Li, H. TRP Channel Classification. Adv. Exp. Med. Biol. 976, 1–8 (2017).
Palmer, C. P. et al. A TRP homolog in Saccharomyces cerevisiae forms an intracellular Ca(2+)-permeable channel in the yeast vacuolar membrane. Proc. Natl Acad. Sci. USA 98, 7801–7805 (2001).
Pan, Z., Yang, H. & Reinach, P. S. Transient receptor potential (TRP) gene superfamily encoding cation channels. Hum. Genomics 5, 108–116 (2011).
Fine, M., Li, X. & Dang, S. Structural insights into group II TRP channels. Cell Calcium 86, 102107 (2020).
He, Z. TRPC channel downstream signaling cascades. Adv. Exp. Med. Biol. 976, 25–33 (2017).
Feng, S. TRPC channel structure and properties. Adv. Exp. Med. Biol. 976, 9–23 (2017).
Latorre, R., Zaelzer, C. & Brauchi, S. Structure-functional intimacies of transient receptor potential channels. Q. Rev. Biophys. 42, 201–246 (2009).
Wes, P. D. et al. TRPC1, a human homolog of a Drosophila store-operated channel. Proc. Natl Acad. Sci. USA 92, 9652–9656 (1995).
Venkatachalam, K. & Montell, C. TRP channels. Annu. Rev. Biochem. 76, 387–417 (2007).
Falcon, D. et al. TRPC Channels: dysregulation and Ca(2+) mishandling in ischemic heart disease. Cells 9, 173 (2020).
Kim, J. et al. TRPC1 as a negative regulator for TRPC4 and TRPC5 channels. Pflug. Arch. 471, 1045–1053 (2019).
Seebohm, G. & Schreiber, J. A. Beyond hot and spicy: TRPV channels and their pharmacological modulation. Cell Physiol. Biochem. 55, 108–130 (2021).
Tomohiro, D. et al. Inhibition by capsaicin and its related vanilloids of compound action potentials in frog sciatic nerves. Life Sci 92, 368–378 (2013).
Huynh, K. W. et al. Structural insight into the assembly of TRPV channels. Structure 22, 260–268 (2014).
Satheesh, N. J. et al. TRPV currents and their role in the nociception and neuroplasticity. Neuropeptides 57, 1–8 (2016).
Phelps, C. B. et al. Structural analyses of the ankyrin repeat domain of TRPV6 and related TRPV ion channels. Biochemistry 47, 2476–2484 (2008).
Muller, C., Morales, P. & Reggio, P. H. Cannabinoid ligands targeting TRP channels. Front. Mol. Neurosci. 11, 487 (2018).
Chen, M. & Li, X. Role of TRPV4 channel in vasodilation and neovascularization. Microcirculation 28, e12703 (2021).
Jaquemar, D., Schenker, T. & Trueb, B. An ankyrin-like protein with transmembrane domains is specifically lost after oncogenic transformation of human fibroblasts. J. Biol. Chem. 274, 7325–7333 (1999).
Montell, C. The TRP superfamily of cation channels. Sci. STKE 2005, re3 (2005).
Corey, D. P. & New, T. R. P. channels in hearing and mechanosensation. Neuron 39, 585–588 (2003).
Meents, J. E., Ciotu, C. I. & Fischer, M. J. M. TRPA1: a molecular view. J. Neurophysiol. 121, 427–443 (2019).
Naert, R., Lopez-Requena, A. & Talavera, K. TRPA1 expression and pathophysiology in immune cells. Int. J. Mol. Sci. 22, 11460 (2021).
Talavera, K. et al. Mammalian transient receptor potential TRPA1 channels: from structure to disease. Physiol. Rev. 100, 725–803 (2020).
Duncan, L. M. et al. Down-regulation of the novel gene melastatin correlates with potential for melanoma metastasis. Cancer Res 58, 1515–1520 (1998).
Huang, Y. et al. A structural overview of the ion channels of the TRPM family. Cell Calcium 85, 102111 (2020).
Kozak, J. A. & Putney, J. W. Jr. (eds) Calcium Entry Channels in Non-Excitable Cells, Boca Raton (FL): CRC Press/Taylor & Francis (2018).
Garcia-Avila, M. & Islas, L. D. What is new about mild temperature sensing? A review of recent findings. Temp. (Austin) 6, 132–141 (2019).
Smani, T. et al. TRP channels in angiogenesis and other endothelial functions. Front. Physiol. 9, 1731 (2018).
Gao, Y. & Liao, P. TRPM4 channel and cancer. Cancer Lett 454, 66–69 (2019).
Belrose, J. C. & Jackson, M. F. TRPM2: a candidate therapeutic target for treating neurological diseases. Acta Pharm. Sin. 39, 722–732 (2018).
Gatica, S. et al. TRPM7 mediates kidney injury, endothelial hyperpermeability and mortality during endotoxemia. Lab Invest 100, 234–249 (2020).
Zierler, S., Hampe, S. & Nadolni, W. TRPM channels as potential therapeutic targets against pro-inflammatory diseases. Cell Calcium 67, 105–115 (2017).
Vennekens, R., Mesuere, M. & Philippaert, K. TRPM5 in the battle against diabetes and obesity. Acta Physiol 222, e12949 (2018).
Zholos, A., Johnson, C., Burdyga, T. & Melanaphy, D. TRPM channels in the vasculature. Adv. Exp. Med. Biol. 704, 707–729 (2011).
Walker, R. G., Willingham, A. T. & Zuker, C. S. A Drosophila mechanosensory transduction channel. Science 287, 2229–2234 (2000).
Lee, J., Moon, S., Cha, Y. & Chung, Y. D. Drosophila TRPN(=NOMPC) channel localizes to the distal end of mechanosensory cilia. PLoS ONE 5, e11012 (2010).
Li, W., Feng, Z., Sternberg, P. W. & Xu, X. Z. A. C. elegans stretch receptor neuron revealed by a mechanosensitive TRP channel homologue. Nature 440, 684–687 (2006).
Al-Sheikh, U. & Kang, L. Mechano-gated channels in C. elegans. J. Neurogenet. 34, 363–368 (2020).
Sidi, S., Friedrich, R. W. & Nicolson, T. NompC TRP channel required for vertebrate sensory hair cell mechanotransduction. Science 301, 96–99 (2003).
Barr, M. M. et al. The Caenorhabditis elegans autosomal dominant polycystic kidney disease gene homologs lov-1 and pkd-2 act in the same pathway. Curr. Biol. 11, 1341–1346 (2001).
Watnick, T. J. et al. A flagellar polycystin-2 homolog required for male fertility in Drosophila. Curr. Biol. 13, 2179–2184 (2003).
Neill, A. T., Moy, G. W. & Vacquier, V. D. Polycystin-2 associates with the polycystin-1 homolog, suREJ3, and localizes to the acrosomal region of sea urchin spermatozoa. Mol. Reprod. Dev. 67, 472–477 (2004).
Palmer, C. P., Aydar, E. & Djamgoz, M. B. A microbial TRP-like polycystic-kidney-disease-related ion channel gene. Biochem. J. 387, 211–219 (2005).
Koulen, P. et al. Polycystin-2 is an intracellular calcium release channel. Nat. Cell Biol. 4, 191–197 (2002).
Harteneck, C. Function and pharmacology of TRPM cation channels. Naunyn Schmiedebergs Arch. Pharm 371, 307–314 (2005).
Puertollano, R. & Kiselyov, K. TRPMLs: in sickness and in health. Am. J. Physiol. Ren. Physiol. 296, F1245–F1254 (2009).
Bargal, R. et al. Identification of the gene causing mucolipidosis type IV. Nat. Genet. 26, 118–123 (2000).
Flores, E. N. & Garcia-Anoveros, J. TRPML2 and the evolution of mucolipins. Adv. Exp. Med. Biol. 704, 221–228 (2011).
Abuammar, H. et al. Ion channels and pumps in autophagy: a reciprocal relationship. Cells. 10, 3537 (2021).
Wu, L. J., Sweet, T. B. & Clapham, D. E. International Union of Basic and Clinical Pharmacology. LXXVI. Current progress in the mammalian TRP ion channel family. Pharm. Rev. 62, 381–404 (2010).
Cao, E. Structural mechanisms of transient receptor potential ion channels. J. Gen. Physiol. 152, e201811998 (2020).
Cheng, W., Sun, C. & Zheng, J. Heteromerization of TRP channel subunits: extending functional diversity. Protein Cell 1, 802–810 (2010).
Jin, X., Touhey, J. & Gaudet, R. Structure of the N-terminal ankyrin repeat domain of the TRPV2 ion channel. J. Biol. Chem. 281, 25006–25010 (2006).
Lishko, P. V. et al. The ankyrin repeats of TRPV1 bind multiple ligands and modulate channel sensitivity. Neuron 54, 905–918 (2007).
Hellmich, U. A. & Gaudet, R. Structural biology of TRP channels. Handb. Exp. Pharm. 223, 963–990 (2014).
Numazaki, M. et al. Structural determinant of TRPV1 desensitization interacts with calmodulin. Proc. Natl Acad. Sci. USA 100, 8002–8006 (2003).
Moiseenkova-Bell, V. Y. et al. Structure of TRPV1 channel revealed by electron cryomicroscopy. Proc. Natl Acad. Sci. USA 105, 7451–7455 (2008).
Liao, M., Cao, E., Julius, D. & Cheng, Y. Structure of the TRPV1 ion channel determined by electron cryo-microscopy. Nature 504, 107–112 (2013).
Cao, E., Liao, M., Cheng, Y. & Julius, D. TRPV1 structures in distinct conformations reveal activation mechanisms. Nature 504, 113–118 (2013).
Zhang, K., Julius, D. & Cheng, Y. Structural snapshots of TRPV1 reveal mechanism of polymodal functionality. Cell 184, 5138–5150.e5112 (2021).
Singh, A. K., McGoldrick, L. L. & Sobolevsky, A. I. Structure and gating mechanism of the transient receptor potential channel TRPV3. Nat. Struct. Mol. Biol. 25, 805–813 (2018).
Zubcevic, L. et al. Cryo-electron microscopy structure of the TRPV2 ion channel. Nat. Struct. Mol. Biol. 23, 180–186 (2016).
Groschner, K. & Tiapko, O. Revelation of an enigmatic signaling machinery-First insights into the mammalian TRPC architecture. Cell Calcium 74, 144–146 (2018).
Asghar, M. Y. & Tornquist, K. Transient receptor potential canonical (TRPC) channels as modulators of migration and invasion. Int. J. Mol. Sci. 21, 1739 (2020).
Duan, J. et al. Cryo-EM structure of TRPC5 at 2.8-A resolution reveals unique and conserved structural elements essential for channel function. Sci. Adv. 5, eaaw7935 (2019).
Duan, J. et al. Structure of the mouse TRPC4 ion channel. Nat. Commun. 9, 3102 (2018).
Tang, Q. et al. Structure of the receptor-activated human TRPC6 and TRPC3 ion channels. Cell Res 28, 746–755 (2018).
Sierra-Valdez, F. et al. Structure-function analyses of the ion channel TRPC3 reveal that its cytoplasmic domain allosterically modulates channel gating. J. Biol. Chem. 293, 16102–16114 (2018).
Bavencoffe, A., Zhu, M. X. & Tian, J. B. New aspects of the contribution of ER to SOCE regulation: TRPC proteins as a link between plasma membrane ion transport and intracellular Ca(2+) stores. Adv. Exp. Med. Biol. 993, 239–255 (2017).
Boulay, G. et al. Cloning and expression of a novel mammalian homolog of Drosophila transient receptor potential (Trp) involved in calcium entry secondary to activation of receptors coupled by the Gq class of G protein. J. Biol. Chem. 272, 29672–29680 (1997).
Dietrich, A., Steinritz, D. & Gudermann, T. Transient receptor potential (TRP) channels as molecular targets in lung toxicology and associated diseases. Cell Calcium 67, 123–137 (2017).
Cornillot, M., Giacco, V. & Hamilton, N. B. The role of TRP channels in white matter function and ischaemia. Neurosci. Lett. 690, 202–209 (2019).
Azumaya, C. M., Sierra-Valdez, F., Cordero-Morales, J. F. & Nakagawa, T. Cryo-EM structure of the cytoplasmic domain of murine transient receptor potential cation channel subfamily C member 6 (TRPC6). J. Biol. Chem. 293, 10381–10391 (2018).
Vinayagam, D. et al. Electron cryo-microscopy structure of the canonical TRPC4 ion channel. Elife 7, e36615 (2018).
Fan, C. et al. Structure of the human lipid-gated cation channel TRPC3. Elife 7, e36852 (2018).
Story, G. M. et al. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 112, 819–829 (2003).
Paulsen, C. E. et al. Structure of the TRPA1 ion channel suggests regulatory mechanisms. Nature 525, 552 (2015).
Paulsen, C. E. et al. Structure of the TRPA1 ion channel suggests regulatory mechanisms. Nature 520, 511–517 (2015).
Zhao, J. et al. Irritant-evoked activation and calcium modulation of the TRPA1 receptor. Nature 585, 141–145 (2020).
Julius, D. TRP channels and pain. Annu. Rev. Cell Dev. Biol. 29, 355–384 (2013).
Zayats, V. et al. Regulation of the transient receptor potential channel TRPA1 by its N-terminal ankyrin repeat domain. J. Mol. Model 19, 4689–4700 (2013).
Kim, D. & Cavanaugh, E. J. Requirement of a soluble intracellular factor for activation of transient receptor potential A1 by pungent chemicals: role of inorganic polyphosphates. J. Neurosci. 27, 6500–6509 (2007).
Nilius, B., Prenen, J. & Owsianik, G. Irritating channels: the case of TRPA1. J. Physiol. 589, 1543–1549 (2011).
Yin, Y. et al. Structure of the cold- and menthol-sensing ion channel TRPM8. Science 359, 237–241 (2018).
Phelps, C. B. & Gaudet, R. The role of the N terminus and transmembrane domain of TRPM8 in channel localization and tetramerization. J. Biol. Chem. 282, 36474–36480 (2007).
Wang, L. et al. Structures and gating mechanism of human TRPM2. Science. 362, eaav4809 (2018).
Eisfeld, J. & Luckhoff, A. Trpm2. Handb. Exp. Pharmacol. 237–252, https://doi.org/10.1007/978-3-540-34891-7_14 (2007).
Huang, Y. et al. Architecture of the TRPM2 channel and its activation mechanism by ADP-ribose and calcium. Nature 562, 145–149 (2018).
Montell, C. Mg2+ homeostasis: the Mg2+nificent TRPM chanzymes. Curr. Biol. 13, R799–R801 (2003).
Nadler, M. J. et al. LTRPC7 is a Mg.ATP-regulated divalent cation channel required for cell viability. Nature 411, 590–595 (2001).
Runnels, L. W., Yue, L. & Clapham, D. E. TRP-PLIK, a bifunctional protein with kinase and ion channel activities. Science 291, 1043–1047 (2001).
Guo, J. et al. Structures of the calcium-activated, non-selective cation channel TRPM4. Nature 552, 205–209 (2017).
Winkler, P. A. et al. Electron cryo-microscopy structure of a human TRPM4 channel. Nature 552, 200–204 (2017).
Fleig, A. & Penner, R. The TRPM ion channel subfamily: molecular, biophysical and functional features. Trends Pharm. Sci. 25, 633–639 (2004).
Autzen, H. E. et al. Structure of the human TRPM4 ion channel in a lipid nanodisc. Science 359, 228–232 (2018).
Yin, Y. et al. Activation mechanism of the mouse cold-sensing TRPM8 channel by cooling agonist and PIP(2). Science 378, eadd1268 (2022).
Yin, Y. et al. Structural basis of cooling agent and lipid sensing by the cold-activated TRPM8 channel. Science. 363, eaav9334 (2019).
Hirschi, M. et al. Cryo-electron microscopy structure of the lysosomal calcium-permeable channel TRPML3. Nature 550, 411–414 (2017).
Diver, M. M., Cheng, Y. & Julius, D. Structural insights into TRPM8 inhibition and desensitization. Science 365, 1434–1440 (2019).
Simon, S. A. & Gutierrez, R. in Neurobiology of TRP Channels Frontiers in Neuroscience (ed Emir, T. L. R.) 113–124 (2017).
Mekahli, D. et al. Polycystin-1 but not polycystin-2 deficiency causes upregulation of the mTOR pathway and can be synergistically targeted with rapamycin and metformin. Pflug. Arch. 466, 1591–1604 (2014).
Sharif-Naeini, R. et al. Polycystin-1 and -2 dosage regulates pressure sensing. Cell 139, 587–596 (2009).
Thivichon-Prince, B., Labert, N., Couble, M. & Bleicher, F. Establishment of a model of murine odontoblasts underexpressing PKD1 using shRNA. Bull. Group Int. Rech. Sci. Stomatol Odontol. 52, e23–e28 (2013).
DeCaen, P. G., Liu, X., Abiria, S. & Clapham, D. E. Atypical calcium regulation of the PKD2-L1 polycystin ion channel. Elife 5, e13413 (2016).
Semmo, M., Kottgen, M. & Hofherr, A. The TRPP subfamily and polycystin-1 proteins. Handb. Exp. Pharm. 222, 675–711 (2014).
Catterall, W. A. Structure and regulation of voltage-gated Ca2+ channels. Annu. Rev. Cell Dev. Biol. 16, 521–555 (2000).
Ishimaru, Y. et al. Transient receptor potential family members PKD1L3 and PKD2L1 form a candidate sour taste receptor. Proc. Natl Acad. Sci. USA 103, 12569–12574 (2006).
Yu, Y. et al. Structural and molecular basis of the assembly of the TRPP2/PKD1 complex. Proc. Natl Acad. Sci. USA 106, 11558–11563 (2009).
Yu, Y. et al. Molecular mechanism of the assembly of an acid-sensing receptor ion channel complex. Nat. Commun. 3, 1252 (2012).
Hughes, J. et al. The polycystic kidney disease 1 (PKD1) gene encodes a novel protein with multiple cell recognition domains. Nat. Genet. 10, 151–160 (1995).
Mochizuki, T. et al. PKD2, a gene for polycystic kidney disease that encodes an integral membrane protein. Science 272, 1339–1342 (1996).
Zhang, P. et al. The multimeric structure of polycystin-2 (TRPP2): structural-functional correlates of homo- and hetero-multimers with TRPC1. Hum. Mol. Genet. 18, 1238–1251 (2009).
Shen, P. S. et al. The structure of the polycystic kidney disease channel PKD2 in lipid nanodiscs. Cell 167, 763–773.e711 (2016).
Su, Q. et al. Structure of the human PKD1-PKD2 complex. Science 361, eaat9819 (2018).
Qian, F. et al. PKD1 interacts with PKD2 through a probable coiled-coil domain. Nat. Genet. 16, 179–183 (1997).
Rossetti, S. et al. Comprehensive molecular diagnostics in autosomal dominant polycystic kidney disease. J. Am. Soc. Nephrol. 18, 2143–2160 (2007).
Perrichot, R. et al. Identification of 3 novel mutations (Y4236X, Q3820X, 11745 + 2 ins3) in autosomal dominant polycystic kidney disease 1 gene (PKD1). Hum. Mutat. 15, 582 (2000).
Torres, V. E., Harris, P. C. & Pirson, Y. Autosomal dominant polycystic kidney disease. Lancet 369, 1287–1301 (2007).
Su, Q. et al. Cryo-EM structure of the polycystic kidney disease-like channel PKD2L1. Nat. Commun. 9, 1192 (2018).
Inada, H. et al. Off-response property of an acid-activated cation channel complex PKD1L3-PKD2L1. EMBO Rep 9, 690–697 (2008).
Fujimoto, C. et al. The single pore residue Asp523 in PKD2L1 determines Ca2+ permeation of the PKD1L3/PKD2L1 complex. Biochem. Biophys. Res. Commun. 404, 946–951 (2011).
Su, Q. et al. Structural basis for Ca(2+) activation of the heteromeric PKD1L3/PKD2L1 channel. Nat. Commun. 12, 4871 (2021).
Littleton, J. T. & Ganetzky, B. Ion channels and synaptic organization: analysis of the Drosophila genome. Neuron 26, 35–43 (2000).
Zhang, W. et al. Ankyrin repeats convey force to gate the NOMPC mechanotransduction channel. Cell 162, 1391–1403 (2015).
Jin, P. et al. Electron cryo-microscopy structure of the mechanotransduction channel NOMPC. Nature 547, 118–122 (2017).
Dong, X. P. et al. The type IV mucolipidosis-associated protein TRPML1 is an endolysosomal iron release channel. Nature 455, 992–996 (2008).
Sun, M. et al. Mucolipidosis type IV is caused by mutations in a gene encoding a novel transient receptor potential channel. Hum. Mol. Genet. 9, 2471–2478 (2000).
Schmiege, P., Fine, M., Blobel, G. & Li, X. Human TRPML1 channel structures in open and closed conformations. Nature 550, 366–370 (2017).
Chen, Q. et al. Structure of mammalian endolysosomal TRPML1 channel in nanodiscs. Nature 550, 415–418 (2017).
Santoni, G. et al. Pathophysiological role of transient receptor potential mucolipin channel 1 in CAlcium-mediated Stress-induced Neurodegenerative Diseases. Front. Physiol. 11, 251 (2020).
Plesch, E. et al. Selective agonist of TRPML2 reveals direct role in chemokine release from innate immune cells. Elife 7, e39720 (2018).
Li, M. et al. Structural basis of dual Ca(2+)/pH regulation of the endolysosomal TRPML1 channel. Nat. Struct. Mol. Biol. 24, 205–213 (2017).
Grieben, M. et al. Structure of the polycystic kidney disease TRP channel Polycystin-2 (PC2). Nat. Struct. Mol. Biol. 24, 114–122 (2017).
Wilkes, M. et al. Molecular insights into lipid-assisted Ca(2+) regulation of the TRP channel Polycystin-2. Nat. Struct. Mol. Biol. 24, 123–130 (2017).
Vincent, F. & Duncton, M. A. TRPV4 agonists and antagonists. Curr. Top. Med. Chem. 11, 2216–2226 (2011).
Brederson, J. D., Kym, P. R. & Szallasi, A. Targeting TRP channels for pain relief. Eur. J. Pharm. 716, 61–76 (2013).
Zhu, R. et al. Cinnamaldehyde in diabetes: a review of pharmacology, pharmacokinetics and safety. Pharm. Res. 122, 78–89 (2017).
Eid, S. R. et al. HC-030031, a TRPA1 selective antagonist, attenuates inflammatory- and neuropathy-induced mechanical hypersensitivity. Mol. Pain 4, 48 (2008).
Knotkova, H., Pappagallo, M. & Szallasi, A. Capsaicin (TRPV1 Agonist) therapy for pain relief: farewell or revival? Clin. J. Pain 24, 142–154 (2008).
Yang, F. & Zheng, J. Understand spiciness: mechanism of TRPV1 channel activation by capsaicin. Protein Cell 8, 169–177 (2017).
Sullivan, M. N. et al. Optical recording reveals novel properties of GSK1016790A-induced vanilloid transient receptor potential channel TRPV4 activity in primary human endothelial cells. Mol. Pharm. 82, 464–472 (2012).
Bandell, M. et al. High-throughput random mutagenesis screen reveals TRPM8 residues specifically required for activation by menthol. Nat. Neurosci. 9, 493–500 (2006).
Xu, L. et al. Molecular mechanisms underlying menthol binding and activation of TRPM8 ion channel. Nat. Commun. 11, 3790 (2020).
Dang, S. et al. Structural insight into TRPV5 channel function and modulation. Proc. Natl Acad. Sci. USA 116, 8869–8878 (2019).
Grimm, C. et al. Molecular and functional characterization of the melastatin-related cation channel TRPM3. J. Biol. Chem. 278, 21493–21501 (2003).
Launay, P. et al. TRPM4 is a Ca2+-activated nonselective cation channel mediating cell membrane depolarization. Cell 109, 397–407 (2002).
Monteilh-Zoller, M. K. et al. TRPM7 provides an ion channel mechanism for cellular entry of trace metal ions. J. Gen. Physiol. 121, 49–60 (2003).
Voets, T. et al. TRPM6 forms the Mg2+ influx channel involved in intestinal and renal Mg2+ absorption. J. Biol. Chem. 279, 19–25 (2004).
Fang, J. Y. & Richardson, B. C. The MAPK signalling pathways and colorectal cancer. Lancet Oncol 6, 322–327 (2005).
Wu, H. H. et al. Asenapine maleate inhibits angiotensin II-induced proliferation and activation of cardiac fibroblasts via the ROS/TGFbeta1/MAPK signaling pathway. Biochem. Biophys. Res. Commun. 553, 172–179 (2021).
Cui, X. et al. Scutellariae radix and coptidis rhizoma improve glucose and lipid metabolism in T2DM rats via regulation of the metabolic profiling and MAPK/PI3K/Akt signaling pathway. Int. J. Mol. Sci. 19, 3634 (2018).
Dalal, P. J., Muller, W. A. & Sullivan, D. P. Endothelial cell calcium signaling during barrier function and inflammation. Am. J. Pathol. 190, 535–542 (2020).
Zitt, C., Halaszovich, C. R. & Luckhoff, A. The TRP family of cation channels: probing and advancing the concepts on receptor-activated calcium entry. Prog. Neurobiol. 66, 243–264 (2002).
Zhang, F. et al. Transient receptor potential vanilloid 1 activation induces inflammatory cytokine release in corneal epithelium through MAPK signaling. J. Cell Physiol. 213, 730–739 (2007).
Wei, Y. et al. The transient receptor potential channel, vanilloid 5, induces chondrocyte apoptosis via Ca2+ CaMKII-dependent MAPK and Akt/ mTOR pathways in a rat osteoarthritis model. Cell Physiol. Biochem. 51, 2309–2323 (2018).
Wang, J., Yang, G., Li, M. & Zhou, X. Transient receptor potential melastatin 8 (TRPM8)-based mechanisms underlie both the cold temperature-induced inflammatory reactions and the synergistic effect of cigarette smoke in human bronchial epithelial (16HBE) cells. Front. Physiol. 10, 285 (2019).
Sakurai, J. et al. Activation of extracellular signal-regulated protein kinase in sensory neurons after noxious gastric distention and its involvement in acute visceral pain in rats. Gastroenterology 134, 1094–1103 (2008).
Kondo, T. et al. Transient receptor potential A1 mediates gastric distention-induced visceral pain in rats. Gut 58, 1342–1352 (2009).
Kondo, T., Sakurai, J., Miwa, H. & Noguchi, K. Activation of p38 MAPK through transient receptor potential A1 in a rat model of gastric distension-induced visceral pain. Neuroreport 24, 68–72 (2013).
Zhu, Z., Li, R., Stricker, R. & Reiser, G. Extracellular alpha-crystallin protects astrocytes from cell death through activation of MAPK, PI3K/Akt signaling pathway and blockade of ROS release from mitochondria. Brain Res 17–28, 2015 (1620).
Jie, P. et al. Activation of transient receptor potential vanilloid 4 induces apoptosis in hippocampus through downregulating PI3K/Akt and upregulating p38 MAPK signaling pathways. Cell Death Dis 6, e1775 (2015).
Cai, S. et al. Carvacrol alleviates liver fibrosis by inhibiting TRPM7 and modulating the MAPK signaling pathway. Eur. J. Pharm. 898, 173982 (2021).
Ahn, M. S. et al. Transient receptor potential channel TRPV4 mediates TGF-beta1-induced differentiation of human ventricular fibroblasts. Cardiol. J. 27, 162–170 (2020).
Wen, X. et al. Signaling pathways in obesity: mechanisms and therapeutic interventions. Signal Transduct. Target Ther 7, 298 (2022).
Massague, J. TGFbeta in cancer. Cell 134, 215–230 (2008).
Liu, S., Ren, J. & Ten Dijke, P. Targeting TGFbeta signal transduction for cancer therapy. Signal Transduct. Target Ther 6, 8 (2021).
Khalil, H. et al. Fibroblast-specific TGF-beta-Smad2/3 signaling underlies cardiac fibrosis. J. Clin. Invest. 127, 3770–3783 (2017).
Liu, Y. et al. Transient receptor potential vanilloid-3 (TRPV3) activation plays a central role in cardiac fibrosis induced by pressure overload in rats via TGF-beta(1) pathway. Naunyn Schmiedebergs Arch. Pharm 391, 131–143 (2018).
Han, L. et al. Protective mechanism of SIRT1 on Hcy-induced atrial fibrosis mediated by TRPC3. J. Cell Mol. Med. 24, 488–510 (2020).
He, R. et al. Upregulation of transient receptor potential canonical type 3 channel via AT1R/TGF-beta1/Smad2/3 induces atrial fibrosis in aging and spontaneously hypertensive rats. Oxid. Med. Cell Longev. 2019, 4025496 (2019).
Yu, S. Q., Ma, S. & Wang, D. H. Ablation of TRPV1-positive nerves exacerbates salt-induced hypertension and tissue injury in rats after renal ischemia-reperfusion via infiltration of macrophages. Clin. Exp. Hypertens. 43, 254–262 (2021).
Qian, K. et al. Transient receptor potential vanilloid-1 (TRPV1) alleviates hepatic fibrosis via TGF-beta signaling. Dis. Markers 2022, 3100943 (2022).
Liu, Z. et al. Capsaicin ameliorates renal fibrosis by inhibiting TGF-beta1-Smad2/3 signaling. Phytomedicine 100, 154067 (2022).
Wang, Y. & Wang, D. H. Protective effect of TRPV1 against renal fibrosis via inhibition of TGF-beta/Smad signaling in DOCA-salt hypertension. Mol. Med. 17, 1204–1212 (2011).
Yu, H. et al. Targeting NF-kappaB pathway for the therapy of diseases: mechanism and clinical study. Signal Transduct. Target Ther 5, 209 (2020).
Kang, J. et al. TRPA1 mediated aggravation of allergic contact dermatitis induced by DINP and regulated by NF-kappaB activation. Sci. Rep. 7, 43586 (2017).
Lee, K. I. et al. Role of transient receptor potential ankyrin 1 channels in Alzheimer’s disease. J. Neuroinflammation 13, 92 (2016).
Yuan, J. et al. TRPA1 promotes cisplatin-induced nephrotoxicity through inflammation mediated by the MAPK/NF-kappaB signaling pathway. Ann. Transl. Med. 9, 1578 (2021).
Wu, C. K. et al. Renal tubular epithelial TRPA1 acts as an oxidative stress sensor to mediate ischemia-reperfusion-induced kidney injury through MAPKs/NF-kappaB signaling. Int. J. Mol. Sci. 22, 2309 (2021).
An, D. et al. Blockage of TRPV4 downregulates the nuclear factor-kappa B signaling pathway to inhibit inflammatory responses and neuronal death in mice with pilocarpine-induced status epilepticus. Cell Mol. Neurobiol. 43, 1283–1300 (2023).
Cao, S. et al. Intrathecal TRPM8 blocking attenuates cold hyperalgesia via PKC and NF-kappaB signaling in the dorsal root ganglion of rats with neuropathic pain. J. Pain. Res. 12, 1287–1296 (2019).
Wang, X. P. et al. TRPM8 in the negative regulation of TNFalpha expression during cold stress. Sci. Rep. 7, 45155 (2017).
Liu, L. et al. TRPC6 attenuates cortical astrocytic apoptosis and inflammation in cerebral ischemic/reperfusion injury. Front. Cell Dev. Biol. 8, 594283 (2020).
Bair, A. M. et al. Ca2+ entry via TRPC channels is necessary for thrombin-induced NF-kappaB activation in endothelial cells through AMP-activated protein kinase and protein kinase Cdelta. J. Biol. Chem. 284, 563–574 (2009).
Herzig, S. & Shaw, R. J. AMPK: guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell Biol. 19, 121–135 (2018).
Lee, G. H. et al. Rutaecarpine increases nitric oxide synthesis via eNOS phosphorylation by TRPV1-dependent CaMKII and CaMKKbeta/AMPK signaling pathway in human endothelial cells. Int. J. Mol. Sci. 22, 9407 (2021).
Chen, M. et al. Targeting TRPV1-mediated autophagy attenuates nitrogen mustard-induced dermal toxicity. Signal Transduct. Target Ther 6, 29 (2021).
Zou, Y. et al. TRPC5-induced autophagy promotes the TMZ-resistance of glioma cells via the CAMMKbeta/AMPKalpha/mTOR pathway. Oncol. Rep. 41, 3413–3423 (2019).
Szrejder, M. et al. Metformin reduces TRPC6 expression through AMPK activation and modulates cytoskeleton dynamics in podocytes under diabetic conditions. Biochim. Biophys. Acta Mol. Basis Dis. 1866, 165610 (2020).
Toro, C. A., Arias, L. A. & Brauchi, S. Sub-cellular distribution and translocation of TRP channels. Curr. Pharm. Biotechnol. 12, 12–23 (2011).
Steglitz, J., Buscemi, J. & Ferguson, M. J. The future of pain research, education, and treatment: a summary of the IOM report “Relieving pain in America: a blueprint for transforming prevention, care, education, and research”. Transl. Behav. Med. 2, 6–8 (2012).
Loeser, J. D. & Melzack, R. Pain: an overview. Lancet 353, 1607–1609 (1999).
Cohen, S. P., Vase, L. & Hooten, W. M. Chronic pain: an update on burden, best practices, and new advances. Lancet 397, 2082–2097 (2021).
Klimas, J. et al. Strategies to identify patient risks of prescription opioid addiction when initiating opioids for pain: a systematic review. JAMA Netw. Open 2, e193365 (2019).
Woolf, C. J. et al. Towards a mechanism-based classification of pain? Pain 77, 227–229 (1998).
Szallasi, A., Cortright, D. N., Blum, C. A. & Eid, S. R. The vanilloid receptor TRPV1: 10 years from channel cloning to antagonist proof-of-concept. Nat. Rev. Drug Discov. 6, 357–372 (2007).
Mickle, A. D., Shepherd, A. J. & Mohapatra, D. P. Nociceptive TRP channels: sensory detectors and transducers in multiple pain pathologies. Pharmaceutical 9, 72 (2016).
Fujita, F. et al. Methyl p-hydroxybenzoate causes pain sensation through activation of TRPA1 channels. Br. J. Pharm. 151, 153–160 (2007).
Namer, B., Seifert, F., Handwerker, H. O. & Maihofner, C. TRPA1 and TRPM8 activation in humans: effects of cinnamaldehyde and menthol. Neuroreport 16, 955–959 (2005).
Mizushima, T. et al. Noxious cold stimulation induces mitogen-activated protein kinase activation in transient receptor potential (TRP) channels TRPA1- and TRPM8-containing small sensory neurons. Neuroscience 140, 1337–1348 (2006).
Koivisto, A. Sustained TRPA1 activation in vivo. Acta Physiol 204, 248–254 (2012).
Zsombok, A. & Derbenev, A. V. TRP channels as therapeutic targets in diabetes and obesity. Pharmaceuticals 9, 50 (2016).
Wei, H. et al. Mechanical antihypersensitivity effect induced by repeated spinal administrations of a TRPA1 antagonist or a gap junction decoupler in peripheral neuropathy. Pharm. Biochem. Behav. 150-151, 57–67 (2016).
Koh, W. U. et al. Perineural pretreatment of bee venom attenuated the development of allodynia in the spinal nerve ligation injured neuropathic pain model; an experimental study. BMC Complement Alter. Med 14, 431 (2014).
Sagalajev, B. et al. Oxidative stress in the amygdala contributes to neuropathic pain. Neuroscience 387, 92–103 (2018).
Hansson, E. Long-term pain, neuroinflammation and glial activation. Scand. J. Pain 1, 67–72 (2010).
Sagalajev, B., Viisanen, H., Wei, H. & Pertovaara, A. Descending antinociception induced by secondary somatosensory cortex stimulation in experimental neuropathy: role of the medullospinal serotonergic pathway. J. Neurophysiol. 117, 1200–1214 (2017).
De Logu, F. et al. Schwann cell TRPA1 mediates neuroinflammation that sustains macrophage-dependent neuropathic pain in mice. Nat. Commun 8, 1887 (2017).
Earley, S. TRPA1 channels in the vasculature. Br. J. Pharm. 167, 13–22 (2012).
McKemy, D. D. How cold is it? TRPM8 and TRPA1 in the molecular logic of cold sensation. Mol. Pain 1, 16 (2005).
Pertovaara, A. & Koivisto, A. TRPA1 ion channel in the spinal dorsal horn as a therapeutic target in central pain hypersensitivity and cutaneous neurogenic inflammation. Eur. J. Pharm. 666, 1–4 (2011).
Kumamoto, E. & Fujita, T. Differential activation of TRP channels in the adult rat spinal substantia gelatinosa by stereoisomers of plant-derived chemicals. Pharmaceuticals 9, 46 (2016).
Bandell, M. et al. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron 41, 849–857 (2004).
Bautista, D. M. et al. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell 124, 1269–1282 (2006).
Wei, H. et al. Spinal transient receptor potential ankyrin 1 channel contributes to central pain hypersensitivity in various pathophysiological conditions in the rat. Pain 152, 582–591 (2011).
da Costa, D. S. M. et al. The involvement of the transient receptor potential A1 (TRPA1) in the maintenance of mechanical and cold hyperalgesia in persistent inflammation. Pain 148, 431–437 (2010).
Koivisto, A. et al. TRPA1: a transducer and amplifier of pain and inflammation. Basic Clin. Pharm. Toxicol. 114, 50–55 (2014).
Abbas, M. A. Modulation of TRPV1 channel function by natural products in the treatment of pain. Chem. Biol. Interact. 330, 109178 (2020).
Bevan, S., Quallo, T. & Andersson, D. A. Trpv1. Handb. Exp. Pharm. 222, 207–245 (2014).
Choi, S. I. et al. Emerging role of spinal cord TRPV1 in pain exacerbation. Neural Plast 2016, 5954890 (2016).
Iftinca, M., Defaye, M. & Altier, C. TRPV1-targeted drugs in development for human pain conditions. Drugs 81, 7–27 (2021).
Caterina, M. J. et al. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 288, 306–313 (2000).
Davis, J. B. et al. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature 405, 183–187 (2000).
Malek, N. et al. The importance of TRPV1-sensitisation factors for the development of neuropathic pain. Mol. Cell Neurosci. 65, 1–10 (2015).
Camprubi-Robles, M., Planells-Cases, R. & Ferrer-Montiel, A. Differential contribution of SNARE-dependent exocytosis to inflammatory potentiation of TRPV1 in nociceptors. FASEB J 23, 3722–3733 (2009).
Serafini, M. et al. Targeting transient receptor potential vanilloid 1 (TRPV1) channel softly: the discovery of passerini adducts as a topical treatment for inflammatory skin disorders. J. Med. Chem. 61, 4436–4455 (2018).
Huang, Y. K. et al. Cytokine activin C ameliorates chronic neuropathic pain in peripheral nerve injury rodents by modulating the TRPV1 channel. Br. J. Pharm. 177, 5642–5657 (2020).
Caterina, M. J. & Pang, Z. TRP channels in skin biology and pathophysiology. Pharmaceuticals 9, 77 (2016).
Mehta, D. & Granstein, R. D. Immunoregulatory effects of neuropeptides on endothelial cells: relevance to dermatological disorders. Dermatology 235, 175–186 (2019).
Brierley, S. M. et al. Selective role for TRPV4 ion channels in visceral sensory pathways. Gastroenterology 134, 2059–2069 (2008).
Moore, C. et al. UVB radiation generates sunburn pain and affects skin by activating epidermal TRPV4 ion channels and triggering endothelin-1 signaling. Proc. Natl Acad. Sci. USA 110, E3225–E3234 (2013).
Moran, M. M., McAlexander, M. A., Biro, T. & Szallasi, A. Transient receptor potential channels as therapeutic targets. Nat. Rev. Drug Discov. 10, 601–620 (2011).
Vergnolle, N. et al. A role for transient receptor potential vanilloid 4 in tonicity-induced neurogenic inflammation. Br. J. Pharm. 159, 1161–1173 (2010).
Alessandri-Haber, N. et al. TRPV4 mediates pain-related behavior induced by mild hypertonic stimuli in the presence of inflammatory mediator. Pain 118, 70–79 (2005).
Levine, J. D. & Alessandri-Haber, N. TRP channels: targets for the relief of pain. Biochim Biophys. Acta 1772, 989–1003 (2007).
Qu, Y. J. et al. Effect of TRPV4-p38 MAPK pathway on neuropathic pain in rats with chronic compression of the dorsal root ganglion. Biomed. Res. Int. 2016, 6978923 (2016).
Lewinter, R. D., Skinner, K., Julius, D. & Basbaum, A. I. Immunoreactive TRPV-2 (VRL-1), a capsaicin receptor homolog, in the spinal cord of the rat. J. Comp. Neurol. 470, 400–408 (2004).
Stokes, A. J. et al. Formation of a physiological complex between TRPV2 and RGA protein promotes cell surface expression of TRPV2. J. Cell Biochem. 94, 669–683 (2005).
Urata, K. et al. Involvement of transient receptor potential vanilloid 2 in intra-oral incisional pain. Oral. Dis. 24, 1093–1100 (2018).
Moqrich, A. et al. Impaired thermosensation in mice lacking TRPV3, a heat and camphor sensor in the skin. Science 307, 1468–1472 (2005).
Smith, G. D. et al. TRPV3 is a temperature-sensitive vanilloid receptor-like protein. Nature 418, 186–190 (2002).
Gunthorpe, M. J., Benham, C. D., Randall, A. & Davis, J. B. The diversity in the vanilloid (TRPV) receptor family of ion channels. Trends Pharm. Sci. 23, 183–191 (2002).
Han, Y. et al. A plant-derived TRPV3 inhibitor suppresses pain and itch. Br. J. Pharm. 178, 1669–1683 (2021).
Bautista, D. M. et al. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature 448, 204–208 (2007).
Colburn, R. W. et al. Attenuated cold sensitivity in TRPM8 null mice. Neuron 54, 379–386 (2007).
Zuo, X., Ling, J. X., Xu, G. Y. & Gu, J. G. Operant behavioral responses to orofacial cold stimuli in rats with chronic constrictive trigeminal nerve injury: effects of menthol and capsazepine. Mol. Pain 9, 28 (2013).
Klein, A. H. et al. Topical application of L-menthol induces heat analgesia, mechanical allodynia, and a biphasic effect on cold sensitivity in rats. Behav. Brain Res. 212, 179–186 (2010).
Klein, A. H. et al. Topical hindpaw application of L-menthol decreases responsiveness to heat with biphasic effects on cold sensitivity of rat lumbar dorsal horn neurons. Neuroscience 219, 234–242 (2012).
Vinuela-Fernandez, I. et al. The TRPM8 channel forms a complex with the 5-HT(1B) receptor and phospholipase D that amplifies its reversal of pain hypersensitivity. Neuropharmacology 79, 136–151 (2014).
Anderson, E. M., Jenkins, A. C., Caudle, R. M. & Neubert, J. K. The effects of a co-application of menthol and capsaicin on nociceptive behaviors of the rat on the operant orofacial pain assessment device. PLoS ONE 9, e89137 (2014).
Silva, R. L., Lopes, A. H., Guimaraes, R. M. & Cunha, T. M. CXCL1/CXCR2 signaling in pathological pain: role in peripheral and central sensitization. Neurobiol. Dis. 105, 109–116 (2017).
Chung, M. K. et al. The role of TRPM2 in hydrogen peroxide-induced expression of inflammatory cytokine and chemokine in rat trigeminal ganglia. Neuroscience 297, 160–169 (2015).
Haraguchi, K. et al. TRPM2 contributes to inflammatory and neuropathic pain through the aggravation of pronociceptive inflammatory responses in mice. J. Neurosci. 32, 3931–3941 (2012).
Su, S. et al. TRPM3 channels play roles in heat hypersensitivity and spontaneous pain after nerve injury. J. Neurosci. 41, 2457–2474 (2021).
Szallasi, A. & Sheta, M. Targeting TRPV1 for pain relief: limits, losers and laurels. Expert Opin. Investig. Drugs 21, 1351–1369 (2012).
Kaplan, G. G. The global burden of IBD: from 2015 to 2025. Nat. Rev. Gastroenterol. Hepatol. 12, 720–727 (2015).
Mentella, M. C. et al. Nutrition, IBD and gut microbiota: a review. Nutrients 12, 944 (2020).
Baumgart, D. C. & Carding, S. R. Inflammatory bowel disease: cause and immunobiology. Lancet 369, 1627–1640 (2007).
Kaplan, G. G. & Windsor, J. W. The four epidemiological stages in the global evolution of inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 18, 56–66 (2021).
Ng, S. C. et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet 390, 2769–2778 (2017).
Ramos, G. P. & Papadakis, K. A. Mechanisms of disease: inflammatory bowel diseases. Mayo Clin. Proc. 94, 155–165 (2019).
Vavricka, S. R. et al. Extraintestinal manifestations of inflammatory bowel disease. Inflamm. Bowel Dis. 21, 1982–1992 (2015).
Argollo, M. et al. Comorbidities in inflammatory bowel disease: a call for action. Lancet Gastroenterol. Hepatol. 4, 643–654 (2019).
Restellini, S., Chazouilleres, O. & Frossard, J. L. Hepatic manifestations of inflammatory bowel diseases. Liver Int 37, 475–489 (2017).
Perez-Carreras, M. et al. Non-alcoholic fatty liver disease in patients with intestinal, pulmonary or skin diseases: inflammatory cross-talk that needs a multidisciplinary approach. World J. Gastroenterol. 27, 7113–7124 (2021).
Fu, Y., Lee, C. H. & Chi, C. C. Association of psoriasis with inflammatory bowel disease: a systematic review and meta-analysis. JAMA Dermatol 154, 1417–1423 (2018).
Zhang, M. & Merlin, D. Nanoparticle-based oral drug delivery systems targeting the colon for treatment of ulcerative colitis. Inflamm. Bowel Dis. 24, 1401–1415 (2018).
Yang, C. & Merlin, D. Nanoparticle-mediated drug delivery systems for the treatment of IBD: current perspectives. Int. J. Nanomed. 14, 8875–8889 (2019).
Neurath, M. F. Current and emerging therapeutic targets for IBD. Nat. Rev. Gastroenterol. Hepatol. 14, 269–278 (2017).
Arora, Z. & Shen, B. Biological therapy for ulcerative colitis. Gastroenterol. Rep. 3, 103–109 (2015).
Dretzke, J. et al. A systematic review and economic evaluation of the use of tumour necrosis factor-alpha (TNF-alpha) inhibitors, adalimumab and infliximab, for Crohn’s disease. Health Technol. Assess. 15, 1–244 (2011).
Vergnolle, N. TRPV4: new therapeutic target for inflammatory bowel diseases. Biochem. Pharm. 89, 157–161 (2014).
D’Aldebert, E. et al. Transient receptor potential vanilloid 4 activated inflammatory signals by intestinal epithelial cells and colitis in mice. Gastroenterology 140, 275–285 (2011).
Cenac, N. et al. Transient receptor potential vanilloid-4 has a major role in visceral hypersensitivity symptoms. Gastroenterology 135, 937–946.946 (2008). e1–2.
Fichna, J. et al. Transient receptor potential vanilloid 4 blockade protects against experimental colitis in mice: a new strategy for inflammatory bowel diseases treatment? Neurogastroenterol. Motil. 24, e557–e560 (2012).
De Petrocellis, L. et al. Cannabinoid actions at TRPV channels: effects on TRPV3 and TRPV4 and their potential relevance to gastrointestinal inflammation. Acta Physiol. 204, 255–266 (2012).
Akbar, A. et al. Expression of the TRPV1 receptor differs in quiescent inflammatory bowel disease with or without abdominal pain. Gut 59, 767–774 (2010).
Kun, J. et al. Upregulation of the transient receptor potential ankyrin 1 ion channel in the inflamed human and mouse colon and its protective roles. PLoS ONE 9, e108164 (2014).
Molodecky, N. A. et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 142, 46–54.e42 (2012).
Basso, L. & Altier, C. Transient Receptor Potential Channels in neuropathic pain. Curr. Opin. Pharm. 32, 9–15 (2017).
Engel, M. A. et al. The proximodistal aggravation of colitis depends on substance P released from TRPV1-expressing sensory neurons. J. Gastroenterol. 47, 256–265 (2012).
Miranda, A. et al. The role of transient receptor potential vanilloid 1 in mechanical and chemical visceral hyperalgesia following experimental colitis. Neuroscience 148, 1021–1032 (2007).
Harrington, A. M. et al. A novel role for TRPM8 in visceral afferent function. Pain 152, 1459–1468 (2011).
Adam, B. et al. A combination of peppermint oil and caraway oil attenuates the post-inflammatory visceral hyperalgesia in a rat model. Scand. J. Gastroenterol. 41, 155–160 (2006).
Ramachandran, R. et al. TRPM8 activation attenuates inflammatory responses in mouse models of colitis. Proc. Natl Acad. Sci. USA 110, 7476–7481 (2013).
Andersson, D. A., Nash, M. & Bevan, S. Modulation of the cold-activated channel TRPM8 by lysophospholipids and polyunsaturated fatty acids. J. Neurosci. 27, 3347–3355 (2007).
Minami, T. et al. Elevation of phospholipase A2 protein in sera of patients with Crohn’s disease and ulcerative colitis. Am. J. Gastroenterol. 88, 1076–1080 (1993).
Braun, A. et al. Alterations of phospholipid concentration and species composition of the intestinal mucus barrier in ulcerative colitis: a clue to pathogenesis. Inflamm. Bowel Dis. 15, 1705–1720 (2009).
Izzo, A. A. et al. Inhibitory effect of cannabichromene, a major non-psychotropic cannabinoid extracted from Cannabis sativa, on inflammation-induced hypermotility in mice. Br. J. Pharm. 166, 1444–1460 (2012).
Yang, J. et al. Transient receptor potential ankyrin-1 participates in visceral hyperalgesia following experimental colitis. Neurosci. Lett. 440, 237–241 (2008).
Engel, M. A., Becker, C., Reeh, P. W. & Neurath, M. F. Role of sensory neurons in colitis: increasing evidence for a neuroimmune link in the gut. Inflamm. Bowel Dis. 17, 1030–1033 (2011).
Romano, B. et al. The cannabinoid TRPA1 agonist cannabichromene inhibits nitric oxide production in macrophages and ameliorates murine colitis. Br. J. Pharm. 169, 213–229 (2013).
Gibiino, G. et al. The other side of malnutrition in inflammatory bowel disease (IBD): Non-alcoholic fatty liver disease. Nutrients 13, 2772 (2021).
Fousekis, F. S. et al. Hepatobiliary manifestations and complications in inflammatory bowel disease: a review. Gastroenterol. Res. 11, 83–94 (2018).
Harbord, M. et al. The First European Evidence-based Consensus on Extra-intestinal Manifestations in Inflammatory Bowel Disease. J. Crohns Colitis 10, 239–254 (2016).
Ritaccio, G. et al. Nonalcoholic fatty liver disease is common in IBD patients however progression to hepatic fibrosis by noninvasive markers is rare. Dig. Dis. Sci. 66, 3186–3191 (2021).
Cotter, T. G. & Rinella, M. Nonalcoholic Fatty Liver Disease 2020: The State of the Disease. Gastroenterology 158, 1851–1864 (2020).
Younossi, Z. M. Non-alcoholic fatty liver disease - A global public health perspective. J. Hepatol. 70, 531–544 (2019).
Ma, L. et al. Activation of TRPV1 reduces vascular lipid accumulation and attenuates atherosclerosis. Cardiovasc. Res. 92, 504–513 (2011).
Iwasaki, Y. et al. TRPV1 agonist monoacylglycerol increases UCP1 content in brown adipose tissue and suppresses accumulation of visceral fat in mice fed a high-fat and high-sucrose diet. Biosci. Biotechnol. Biochem. 75, 904–909 (2011).
Tobita, N. et al. Sweet scent lactones activate hot capsaicin receptor, TRPV1. Biochem. Biophys. Res. Commun. 534, 547–552 (2021).
Li, L. et al. TRPV1 activation prevents nonalcoholic fatty liver through UCP2 upregulation in mice. Pflug. Arch. 463, 727–732 (2012).
Sun, X. D. & Yu, Y. R. The protective effects of rosiglitazone on kidney in diet-induced obese rats. Sichuan Da Xue Xue Bao Yi Xue Ban 45, 24–28 (2014).
Song, Y. et al. TRPV4 channel inhibits TGF-beta1-induced proliferation of hepatic stellate cells. PLoS ONE 9, e101179 (2014).
Seth, R. K. et al. TRPV4 activation of endothelial nitric oxide synthase resists nonalcoholic fatty liver disease by blocking CYP2E1-mediated redox toxicity. Free Radic. Biol. Med. 102, 260–273 (2017).
Kheradpezhouh, E. et al. TRPM2 channels mediate acetaminophen-induced liver damage. Proc. Natl Acad. Sci. USA 111, 3176–3181 (2014).
Malko, P. & Jiang, L. H. TRPM2 channel-mediated cell death: an important mechanism linking oxidative stress-inducing pathological factors to associated pathological conditions. Redox Biol 37, 101755 (2020).
Zhu, Y. et al. Blockage of TRPM7 channel induces hepatic stellate cell death through endoplasmic reticulum stress-mediated apoptosis. Life Sci 94, 37–44 (2014).
Smedlund, K., Dube, P. & Vazquez, G. Early steatohepatitis in hyperlipidemic mice with endothelial-specific gain of TRPC3 function precedes changes in aortic atherosclerosis. Physiol. Genomics 48, 644–649 (2016).
Nishiyama, K. et al. Deletion of TRPC3 or TRPC6 fails to attenuate the formation of inflammation and fibrosis in non-alcoholic steatohepatitis. Biol. Pharm. Bull. 44, 431–436 (2021).
Yu, C. J. et al. TRUSS exacerbates NAFLD development by promoting ikappabalpha degradation in mice. Hepatology 68, 1769–1785 (2018).
Alinaghi, F. et al. Global prevalence and bidirectional association between psoriasis and inflammatory bowel disease-a systematic review and meta-analysis. J. Crohns Colitis 14, 351–360 (2020).
Bernstein, C. N., Wajda, A. & Blanchard, J. F. The clustering of other chronic inflammatory diseases in inflammatory bowel disease: a population-based study. Gastroenterology 129, 827–836 (2005).
Koivisto, A. P., Belvisi, M. G., Gaudet, R. & Szallasi, A. Advances in TRP channel drug discovery: from target validation to clinical studies. Nat. Rev. Drug Discov. 21, 41–59 (2022).
Kambiz, S. et al. Thermo-sensitive TRP channels in peripheral nerve injury: a review of their role in cold intolerance. J. Plast. Reconstr. Aesthet. Surg. 67, 591–599 (2014).
Ho, J. C. & Lee, C. H. TRP channels in skin: from physiological implications to clinical significances. Biophysics (Nagoya-shi) 11, 17–24 (2015).
Neuberger, A., Nadezhdin, K. D. & Sobolevsky, A. I. TRPV3 expression and purification for structure determination by Cryo-EM. Methods Enzymol 652, 31–48 (2021).
Okada, Y. et al. Roles of epithelial and mesenchymal TRP channels in mediating inflammatory fibrosis. Front Immunol 12, 731674 (2021).
Bertin, S. & Raz, E. Transient receptor potential (TRP) channels in T cells. Semin Immunopathol 38, 309–319 (2016).
Maglie, R. et al. The Role of TRPA1 in Skin Physiology and Pathology. Int. J. Mol. Sci. 22, 3065 (2021).
Toth, B. I., Olah, A., Szollosi, A. G. & Biro, T. TRP channels in the skin. Br. J. Pharm. 171, 2568–2581 (2014).
Feng, J. et al. Sensory TRP channels contribute differentially to skin inflammation and persistent itch. Nat. Commun. 8, 980 (2017).
Abrahamsen, B. et al. The cell and molecular basis of mechanical, cold, and inflammatory pain. Science 321, 702–705 (2008).
Lee, S. H. et al. Resolvin D3 controls mouse and human TRPV1-positive neurons and preclinical progression of psoriasis. Theranostics 10, 12111–12126 (2020).
Riol-Blanco, L. et al. Nociceptive sensory neurons drive interleukin-23-mediated psoriasiform skin inflammation. Nature 510, 157–161 (2014).
Imamachi, N. et al. TRPV1-expressing primary afferents generate behavioral responses to pruritogens via multiple mechanisms. Proc. Natl Acad. Sci. USA 106, 11330–11335 (2009).
Cavanaugh, D. J. et al. Distinct subsets of unmyelinated primary sensory fibers mediate behavioral responses to noxious thermal and mechanical stimuli. Proc. Natl Acad. Sci. USA 106, 9075–9080 (2009).
Bromm, B., Scharein, E., Darsow, U. & Ring, J. Effects of menthol and cold on histamine-induced itch and skin reactions in man. Neurosci. Lett. 187, 157–160 (1995).
Palkar, R. et al. Cooling relief of acute and chronic itch requires TRPM8 channels and neurons. J. Invest. Dermatol. 138, 1391–1399 (2018).
Caceres, A. I. et al. Transient Receptor Potential Cation Channel Subfamily M Member 8 channels mediate the anti-inflammatory effects of eucalyptol. Br. J. Pharm. 174, 867–879 (2017).
Liu, B. & Jordt, S. E. Cooling the Itch via TRPM8. J. Invest. Dermatol. 138, 1254–1256 (2018).
van der Fits, L. et al. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J. Immunol. 182, 5836–5845 (2009).
Wang, W. et al. Thymol activates TRPM8-mediated Ca(2+) influx for its antipruritic effects and alleviates inflammatory response in Imiquimod-induced mice. Toxicol. Appl. Pharm. 407, 115247 (2020).
Liang, D. et al. Thymol inhibits LPS-stimulated inflammatory response via down-regulation of NF-kappaB and MAPK signaling pathways in mouse mammary epithelial cells. Inflammation 37, 214–222 (2014).
Younes, S. F. & Bakry, O. A. Immunohistochemical evaluation of role of serotonin in pathogenesis of psoriasis. J. Clin. Diagn. Res. 10, EC05–EC09 (2016).
Lee, S. H. et al. Peripheral serotonin receptor 2B and transient receptor potential channel 4 mediate pruritus to serotonergic antidepressants in mice. J. Allergy Clin. Immunol. 142, 1349–1352.e1316 (2018).
Lee, S. H. et al. Sensory neuron-expressed TRPC4 is a target for the relief of psoriasiform itch and skin inflammation in mice. J. Invest. Dermatol. 140, 2221–2229.e2226 (2020).
Belvisi, M. G. & Birrell, M. A. The emerging role of transient receptor potential channels in chronic lung disease. Eur. Respir. J. 50, 1601357 (2017).
Safiabadi Tali, S. H. et al. Tools and techniques for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)/COVID-19 detection. Clin. Microbiol. Rev. 34, https://doi.org/10.1128/CMR.00228-20 (2021).
Straus, M. R. et al. Inhibitors of L-type calcium channels show therapeutic potential for treating SARS-CoV-2 infections by preventing virus entry and spread. ACS Infect. Dis. 7, 2807–2815 (2021).
Yelshanskaya, M. V., Nadezhdin, K. D., Kurnikova, M. G. & Sobolevsky, A. I. Structure and function of the calcium-selective TRP channel TRPV6. J. Physiol. 599, 2673–2697 (2021).
Singh, A. K., McGoldrick, L. L., Twomey, E. C. & Sobolevsky, A. I. Mechanism of calmodulin inactivation of the calcium-selective TRP channel TRPV6. Sci. Adv. 4, eaau6088 (2018).
Leidinger, G. et al. TRPC6 is altered in COVID-19 pneumonia. Chem. Biol. Interact. 362, 109982 (2022).
Meyer, N. J., Gattinoni, L. & Calfee, C. S. Acute respiratory distress syndrome. Lancet 398, 622–637 (2021).
Gibson, P. G., Qin, L. & Puah, S. H. COVID-19 acute respiratory distress syndrome (ARDS): clinical features and differences from typical pre-COVID-19 ARDS. Med. J. Aust. 213, 54–56 e51 (2020).
Khodadadi, H. et al. A potential role for cannabichromene in modulating TRP channels during acute respiratory distress syndrome. J. Cannabis Res. 3, 45 (2021).
Wong, T. L. & Weitzer, D. J. Long COVID and myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS)-a systemic review and comparison of clinical presentation and symptomatology. Medicina (Kaunas) 57, 418 (2021).
Paul, B. D., Lemle, M. D., Komaroff, A. L. & Snyder, S. H. Redox imbalance links COVID-19 and myalgic encephalomyelitis/chronic fatigue syndrome. Proc. Natl Acad. Sci. USA 118, https://doi.org/10.1073/pnas.2024358118 (2021).
Haffke, M. et al. Endothelial dysfunction and altered endothelial biomarkers in patients with post-COVID-19 syndrome and chronic fatigue syndrome (ME/CFS). J. Transl. Med. 20, 138 (2022).
Sasso, E. M. et al. Transient receptor potential melastatin 3 dysfunction in post COVID-19 condition and myalgic encephalomyelitis/chronic fatigue syndrome patients. Mol. Med. 28, 98 (2022).
Hu, Y. et al. Prevalence and severity of corona virus disease 2019 (COVID-19): a systematic review and meta-analysis. J. Clin. Virol. 127, 104371 (2020).
Lechien, J. R. et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur. Arch. Otorhinolaryngol. 277, 2251–2261 (2020).
Izquierdo-Dominguez, A., Rojas-Lechuga, M. J., Mullol, J. & Alobid, I. Olfactory dysfunction in the COVID-19 outbreak. J. Investig. Allergol. Clin. Immunol. 30, 317–326 (2020).
Nepal, G. et al. Neurological manifestations of COVID-19: a systematic review. Crit. Care 24, 421 (2020).
Salmon Ceron, D. et al. Self-reported loss of smell without nasal obstruction to identify COVID-19. The multicenter Coranosmia cohort study. J. Infect. 81, 614–620 (2020).
Mao, R. et al. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: a systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 5, 667–678 (2020).
Fuhrer, M., Dejaco, C., Kopp, B. & Hammer, J. Gastric administration of garlic powder containing the trpa1- agonist allicin induces specific epigastric symptoms and gastric relaxation in healthy subjects. Neurogastroenterol. Motil. 31, e13470 (2019).
Holzer, P. Transient receptor potential (TRP) channels as drug targets for diseases of the digestive system. Pharm. Ther. 131, 142–170 (2011).
Bousquet, J. et al. Potential Interplay between Nrf2, TRPA1, and TRPV1 in nutrients for the control of COVID-19. Int. Arch. Allergy Immunol. 182, 324–338 (2021).
Nahama, A., Ramachandran, R., Cisternas, A. F. & Ji, H. The role of afferent pulmonary innervation in ARDS associated with COVID-19 and potential use of resiniferatoxin to improve prognosis: a review. Med. Drug Discov. 5, 100033 (2020).
Balakrishna, S. et al. TRPV4 inhibition counteracts edema and inflammation and improves pulmonary function and oxygen saturation in chemically induced acute lung injury. Am. J. Physiol. Lung Cell Mol. Physiol. 307, L158–L172 (2014).
Rosenbaum, T. et al. TRPV4: a physio and pathophysiologically significant ion channel. Int. J. Mol. Sci. 21, 3837 (2020).
Mazzotta, S. et al. Design, synthesis and in vitro experimental validation of novel TRPV4 antagonists inspired by labdane diterpenes. Mar. Drugs. 18, 519 (2020).
Chung, K. F. & Pavord, I. D. Prevalence, pathogenesis, and causes of chronic cough. Lancet 371, 1364–1374 (2008).
Chung, K. F. et al. Cough hypersensitivity and chronic cough. Nat. Rev. Dis. Prim. 8, 45 (2022).
Undem, B. J. & Sun, H. Molecular/ionic basis of vagal bronchopulmonary C-fiber activation by inflammatory mediators. Physiology 35, 57–68 (2020).
Grace, M. S. et al. Transient receptor potential (TRP) channels in the airway: role in airway disease. Br. J. Pharm. 171, 2593–2607 (2014).
Trevisani, M. et al. Antitussive activity of iodo-resiniferatoxin in guinea pigs. Thorax 59, 769–772 (2004).
Anderson, G. P. TRPV1 and cough. Thorax 59, 730–731 (2004).
Bonvini, S. J., Birrell, M. A., Smith, J. A. & Belvisi, M. G. Targeting TRP channels for chronic cough: from bench to bedside. Naunyn Schmiedebergs Arch. Pharm 388, 401–420 (2015).
Bonvini, S. J. et al. Transient receptor potential cation channel, subfamily V, member 4 and airway sensory afferent activation: role of adenosine triphosphate. J. Allergy Clin. Immunol. 138, 249–261.e212 (2016).
Buday, T., Kovacikova, L., Ruzinak, R. & Plevkova, J. TRPV4 antagonist GSK2193874 does not modulate cough response to osmotic stimuli. Respir. Physiol. Neurobiol. 236, 1–4 (2017).
Morice, A. H. TRPA1 receptors in chronic cough. Pulm. Pharm. Ther. 47, 42–44 (2017).
Birrell, M. A. et al. TRPA1 agonists evoke coughing in guinea pig and human volunteers. Am. J. Respir. Crit. Care Med. 180, 1042–1047 (2009).
Mukhopadhyay, I. et al. Transient receptor potential ankyrin 1 receptor activation in vitro and in vivo by pro-tussive agents: GRC 17536 as a promising anti-tussive therapeutic. PLoS ONE 9, e97005 (2014).
Ji, Z. et al. Melatonin attenuates chronic cough mediated by oxidative stress via transient receptor potential melastatin-2 in guinea pigs exposed to particulate matter 2.5. Physiol. Res. 67, 293–305 (2018).
Plevkova, J. et al. The role of trigeminal nasal TRPM8-expressing afferent neurons in the antitussive effects of menthol. J. Appl. Physiol. 115, 268–274 (2013).
Fisher, J. T. TRPM8 and dyspnea: from the frigid and fascinating past to the cool future? Curr. Opin. Pharm. 11, 218–223 (2011).
Nassenstein, C. et al. Expression and function of the ion channel TRPA1 in vagal afferent nerves innervating mouse lungs. J. Physiol. 586, 1595–1604 (2008).
Barnes, P. J. Cellular and molecular mechanisms of asthma and COPD. Clin. Sci. 131, 1541–1558 (2017).
Barnes, P. J. Asthma-COPD overlap. Chest 149, 7–8 (2016).
Bateman, E. D., Reddel, H. K., van Zyl-Smit, R. N. & Agusti, A. The asthma-COPD overlap syndrome: towards a revised taxonomy of chronic airways diseases? Lancet Respir. Med. 3, 719–728 (2015).
Zhou, J. S. et al. Cigarette smoke-initiated autoimmunity facilitates sensitisation to elastin-induced COPD-like pathologies in mice. Eur. Respir. J. 56, 2000404 (2020).
Papi, A., Brightling, C., Pedersen, S. E. & Reddel, H. K. Asthma. Lancet 391, 783–800 (2018).
Baxter, M. et al. Role of transient receptor potential and pannexin channels in cigarette smoke-triggered ATP release in the lung. Thorax 69, 1080–1089 (2014).
Basoglu, O. K., Barnes, P. J., Kharitonov, S. A. & Pelleg, A. Effects of aerosolized adenosine 5’-triphosphate in smokers and patients with COPD. Chest 148, 430–435 (2015).
Zhu, G. et al. Association of TRPV4 gene polymorphisms with chronic obstructive pulmonary disease. Hum. Mol. Genet. 18, 2053–2062 (2009).
Jia, Y. et al. Functional TRPV4 channels are expressed in human airway smooth muscle cells. Am. J. Physiol. Lung Cell Mol. Physiol. 287, L272–L278 (2004).
McAlexander, M. A., Luttmann, M. A., Hunsberger, G. E. & Undem, B. J. Transient receptor potential vanilloid 4 activation constricts the human bronchus via the release of cysteinyl leukotrienes. J. Pharm. Exp. Ther. 349, 118–125 (2014).
Hwang, S. W. et al. Direct activation of capsaicin receptors by products of lipoxygenases: endogenous capsaicin-like substances. Proc. Natl Acad. Sci. USA 97, 6155–6160 (2000).
Lin, Y. S. et al. Sensitization of capsaicin-sensitive lung vagal afferents by anandamide in rats: role of transient receptor potential vanilloid 1 receptors. J. Appl Physiol. 106, 1142–1152 (2009).
Doherty, M. J., Mister, R., Pearson, M. G. & Calverley, P. M. Capsaicin induced cough in cryptogenic fibrosing alveolitis. Thorax 55, 1028–1032 (2000).
Banner, K. H., Igney, F. & Poll, C. TRP channels: emerging targets for respiratory disease. Pharm. Ther. 130, 371–384 (2011).
Link, T. M. et al. TRPV2 has a pivotal role in macrophage particle binding and phagocytosis. Nat. Immunol. 11, 232–239 (2010).
Andre, E. et al. Cigarette smoke-induced neurogenic inflammation is mediated by alpha,beta-unsaturated aldehydes and the TRPA1 receptor in rodents. J. Clin. Invest. 118, 2574–2582 (2008).
Barnes, P. J. Alveolar macrophages as orchestrators of COPD. COPD 1, 59–70 (2004).
Finney-Hayward, T. K. et al. Expression of transient receptor potential C6 channels in human lung macrophages. Am. J. Respir. Cell Mol. Biol. 43, 296–304 (2010).
Sabnis, A. S., Shadid, M., Yost, G. S. & Reilly, C. A. Human lung epithelial cells express a functional cold-sensing TRPM8 variant. Am. J. Respir. Cell Mol. Biol. 39, 466–474 (2008).
Peier, A. M. et al. A TRP channel that senses cold stimuli and menthol. Cell 108, 705–715 (2002).
Richeldi, L., Collard, H. R. & Jones, M. G. Idiopathic pulmonary fibrosis. Lancet 389, 1941–1952 (2017).
Rahaman, S. O. et al. TRPV4 mediates myofibroblast differentiation and pulmonary fibrosis in mice. J. Clin. Invest. 124, 5225–5238 (2014).
Hamanaka, K. et al. TRPV4 channels augment macrophage activation and ventilator-induced lung injury. Am. J. Physiol. Lung Cell Mol. Physiol. 299, L353–L362 (2010).
Alvarez, D. F. et al. Transient receptor potential vanilloid 4-mediated disruption of the alveolar septal barrier: a novel mechanism of acute lung injury. Circ. Res. 99, 988–995 (2006).
Weissmann, N. et al. Activation of TRPC6 channels is essential for lung ischaemia-reperfusion induced oedema in mice. Nat. Commun. 3, 649 (2012).
Hafner, S. et al. A (+)-Larixol congener with high affinity and subtype selectivity toward TRPC6. ChemMedChem 13, 1028–1035 (2018).
Feigin, V. L. et al. The global burden of neurological disorders: translating evidence into policy. Lancet Neurol 19, 255–265 (2020).
Sisignano, M., Bennett, D. L., Geisslinger, G. & Scholich, K. TRP-channels as key integrators of lipid pathways in nociceptive neurons. Prog. Lipid Res. 53, 93–107 (2014).
Kuppusamy, M., Ottolini, M. & Sonkusare, S. K. Role of TRP ion channels in cerebral circulation and neurovascular communication. Neurosci. Lett. 765, 136258 (2021).
Yao, H. et al. Involvement of TRPC channels in CCL2-mediated neuroprotection against tat toxicity. J. Neurosci. 29, 1657–1669 (2009).
Bollimuntha, S., Ebadi, M. & Singh, B. B. TRPC1 protects human SH-SY5Y cells against salsolinol-induced cytotoxicity by inhibiting apoptosis. Brain Res 1099, 141–149 (2006).
Bollimuntha, S. et al. TRPC1-mediated inhibition of 1-methyl-4-phenylpyridinium ion neurotoxicity in human SH-SY5Y neuroblastoma cells. J. Biol. Chem. 280, 2132–2140 (2005).
Kim, J. H. et al. Capacitative Ca2+ entry is involved in regulating soluble amyloid precursor protein (sAPPalpha) release mediated by muscarinic acetylcholine receptor activation in neuroblastoma SH-SY5Y cells. J. Neurochem. 97, 245–254 (2006).
Li, H. S., Xu, X. Z. & Montell, C. Activation of a TRPC3-dependent cation current through the neurotrophin BDNF. Neuron 24, 261–273 (1999).
Yamamoto, S. et al. Transient receptor potential channels in Alzheimer’s disease. Biochim. Biophys. Acta 1772, 958–967 (2007).
Griesi-Oliveira, K., Suzuki, A. M. & Muotri, A. R. TRPC channels and mental disorders. Adv. Exp. Med. Biol. 976, 137–148 (2017).
Griesi-Oliveira, K. et al. Modeling non-syndromic autism and the impact of TRPC6 disruption in human neurons. Mol. Psychiatry 20, 1350–1365 (2015).
Shinozaki, G. & Potash, J. B. New developments in the genetics of bipolar disorder. Curr. Psychiatry Rep. 16, 493 (2014).
Yoon, I. S. et al. Altered TRPC7 gene expression in bipolar-I disorder. Biol. Psychiatry 50, 620–626 (2001).
Andreopoulos, S. et al. Chronic lithium treatment of B lymphoblasts from bipolar disorder patients reduces transient receptor potential channel 3 levels. Pharmacogenomics J 4, 365–373 (2004).
Zaeri, S., Farjadian, S. & Emamghoreishi, M. Decreased levels of canonical transient receptor potential channel 3 protein in the rat cerebral cortex after chronic treatment with lithium or valproate. Res. Pharm. Sci. 10, 397–406 (2015).
Roedding, A. S. et al. Effect of oxidative stress on TRPM2 and TRPC3 channels in B lymphoblast cells in bipolar disorder. Bipolar Disord 14, 151–161 (2012).
Snaith, R. P. Defining “depression”. Am. J. Psychiatry 144, 828–829 (1987).
Ng, Q. X., Venkatanarayanan, N. & Ho, C. Y. Clinical use of Hypericum perforatum (St John’s wort) in depression: a meta-analysis. J. Affect Disord. 210, 211–221 (2017).
Leuner, K. et al. Hyperforin modulates dendritic spine morphology in hippocampal pyramidal neurons by activating Ca(2+) -permeable TRPC6 channels. Hippocampus 23, 40–52 (2013).
Malhi, G. S. & Mann, J. J. Depression Lancet 392, 2299–2312 (2018).
Amaral, M. D. & Pozzo-Miller, L. TRPC3 channels are necessary for brain-derived neurotrophic factor to activate a nonselective cationic current and to induce dendritic spine formation. J. Neurosci. 27, 5179–5189 (2007).
Huang, J. TRPC channels and stroke. Adv. Exp. Med. Biol. 976, 61–71 (2017).
Shirakawa, H. et al. Sphingosine-1-phosphate induces Ca(2+) signaling and CXCL1 release via TRPC6 channel in astrocytes. Glia 65, 1005–1016 (2017).
Chahine, L. M. et al. In vivo distribution of alpha-synuclein in multiple tissues and biofluids in Parkinson disease. Neurology 95, e1267–e1284 (2020).
Yuan, J. et al. Controlled activation of TRPV1 channels on microglia to boost their autophagy for clearance of alpha-synuclein and enhance therapy of Parkinson’s disease. Adv. Mater. 34, e2108435 (2022).
Liu, N. et al. TRPV4 contributes to ER stress and inflammation: implications for Parkinson’s disease. J. Neuroinflammation 19, 26 (2022).
Nam, J. H. et al. TRPV1 on astrocytes rescues nigral dopamine neurons in Parkinson’s disease via CNTF. Brain 138, 3610–3622 (2015).
Sevigny, J. et al. The antibody aducanumab reduces Abeta plaques in Alzheimer’s disease. Nature 537, 50–56 (2016).
Qiang, W. et al. Structural variation in amyloid-beta fibrils from Alzheimer’s disease clinical subtypes. Nature 541, 217–221 (2017).
Liu, P. P., Xie, Y., Meng, X. Y. & Kang, J. S. History and progress of hypotheses and clinical trials for Alzheimer’s disease. Signal Transduct. Target Ther 4, 29 (2019).
Luo, R. et al. A novel missense variant in ACAA1 contributes to early-onset Alzheimer’s disease, impairs lysosomal function, and facilitates amyloid-beta pathology and cognitive decline. Signal Transduct. Target Ther 6, 325 (2021).
Lu, J. et al. TRPV1 sustains microglial metabolic reprogramming in Alzheimer’s disease. EMBO Rep 22, e52013 (2021).
Du, Y. et al. TRPV1 activation alleviates cognitive and synaptic plasticity impairments through inhibiting AMPAR endocytosis in APP23/PS45 mouse model of Alzheimer’s disease. Aging Cell 19, e13113 (2020).
Deng, Y. et al. Amelioration of scopolamine-induced learning and memory impairment by the TRPV4 inhibitor HC067047 in ICR mice. Neurosci. Lett. 767, 136209 (2022).
Luo, H. et al. Cannabidiol increases proliferation, migration, tubulogenesis, and integrity of human brain endothelial cells through TRPV2 activation. Mol. Pharm. 16, 1312–1326 (2019).
Li, L. et al. Activation of transient receptor potential vanilloid 4 increases NMDA-activated current in hippocampal pyramidal neurons. Front. Cell Neurosci. 7, 17 (2013).
Tanaka, K. et al. Reduced post-ischemic brain injury in transient receptor potential vanilloid 4 knockout mice. Front. Neurosci. 14, 453 (2020).
Paumier, A. et al. Astrocyte-neuron interplay is critical for Alzheimer’s disease pathogenesis and is rescued by TRPA1 channel blockade. Brain 145, 388–405 (2022).
Pradeepkiran, J. A. & Reddy, P. H. Defective mitophagy in Alzheimer’s disease. Ageing Res. Rev. 64, 101191 (2020).
Ozsimsek, A. & Ovey, I. S. Potential effects of melatonin on TRPA1 channels in the prevention and treatment of Alzheimer’s disease. Noro Psikiyatr Ars 59, 188–192 (2022).
Gunaydin, C., Arslan, G. & Bilge, S. S. Proconvulsant effect of trans-cinnamaldehyde in pentylenetetrazole-induced kindling model of epilepsy: The role of TRPA1 channels. Neurosci. Lett. 721, 134823 (2020).
Heydari, F. S. et al. The blockade of transient receptor potential ankyrin 1 (TRPA1) protects against PTZ-induced seizure. Metab. Brain Dis. 38, 621–630 (2023).
Peres, D. S. et al. TRPA1 involvement in depression- and anxiety-like behaviors in a progressive multiple sclerosis model in mice. Brain Res. Bull. 175, 1–15 (2021).
de Moura, J. C. et al. The blockade of transient receptor potential ankirin 1 (TRPA1) signalling mediates antidepressant- and anxiolytic-like actions in mice. Br. J. Pharm. 171, 4289–4299 (2014).
Pires, P. W. & Earley, S. Neuroprotective effects of TRPA1 channels in the cerebral endothelium following ischemic stroke. Elife 7, https://doi.org/10.7554/eLife.35316 (2018).
Jiang, L. H. et al. The TRPM2 channel nexus from oxidative damage to Alzheimer’s pathologies: an emerging novel intervention target for age-related dementia. Ageing Res. Rev. 47, 67–79 (2018).
Huang, Q. et al. The role of transient receptor potential channels in blood-brain barrier dysfunction after ischemic stroke. Biomed. Pharmacother. 131, 110647 (2020).
Xie, Y. F., Macdonald, J. F. & Jackson, M. F. TRPM2, calcium and neurodegenerative diseases. Int. J. Physiol. Pathophysiol. Pharm. 2, 95–103 (2010).
Hazalin, N., Liao, P. & Hassan, Z. TRPM4 inhibition improves spatial memory impairment and hippocampal long-term potentiation deficit in chronic cerebral hypoperfused rats. Behav. Brain Res. 393, 112781 (2020).
Chen, B. et al. TRPM4-specific blocking antibody attenuates reperfusion injury in a rat model of stroke. Pflug. Arch. 471, 1455–1466 (2019).
Chen, B. et al. Non-invasive multimodality imaging directly shows TRPM4 inhibition ameliorates stroke reperfusion injury. Transl. Stroke Res. 10, 91–103 (2019).
Inoue, R. et al. The transient receptor potential protein homologue TRP6 is the essential component of vascular alpha(1)-adrenoceptor-activated Ca(2+)-permeable cation channel. Circ. Res. 88, 325–332 (2001).
Gan, L. et al. CD38 deficiency alleviates Ang II-induced vascular remodeling by inhibiting small extracellular vesicle-mediated vascular smooth muscle cell senescence in mice. Signal Transduct. Target Ther 6, 223 (2021).
Zhao, H. et al. Inhibition of intermittent calcium-activated potassium channel (SK4) attenuates Ang II-induced hypertrophy of human-induced stem cell-derived cardiomyocytes via targeting Ras-Raf-MEK1/2-ERK1/2 and CN-NFAT signaling pathways. Cell Biol. Int. 47, 480–491 (2023).
Onohara, N. et al. TRPC3 and TRPC6 are essential for angiotensin II-induced cardiac hypertrophy. EMBO J 25, 5305–5316 (2006).
Heineke, J. & Molkentin, J. D. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat. Rev. Mol. Cell Biol. 7, 589–600 (2006).
Satoh, S. et al. Transient receptor potential (TRP) protein 7 acts as a G protein-activated Ca2+ channel mediating angiotensin II-induced myocardial apoptosis. Mol. Cell Biochem. 294, 205–215 (2007).
Nakayama, H., Wilkin, B. J., Bodi, I. & Molkentin, J. D. Calcineurin-dependent cardiomyopathy is activated by TRPC in the adult mouse heart. FASEB J 20, 1660–1670 (2006).
Seth, M. et al. TRPC1 channels are critical for hypertrophic signaling in the heart. Circ. Res. 105, 1023–1030 (2009).
Zhang, Q. et al. Activation of transient receptor potential vanilloid 3 channel (TRPV3) aggravated pathological cardiac hypertrophy via calcineurin/NFATc3 pathway in rats. J. Cell Mol. Med. 22, 6055–6067 (2018).
Feng, J. et al. Upregulation of transient receptor potential melastatin 4 (TRPM4) in ventricular fibroblasts from heart failure patients. Pflug. Arch. 473, 521–531 (2021).
Lang, H. et al. Activation of TRPV1 attenuates high salt-induced cardiac hypertrophy through improvement of mitochondrial function. Br. J. Pharm. 172, 5548–5558 (2015).
Poulter, N. R. & Prabhakaran, D. & Caulfield, M. Hypertension. Lancet 386, 801–812 (2015).
Desai, A. N. High blood pressure. JAMA 324, 1254–1255 (2020).
Hollis, M. & Wang, D. H. Transient receptor potential vanilloid in blood pressure regulation. Curr. Opin. Nephrol. Hypertens. 22, 170–176 (2013).
Zygmunt, P. M. et al. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature 400, 452–457 (1999).
Poblete, I. M. et al. Anandamide elicits an acute release of nitric oxide through endothelial TRPV1 receptor activation in the rat arterial mesenteric bed. J. Physiol. 568, 539–551 (2005).
Mathar, I. et al. Increased catecholamine secretion contributes to hypertension in TRPM4-deficient mice. J. Clin. Invest. 120, 3267–3279 (2010).
Earley, S. et al. TRPV4-dependent dilation of peripheral resistance arteries influences arterial pressure. Am. J. Physiol. Heart Circ. Physiol. 297, H1096–H1102 (2009).
Basatemur, G. L. et al. Vascular smooth muscle cells in atherosclerosis. Nat. Rev. Cardiol. 16, 727–744 (2019).
Kong, P. et al. Inflammation and atherosclerosis: signaling pathways and therapeutic intervention. Signal Transduct. Target Ther 7, 131 (2022).
Simion, V. et al. A macrophage-specific lncRNA regulates apoptosis and atherosclerosis by tethering HuR in the nucleus. Nat. Commun. 11, 6135 (2020).
Tano, J. Y. et al. Bone marrow deficiency of TRPC3 channel reduces early lesion burden and necrotic core of advanced plaques in a mouse model of atherosclerosis. Cardiovasc. Res. 101, 138–144 (2014).
Zhao, J. F. et al. Transient receptor potential ankyrin 1 channel involved in atherosclerosis and macrophage-foam cell formation. Int. J. Biol. Sci. 12, 812–823 (2016).
Dai, Y. Q. et al. Transient receptor potential melastatin 2 enhances vascular reactivity during development of atherosclerosis in mice. Am. J. Hypertens. 34, 121–122 (2021).
Perrone, R. D., Malek, A. M. & Watnick, T. Vascular complications in autosomal dominant polycystic kidney disease. Nat. Rev. Nephrol. 11, 589–598 (2015).
Bergmann, C. et al. Polycystic kidney disease. Nat. Rev. Dis. Prim. 4, 50 (2018).
Qian, Q. et al. Pkd2 haploinsufficiency alters intracellular calcium regulation in vascular smooth muscle cells. Hum. Mol. Genet. 12, 1875–1880 (2003).
Yue, Z. et al. Role of TRP channels in the cardiovascular system. Am. J. Physiol. Heart Circ. Physiol. 308, H157–H182 (2015).
Wang, S. et al. The molecular biology of pancreatic adenocarcinoma: translational challenges and clinical perspectives. Signal Transduct. Target Ther 6, 249 (2021).
Yin, X. et al. The evolving view of thermogenic fat and its implications in cancer and metabolic diseases. Signal Transduct. Target Ther 7, 324 (2022).
Yang, D. & Kim, J. Emerging role of transient receptor potential (TRP) channels in cancer progression. BMB Rep 53, 125–132 (2020).
Perrouin-Verbe, M. A. et al. Overexpression of certain transient receptor potential and Orai channels in prostate cancer is associated with decreased risk of systemic recurrence after radical prostatectomy. Prostate 79, 1793–1804 (2019).
So, C. L., Milevskiy, M. J. G. & Monteith, G. R. Transient receptor potential cation channel subfamily V and breast cancer. Lab Invest 100, 199–206 (2020).
Rizopoulos, T. & Assimakopoulou, M. Transient receptor potential (TRP) channels in human colorectal cancer: evidence and perspectives. Histol. Histopathol. 36, 515–526 (2021).
Huang, J., Liu, J. & Qiu, L. Transient receptor potential vanilloid 1 promotes EGFR ubiquitination and modulates EGFR/MAPK signalling in pancreatic cancer cells. Cell Biochem. Funct. 38, 401–408 (2020).
Hu, Z. et al. Transient receptor potential vanilloid-type 2 targeting on stemness in liver cancer. Biomed. Pharmacother. 105, 697–706 (2018).
Kim, B. J. et al. The role of transient receptor potential channel blockers in human gastric cancer cell viability. Can. J. Physiol. Pharm. 90, 175–186 (2012).
Rybarczyk, P. et al. The transient receptor potential melastatin 7 channel regulates pancreatic cancer cell invasion through the Hsp90alpha/uPA/MMP2 pathway. Neoplasia 19, 288–300 (2017).
Zeng, X. et al. Novel role for the transient receptor potential channel TRPM2 in prostate cancer cell proliferation. Prostate Cancer Prostatic Dis 13, 195–201 (2010).
Sun, W. et al. Involvement of TRP channels in adipocyte thermogenesis: an update. Front. Cell Dev. Biol. 9, 686173 (2021).
Yoneshiro, T. & Saito, M. Transient receptor potential activated brown fat thermogenesis as a target of food ingredients for obesity management. Curr. Opin. Clin. Nutr. Metab. Care 16, 625–631 (2013).
Snitker, S. et al. Effects of novel capsinoid treatment on fatness and energy metabolism in humans: possible pharmacogenetic implications. Am. J. Clin. Nutr. 89, 45–50 (2009).
Yoneshiro, T. et al. Nonpungent capsaicin analogs (capsinoids) increase energy expenditure through the activation of brown adipose tissue in humans. Am. J. Clin. Nutr. 95, 845–850 (2012).
Lejeune, M. P., Kovacs, E. M. & Westerterp-Plantenga, M. S. Effect of capsaicin on substrate oxidation and weight maintenance after modest body-weight loss in human subjects. Br. J. Nutr. 90, 651–659 (2003).
Inoue, N., Matsunaga, Y., Satoh, H. & Takahashi, M. Enhanced energy expenditure and fat oxidation in humans with high BMI scores by the ingestion of novel and non-pungent capsaicin analogues (capsinoids). Biosci. Biotechnol. Biochem. 71, 380–389 (2007).
Moller, C. C. et al. Induction of TRPC6 channel in acquired forms of proteinuric kidney disease. J. Am. Soc. Nephrol. 18, 29–36 (2007).
Reiser, J. et al. TRPC6 is a glomerular slit diaphragm-associated channel required for normal renal function. Nat. Genet. 37, 739–744 (2005).
Yu, S. & Yu, L. Dexamethasone resisted podocyte injury via stabilizing TRPC6 expression and distribution. Evid. Based Complement. Altern. Med. 2012, 652059 (2012).
Sonneveld, R. et al. Glucose specifically regulates TRPC6 expression in the podocyte in an AngII-dependent manner. Am. J. Pathol. 184, 1715–1726 (2014).
Zhang, Q. et al. Screening of ACTN4 and TRPC6 mutations in a Chinese cohort of patients with adult-onset familial focal segmental glomerulosclerosis. Contrib. Nephrol. 181, 91–100 (2013).
Kistler, A. D. et al. Transient receptor potential channel 6 (TRPC6) protects podocytes during complement-mediated glomerular disease. J. Biol. Chem. 288, 36598–36609 (2013).
Eckel, R. H., Grundy, S. M. & Zimmet, P. Z. The metabolic syndrome. Lancet 365, 1415–1428 (2005).
Liu, D., Zhu, Z. & Tepel, M. The role of transient receptor potential channels in metabolic syndrome. Hypertens. Res. 31, 1989–1995 (2008).
Colsoul, B., Nilius, B. & Vennekens, R. Transient receptor potential (TRP) cation channels in diabetes. Curr. Top. Med. Chem. 13, 258–269 (2013).
Chung, A. W. et al. Diabetes modulates capacitative calcium entry and expression of transient receptor potential canonical channels in human saphenous vein. Eur. J. Pharm. 613, 114–118 (2009).
Billert, M. et al. TRPV4 regulates insulin mRNA expression and INS-1E cell death via ERK1/2 and NO-dependent mechanisms. Cell Signal 35, 242–249 (2017).
Deruyver, Y. et al. Intravesical activation of the cation channel TRPV4 improves bladder function in a rat model for detrusor underactivity. Eur. Urol. 74, 336–345 (2018).
Everaerts, W. et al. Inhibition of the cation channel TRPV4 improves bladder function in mice and rats with cyclophosphamide-induced cystitis. Proc. Natl Acad. Sci. USA 107, 19084–19089 (2010).
Szallasi, A. & Blumberg, P. M. Vanilloid (Capsaicin) receptors and mechanisms. Pharm. Rev. 51, 159–212 (1999).
Liu, B. L. et al. Increased severity of inflammation correlates with elevated expression of TRPV1 nerve fibers and nerve growth factor on interstitial cystitis/bladder pain syndrome. Urol. Int. 92, 202–208 (2014).
Kawate, T. P2X receptor activation. Adv. Exp. Med. Biol. 1051, 55–69 (2017).
Song, J. et al. Posterior tibial nerve stimulation improves neurogenic bladder in rats with spinal cord injury through transient receptor potential/P2X signaling pathway. Neurourol. Urodyn. 41, 756–764 (2022).
Wu, L., Zhang, J., Zhou, F. & Zhang, P. Increased transient receptor potential melastatin 8 expression in the development of bladder pain in patients with interstitial cystitis/bladder pain syndrome. Urology 146, 301.e301–301 e306 (2020).
Furuta, A. et al. Transient receptor potential A1 receptor-mediated neural cross-talk and afferent sensitization induced by oxidative stress: implication for the pathogenesis of interstitial cystitis/bladder pain syndrome. Int. J. Urol. 19, 429–436 (2012).
Andersson, K. E., Gratzke, C. & Hedlund, P. The role of the transient receptor potential (TRP) superfamily of cation-selective channels in the management of the overactive bladder. BJU Int 106, 1114–1127 (2010).
Zhang, H. Y. et al. Expression and diagnosis of transient receptor potential vanilloid1 in urothelium of patients with overactive bladder. J. Biol. Regul. Homeost. Agents 29, 875–879 (2015).
Frias, B. et al. Transient receptor potential vanilloid 1 mediates nerve growth factor-induced bladder hyperactivity and noxious input. BJU Int 110, E422–E428 (2012).
Nakanishi, O. et al. KPR-5714, a novel transient receptor potential melastatin 8 antagonist, improves overactive bladder via inhibition of bladder afferent hyperactivity in rats. J. Pharm. Exp. Ther. 373, 239–247 (2020).
Khalil, M. et al. Functional role of transient receptor potential channels in immune cells and epithelia. Front. Immunol. 9, 174 (2018).
Kuzumi, A. et al. Interleukin-31 promotes fibrosis and T helper 2 polarization in systemic sclerosis. Nat. Commun. 12, 5947 (2021).
Cevikbas, F. et al. A sensory neuron-expressed IL-31 receptor mediates T helper cell-dependent itch: Involvement of TRPV1 and TRPA1. J. Allergy Clin. Immunol. 133, 448–460 (2014).
Facchinetti, F. et al. Alpha,beta-unsaturated aldehydes in cigarette smoke release inflammatory mediators from human macrophages. Am. J. Respir. Cell Mol. Biol. 37, 617–623 (2007).
Wu, S. N., Wu, P. Y. & Tsai, M. L. Characterization of TRPM8-like channels activated by the cooling agent icilin in the macrophage cell line RAW 264.7. J. Membr. Biol. 241, 11–20 (2011).
Khalil, M. et al. Transient receptor potential melastatin 8 ion channel in macrophages modulates colitis through a balance-shift in TNF-alpha and interleukin-10 production. Mucosal Immunol 9, 1500–1513 (2016).
Juergens, U. R. et al. Inhibitory activity of 1,8-cineol (eucalyptol) on cytokine production in cultured human lymphocytes and monocytes. Pulm. Pharm. Ther. 17, 281–287 (2004).
Zhao, J. F. et al. Activation of TRPV1 prevents OxLDL-induced lipid accumulation and TNF-alpha-induced inflammation in macrophages: role of liver X receptor alpha. Mediators Inflamm 2013, 925171 (2013).
Kim, C. S. et al. Capsaicin exhibits anti-inflammatory property by inhibiting IkB-a degradation in LPS-stimulated peritoneal macrophages. Cell Signal 15, 299–306 (2003).
Acharya, N. et al. Endocannabinoid system acts as a regulator of immune homeostasis in the gut. Proc. Natl Acad. Sci. USA 114, 5005–5010 (2017).
Henry, C. O. et al. In vitro and in vivo evidence for an inflammatory role of the calcium channel TRPV4 in lung epithelium: potential involvement in cystic fibrosis. Am. J. Physiol. Lung Cell Mol. Physiol. 311, L664–675 (2016).
Majhi, R. K. et al. Functional expression of TRPV channels in T cells and their implications in immune regulation. FEBS J 282, 2661–2681 (2015).
Santoni, G. et al. “Immuno-transient receptor potential ion channels”: the role in monocyte- and macrophage-mediated inflammatory responses. Front. Immunol. 9, 1273 (2018).
Beceiro, S. et al. TRPM2 ion channels regulate macrophage polarization and gastric inflammation during Helicobacter pylori infection. Mucosal Immunol 10, 493–507 (2017).
Yamamoto, S. et al. TRPM2-mediated Ca2+influx induces chemokine production in monocytes that aggravates inflammatory neutrophil infiltration. Nat. Med. 14, 738–747 (2008).
Serafini, N. et al. The TRPM4 channel controls monocyte and macrophage, but not neutrophil, function for survival in sepsis. J. Immunol. 189, 3689–3699 (2012).
Surowy, C. S. et al. (R)-(5-tert-butyl-2,3-dihydro-1H-inden-1-yl)-3-(1H-indazol-4-yl)-urea (ABT-102) blocks polymodal activation of transient receptor potential vanilloid 1 receptors in vitro and heat-evoked firing of spinal dorsal horn neurons in vivo. J. Pharm. Exp. Ther. 326, 879–888 (2008).
Honore, P. et al. Repeated dosing of ABT-102, a potent and selective TRPV1 antagonist, enhances TRPV1-mediated analgesic activity in rodents, but attenuates antagonist-induced hyperthermia. Pain 142, 27–35 (2009).
Garami, A. et al. Hyperthermia induced by transient receptor potential vanilloid-1 (TRPV1) antagonists in human clinical trials: Insights from mathematical modeling and meta-analysis. Pharm. Ther. 208, 107474 (2020).
Krarup, A. L. et al. Randomized clinical trial: inhibition of the TRPV1 system in patients with nonerosive gastroesophageal reflux disease and a partial response to PPI treatment is not associated with analgesia to esophageal experimental pain. Scand. J. Gastroenterol. 48, 274–284 (2013).
Miller, F., Bjornsson, M., Svensson, O. & Karlsten, R. Experiences with an adaptive design for a dose-finding study in patients with osteoarthritis. Contemp. Clin. Trials 37, 189–199 (2014).
Munjuluri, S. et al. Capsaicin and TRPV1 channels in the cardiovascular system: the role of inflammation. Cells 11, 18 (2022).
Sharma, S. K., Vij, A. S. & Sharma, M. Mechanisms and clinical uses of capsaicin. Eur. J. Pharm. 720, 55–62 (2013).
Gibson, R. A. et al. A randomised trial evaluating the effects of the TRPV1 antagonist SB705498 on pruritus induced by histamine, and cowhage challenge in healthy volunteers. PLoS ONE 9, e100610 (2014).
Szallasi, A., Cruz, F. & Geppetti, P. TRPV1: a therapeutic target for novel analgesic drugs? Trends Mol. Med. 12, 545–554 (2006).
Belvisi, M. G. et al. XEN-D0501, a novel transient receptor potential vanilloid 1 antagonist, does not reduce cough in patients with refractory cough. Am. J. Respir. Crit. Care Med. 196, 1255–1263 (2017).
Lim, K. M. & Park, Y. H. Development of PAC-14028, a novel transient receptor potential vanilloid type 1 (TRPV1) channel antagonist as a new drug for refractory skin diseases. Arch. Pharm. Res. 35, 393–396 (2012).
Song, P. I. & Armstrong, C. A. Novel therapeutic approach with PAC-14028 cream, a TRPV1 antagonist, for patients with mild-to-moderate atopic dermatitis. Br. J. Dermatol. 180, 971–972 (2019).
Chen, H. & Terrett, J. A. Transient receptor potential ankyrin 1 (TRPA1) antagonists: a patent review (2015-2019). Expert Opin. Ther. Pat. 30, 643–657 (2020).
Balestrini, A. et al. A TRPA1 inhibitor suppresses neurogenic inflammation and airway contraction for asthma treatment. J. Exp. Med. 218, e20201637 (2021).
Preti, D., Saponaro, G. & Szallasi, A. Transient receptor potential ankyrin 1 (TRPA1) antagonists. Pharm. Pat. Anal. 4, 75–94 (2015).
Skerratt, S. Recent progress in the discovery and development of TRPA1 modulators. Prog. Med. Chem. 56, 81–115 (2017).
Gosset, J. R. et al. A cross-species translational pharmacokinetic-pharmacodynamic evaluation of core body temperature reduction by the TRPM8 blocker PF-05105679. Eur. J. Pharm. Sci. 109S, S161–S167 (2017).
Winchester, W. J. et al. Inhibition of TRPM8 channels reduces pain in the cold pressor test in humans. J. Pharm. Exp. Ther. 351, 259–269 (2014).
Barkin, R. L. The pharmacology of topical analgesics. Postgrad. Med. 125, 7–18 (2013).
Craighead, D. H., McCartney, N. B., Tumlinson, J. H. & Alexander, L. M. Mechanisms and time course of menthol-induced cutaneous vasodilation. Microvasc. Res. 110, 43–47 (2017).
Lasanen, R., Julkunen, P., Airaksinen, O. & Toyras, J. Menthol concentration in topical cold gel does not have significant effect on skin cooling. Ski. Res. Technol. 22, 40–45 (2016).
Tomsen, N. et al. A randomized clinical trial on the acute therapeutic effect of TRPA1 and TRPM8 agonists in patients with oropharyngeal dysphagia. Neurogastroenterol. Motil. 32, e13821 (2020).
Peiris, M. et al. A putative anti-inflammatory role for TRPM8 in irritable bowel syndrome-An exploratory study. Neurogastroenterol. Motil. 33, e14170 (2021).
Brooks, C. A. et al. Discovery of GSK2798745: a clinical candidate for inhibition of transient receptor potential vanilloid 4 (TRPV4). ACS Med. Chem. Lett. 10, 1228–1233 (2019).
Goyal, N. et al. Clinical pharmacokinetics, safety, and tolerability of a novel, first-in-class TRPV4 ion channel inhibitor, GSK2798745, in healthy and heart failure subjects. Am. J. Cardiovasc. Drugs 19, 335–342 (2019).
Nilius, B. & Biro, T. TRPV3: a ‘more than skinny’ channel. Exp. Dermatol 22, 447–452 (2013).
Broad, L. M. et al. TRPV3 in drug development. Pharmaceuticals 9, 55 (2016).
Acar, H. V. Acupuncture and related techniques during perioperative period: a literature review. Complement Ther. Med. 29, 48–55 (2016).
Mao, J. J. et al. Effectiveness of electroacupuncture or auricular acupuncture vs usual care for chronic musculoskeletal pain among cancer survivors: the PEACE randomized clinical trial. JAMA Oncol 7, 720–727 (2021).
Ulloa, L. Electroacupuncture activates neurons to switch off inflammation. Nature 598, 573–574 (2021).
Jiang, J., Wang, X., Wu, X. & Yu, Z. Analysis of factors influencing moxibustion efficacy by affecting heat-activated transient receptor potential vanilloid channels. J. Tradit. Chin. Med. 36, 255–260 (2016).
Li, J. & Jiang, J. Role of transient receptor potential vanilloid subetype 1 in the increase of thermal pain threshold by moxibustion. J. Tradit. Chin. Med. 35, 583–587 (2015).
Zou, W. et al. Moxibustion relieves visceral hyperalgesia via inhibition of transient receptor potential vanilloid 1 (TRPV1) and heat shock protein (HSP) 70 expression in rat bone marrow cells. Acupunct. Med. 34, 114–119 (2016).
Inprasit, C. et al. Targeting TRPV1 to relieve motion sickness symptoms in mice by electroacupuncture and gene deletion. Sci. Rep. 8, 10365 (2018).
Ji, B., Hu, J. & Ma, S. Effects of electroacupuncture Zusanli (ST36) on food intake and expression of POMC and TRPV1 through afferents-medulla pathway in obese prone rats. Peptides 40, 188–194 (2013).
Huang, M. et al. Critical roles of TRPV2 channels, histamine H1 and adenosine A1 receptors in the initiation of acupoint signals for acupuncture analgesia. Sci. Rep. 8, 6523 (2018).
Yen, C. M., Hsieh, C. L. & Lin, Y. W. Electroacupuncture reduces chronic fibromyalgia pain through attenuation of transient receptor potential vanilloid 1 signaling pathway in mouse brains. Iran. J. Basic Med. Sci. 23, 894–900 (2020).
Zhong, X. et al. Effects of electroacupuncture on gastrointestinal motility function, pain, and inflammation via transient receptor potential vanilloid 1 in a rat model after colonic anastomoses. Dis. Markers 2022, 5113473 (2022).
Lin, Y. W., Chou, A. I. W., Su, H. & Su, K. P. Transient receptor potential V1 (TRPV1) modulates the therapeutic effects for comorbidity of pain and depression: the common molecular implication for electroacupuncture and omega-3 polyunsaturated fatty acids. Brain Behav. Immun. 89, 604–614 (2020).
Yu, Z. et al. Electroacupuncture at ST25 inhibits jejunal motility: role of sympathetic pathways and TRPV1. World J. Gastroenterol. 22, 1834–1843 (2016).
Tsai, S. T. et al. Transient receptor potential V1 modulates neuroinflammation in Parkinson’s disease dementia: molecular implications for electroacupuncture and rivastigmine. Iran. J. Basic Med. Sci. 24, 1336–1345 (2021).
Li, Y. et al. Electroacupuncture alleviates paclitaxel-induced peripheral neuropathic pain in rats via suppressing TLR4 signaling and TRPV1 upregulation in sensory neurons. Int. J. Mol. Sci. 20, 5917 (2019).
Wu, J. H. et al. [Effect of electroacupuncture pretreatment on transient receptor potential vanilloid 1(TRPV1)/calcitonin gene-related peptide(CGRP) signal and NF-kappaB p65 protein expression in rats with acute myocardial ischemia]. Zhen Ci Yan Jiu 46, 58–63 (2021).
Zheng, J. et al. Gold nanorods: the most versatile plasmonic nanoparticles. Chem. Rev. 121, 13342–13453 (2021).
Zhou, C. et al. Gold nanorods-based thermosensitive hydrogel produces selective long-lasting regional anesthesia triggered by photothermal activation of transient receptor potential vanilloid type-1 channels. Colloids Surf. B Biointerfaces 171, 17–23 (2018).
Zhen, X. et al. Semiconducting photothermal nanoagonist for remote-controlled specific cancer therapy. Nano Lett 18, 1498–1505 (2018).
Maggi, C. A. et al. Cystometric evidence that capsaicin-sensitive nerves modulate the afferent branch of micturition reflex in humans. J. Urol. 142, 150–154 (1989).
Szallasi, A. Some like it hot (ever more so in the tropics): a puzzle with no solution. Temp. (Austin) 3, 54–55 (2016).
Szallasi, A. Vanilloid-sensitive neurons: a fundamental subdivision of the peripheral nervous system. J. Peripher. Nerv. Syst. 1, 6–18 (1996).
Krarup, A. L. et al. Randomised clinical trial: the efficacy of a transient receptor potential vanilloid 1 antagonist AZD1386 in human oesophageal pain. Aliment Pharm. Ther. 33, 1113–1122 (2011).
Gavva, N. R. et al. Pharmacological blockade of the vanilloid receptor TRPV1 elicits marked hyperthermia in humans. Pain 136, 202–210 (2008).
Eid, S. R. Therapeutic targeting of TRP channels–the TR(i)P to pain relief. Curr. Top. Med. Chem. 11, 2118–2130 (2011).
Moran, M. M. & Szallasi, A. Targeting nociceptive transient receptor potential channels to treat chronic pain: current state of the field. Br. J. Pharm. 175, 2185–2203 (2018).
Materazzi, S. et al. TRPA1 and TRPV4 mediate paclitaxel-induced peripheral neuropathy in mice via a glutathione-sensitive mechanism. Pflug. Arch. 463, 561–569 (2012).
Schulze, A., Hartung, P., Schaefer, M. & Hill, K. Transient receptor potential ankyrin 1 (TRPA1) channel activation by the thienopyridine-type drugs ticlopidine, clopidogrel, and prasugrel. Cell Calcium 55, 200–207 (2014).
Rudd, J. A. et al. The involvement of TRPV1 in emesis and anti-emesis. Temp. (Austin) 2, 258–276 (2015).
Brown, W. et al. Safety, pharmacokinetics, and pharmacodynamics study in healthy subjects of oral NEO6860, a modality selective transient receptor potential vanilloid subtype 1 antagonist. J. Pain 18, 726–738 (2017).
Gerhold, K. A. & Bautista, D. M. Molecular and cellular mechanisms of trigeminal chemosensation. Ann. N. Y Acad. Sci. 1170, 184–189 (2009).
Terada, Y. et al. Activation and inhibition of thermosensitive TRP channels by voacangine, an alkaloid present in Voacanga africana, an African tree. J. Nat. Prod. 77, 285–297 (2014).
Elzamzamy, O. M., Penner, R. & Hazlehurst, L. A. The role of TRPC1 in modulating cancer progression. Cells 9, 388 (2020).
Yildirim, E. et al. Severely blunted allergen-induced pulmonary Th2 cell response and lung hyperresponsiveness in type 1 transient receptor potential channel-deficient mice. Am. J. Physiol. Lung Cell Mol. Physiol. 303, L539–549 (2012).
Lof, C., Viitanen, T., Sukumaran, P. & Tornquist, K. TRPC2: of mice but not men. Adv. Exp. Med. Biol. 704, 125–134 (2011).
Tornquist, K. et al. Canonical transient receptor potential channel 2 (TRPC2): old name-new games. Importance in regulating of rat thyroid cell physiology. Pflug. Arch. 466, 2025–2034 (2014).
Zhu, X. et al. trp, a novel mammalian gene family essential for agonist-activated capacitative Ca2+ entry. Cell 85, 661–671 (1996).
Hofmann, T., Schaefer, M., Schultz, G. & Gudermann, T. Subunit composition of mammalian transient receptor potential channels in living cells. Proc. Natl Acad. Sci. USA 99, 7461–7466 (2002).
Tiruppathi, C. et al. Impairment of store-operated Ca2+ entry in TRPC4(-/-) mice interferes with increase in lung microvascular permeability. Circ. Res. 91, 70–76 (2002).
Veliceasa, D. et al. Transient potential receptor channel 4 controls thrombospondin-1 secretion and angiogenesis in renal cell carcinoma. FEBS J 274, 6365–6377 (2007).
Zhu, Y. et al. Enhancement of vascular endothelial growth factor release in long-term drug-treated breast cancer via transient receptor potential channel 5-Ca(2+)-hypoxia-inducible factor 1alpha pathway. Pharm. Res. 93, 36–42 (2015).
Lau, O. C. et al. TRPC5 channels participate in pressure-sensing in aortic baroreceptors. Nat. Commun. 7, 11947 (2016).
Ma, X. et al. Essential role for TrpC5-containing extracellular vesicles in breast cancer with chemotherapeutic resistance. Proc. Natl Acad. Sci. USA 111, 6389–6394 (2014).
Corteling, R. L. et al. Expression of transient receptor potential C6 and related transient receptor potential family members in human airway smooth muscle and lung tissue. Am. J. Respir. Cell Mol. Biol. 30, 145–154 (2004).
Staruschenko, A., Spires, D. & Palygin, O. Role of TRPC6 in progression of diabetic kidney disease. Curr. Hypertens. Rep. 21, 48 (2019).
Okada, T. et al. Molecular and functional characterization of a novel mouse transient receptor potential protein homologue TRP7. Ca(2+)-permeable cation channel that is constitutively activated and enhanced by stimulation of G protein-coupled receptor. J. Biol. Chem. 274, 27359–27370 (1999).
Caterina, M. J. et al. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389, 816–824 (1997).
Voets, T. et al. The principle of temperature-dependent gating in cold- and heat-sensitive TRP channels. Nature 430, 748–754 (2004).
Tominaga, M. et al. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron 21, 531–543 (1998).
Becker, D., Blase, C., Bereiter-Hahn, J. & Jendrach, M. TRPV4 exhibits a functional role in cell-volume regulation. J. Cell Sci. 118, 2435–2440 (2005).
de Groot, T., Bindels, R. J. & Hoenderop, J. G. TRPV5: an ingeniously controlled calcium channel. Kidney Int 74, 1241–1246 (2008).
Bachinger, D. et al. Immunolocalization of calcium sensing and transport proteins in the murine endolymphatic sac indicates calciostatic functions within the inner ear. Cell Tissue Res 378, 163–173 (2019).
Lieben, L. et al. Trpv6 mediates intestinal calcium absorption during calcium restriction and contributes to bone homeostasis. Bone 47, 301–308 (2010).
Oancea, E. & Wicks, N. L. TRPM1: new trends for an old TRP. Adv. Exp. Med. Biol. 704, 135–145 (2011).
Hirschler-Laszkiewicz, I. et al. The human ion channel TRPM2 modulates neuroblastoma cell survival and mitochondrial function through Pyk2, CREB, and MCU activation. Am. J. Physiol. Cell Physiol. 315, C571–C586 (2018).
Behrendt, M. Transient receptor potential channels in the context of nociception and pain - recent insights into TRPM3 properties and function. Biol. Chem. 400, 917–926 (2019).
Shiels, A. TRPM3_miR-204: a complex locus for eye development and disease. Hum. Genomics 14, 7 (2020).
Wang, H. et al. Gain-of-function mutations in TRPM4 activation gate cause progressive symmetric erythrokeratodermia. J. Invest. Dermatol. 139, 1089–1097 (2019).
Guinamard, R., Salle, L. & Simard, C. The non-selective monovalent cationic channels TRPM4 and TRPM5. Adv. Exp. Med. Biol. 704, 147–171 (2011).
Gwanyanya, A. et al. Magnesium-inhibited, TRPM6/7-like channel in cardiac myocytes: permeation of divalent cations and pH-mediated regulation. J. Physiol. 559, 761–776 (2004).
Zou, Z. G., Rios, F. J., Montezano, A. C. & Touyz, R. M. TRPM7, magnesium, and signaling. Int. J. Mol. Sci. 20, 1877 (2019).
Gonzalez-Muniz, R., Bonache, M. A., Martin-Escura, C. & Gomez-Monterrey, I. Recent progress in TRPM8 modulation: an update. Int. J. Mol. Sci. 20, 2618 (2019).
Cantiello, H. F. Regulation of calcium signaling by polycystin-2. Am. J. Physiol. Ren. Physiol. 286, F1012–1029 (2004).
Huang, A. L. et al. The cells and logic for mammalian sour taste detection. Nature 442, 934–938 (2006).
Chen, Y. et al. Expression of Pkd2l2 in testis is implicated in spermatogenesis. Biol. Pharm. Bull. 31, 1496–1500 (2008).
Taft, R. A., Denegre, J. M., Pendola, F. L. & Eppig, J. J. Identification of genes encoding mouse oocyte secretory and transmembrane proteins by a signal sequence trap. Biol. Reprod. 67, 953–960 (2002).
Chen, C. S., Bach, G. & Pagano, R. E. Abnormal transport along the lysosomal pathway in mucolipidosis, type IV disease. Proc. Natl Acad. Sci. USA 95, 6373–6378 (1998).
Song, Y., Dayalu, R., Matthews, S. A. & Scharenberg, A. M. TRPML cation channels regulate the specialized lysosomal compartment of vertebrate B-lymphocytes. Eur. J. Cell Biol. 85, 1253–1264 (2006).
Grimm, C. et al. A helix-breaking mutation in TRPML3 leads to constitutive activity underlying deafness in the varitint-waddler mouse. Proc. Natl Acad. Sci. USA 104, 19583–19588 (2007).
Nagata, K. et al. The varitint-waddler (Va) deafness mutation in TRPML3 generates constitutive, inward rectifying currents and causes cell degeneration. Proc. Natl Acad. Sci. USA 105, 353–358 (2008).
Vinayagam, D. et al. Structural basis of TRPC4 regulation by calmodulin and pharmacological agents. Elife 9, https://doi.org/10.7554/eLife.60603 (2020).
Song, K. et al. Structural basis for human TRPC5 channel inhibition by two distinct inhibitors. Elife 10, https://doi.org/10.7554/eLife.63429 (2021).
Kwon, D. H. et al. Heat-dependent opening of TRPV1 in the presence of capsaicin. Nat. Struct. Mol. Biol. 28, 554–563 (2021).
Pumroy, R. A. et al. Molecular mechanism of TRPV2 channel modulation by cannabidiol. Elife 8, https://doi.org/10.7554/eLife.48792 (2019).
Shimada, H. et al. The structure of lipid nanodisc-reconstituted TRPV3 reveals the gating mechanism. Nat. Struct. Mol. Biol. 27, 645–652 (2020).
Inada, H., Procko, E., Sotomayor, M. & Gaudet, R. Structural and biochemical consequences of disease-causing mutations in the ankyrin repeat domain of the human TRPV4 channel. Biochemistry 51, 6195–6206 (2012).
Fluck, E. C., Yazici, A. T., Rohacs, T. & Moiseenkova-Bell, V. Y. Structural basis of TRPV5 regulation by physiological and pathophysiological modulators. Cell Rep 39, 110737 (2022).
McGoldrick, L. L. et al. Opening of the human epithelial calcium channel TRPV6. Nature 553, 233–237 (2018).
Suo, Y. et al. Structural insights into electrophile irritant sensing by the human TRPA1 channel. Neuron 105, 882–894.e885 (2020).
Zhao, C. & MacKinnon, R. Structural and functional analyses of a GPCR-inhibited ion channel TRPM3. Neuron 111, 81–91.e87 (2023).
Ruan, Z. et al. Structures of the TRPM5 channel elucidate mechanisms of activation and inhibition. Nat. Struct. Mol. Biol. 28, 604–613 (2021).
Duan, J. et al. Structure of the mammalian TRPM7, a magnesium channel required during embryonic development. Proc. Natl Acad. Sci. USA 115, E8201–E8210 (2018).
Zhao, C. et al. Structures of a mammalian TRPM8 in closed state. Nat. Commun. 13, 3113 (2022).
Gan, N. et al. Structural mechanism of allosteric activation of TRPML1 by PI(3,5)P(2) and rapamycin. Proc. Natl Acad. Sci. USA 119, https://doi.org/10.1073/pnas.2120404119 (2022).
Viet, K. K. et al. Structure of the human TRPML2 Ion channel extracytosolic/lumenal domain. Structure 27, 1246–1257.e1245 (2019).
Thapak, P., Bishnoi, M. & Sharma, S. S. Tranilast, a transient receptor potential vanilloid 2 channel (TRPV2) inhibitor attenuates amyloid beta-induced cognitive impairment: possible mechanisms. Neuromol. Med. 24, 183–194 (2022).
Laragione, T., Harris, C. & Gulko, P. S. Combination therapy of a TRPV2 agonist with a TNF inhibitor achieves sustained suppression of disease severity and reduced joint damage. Clin. Exp. Immunol. 211, 233–238 (2022).
Zhang, L. et al. Cannabinoid non-cannabidiol site modulation of TRPV2 structure and function. Nat. Commun. 13, 7483 (2022).
Wang, Y. et al. TRPV3 enhances skin keratinocyte proliferation through EGFR-dependent signaling pathways. Cell Biol. Toxicol. 37, 313–330 (2021).
Su, W. et al. TRPV3: structure, diseases and modulators. Molecules 28, 774 (2023).
Lawhorn, B. G., Brnardic, E. J. & Behm, D. J. Recent advances in TRPV4 agonists and antagonists. Bioorg. Med. Chem. Lett. 30, 127022 (2020).
Xing, Y. et al. Stimulating TRPM7 suppresses cancer cell proliferation and metastasis by inhibiting autophagy. Cancer Lett 525, 179–197 (2022).
Cabanas, H. et al. Potential therapeutic benefit of low dose naltrexone in myalgic encephalomyelitis/chronic fatigue syndrome: role of transient receptor potential melastatin 3 ion channels in pathophysiology and treatment. Front. Immunol. 12, 687806 (2021).
Jiang, L. H., Gamper, N. & Beech, D. J. Properties and therapeutic potential of transient receptor potential channels with putative roles in adversity: focus on TRPC5, TRPM2 and TRPA1. Curr. Drug Targets 12, 724–736 (2011).
Lu, M. et al. A Selective TRPC3 inhibitor Pyr3 attenuates myocardial ischemia/reperfusion injury in mice. Curr. Med. Sci. 40, 1107–1113 (2020).
Nagib, M. M. et al. Inhibition of TRPC3 channels by a novel pyrazole compound confers antiseizure effects. Epilepsia 63, 1003–1015 (2022).
Doleschal, B. et al. TRPC3 contributes to regulation of cardiac contractility and arrhythmogenesis by dynamic interaction with NCX1. Cardiovasc. Res. 106, 163–173 (2015).
Zernov, N. et al. New positive TRPC6 modulator penetrates blood-brain barrier, eliminates synaptic deficiency and restores memory deficit in 5xFAD mice. Int. J. Mol. Sci. 23, 13552 (2022).
Pu, Y. et al. TRPC6 ameliorates renal ischemic reperfusion injury by inducing Zn(2+) influx and activating autophagy to resist necrosis. Ann. Transl. Med. 10, 249 (2022).
Ding, M. et al. Pyrazolo[1,5-a]pyrimidine TRPC6 antagonists for the treatment of gastric cancer. Cancer Lett 432, 47–55 (2018).
Morelli, M. B. et al. Transient receptor potential mucolipin-1 channels in glioblastoma: role in patient’s survival. Cancers 11, 525 (2019).
Xia, Z. et al. ML-SA1, a selective TRPML agonist, inhibits DENV2 and ZIKV by promoting lysosomal acidification and protease activity. Antivir. Res. 182, 104922 (2020).
Kriegler, K., Leser, C., Mayer, P. & Bracher, F. Effective chiral pool synthesis of both enantiomers of the TRPML inhibitor trans-ML-SI3. Arch. Pharm. 355, e2100362 (2022).
Brown, D. C., Agnello, K. & Iadarola, M. J. Intrathecal resiniferatoxin in a dog model: efficacy in bone cancer pain. Pain 156, 1018–1024 (2015).
Peppin, J. F. et al. Tolerability of NGX-4010, a capsaicin 8% patch for peripheral neuropathic pain. J. Pain Res 4, 385–392 (2011).
Inoue, M., Fujita, T. & Goto, M. & Kumamoto, E. Presynaptic enhancement by eugenol of spontaneous excitatory transmission in rat spinal substantia gelatinosa neurons is mediated by transient receptor potential A1 channels. Neuroscience 210, 403–415 (2012).
Brown, D. C. Resiniferatoxin: the evolution of the “molecular scalpel” for chronic pain relief. Pharmaceuticals. 9, 47 (2016).
Manitpisitkul, P. et al. A multiple-dose double-blind randomized study to evaluate the safety, pharmacokinetics, pharmacodynamics and analgesic efficacy of the TRPV1 antagonist JNJ-39439335 (mavatrep). Scand. J. Pain 18, 151–164 (2018).
Arsenault, P. et al. NEO6860, modality-selective TRPV1 antagonist: a randomized, controlled, proof-of-concept trial in patients with osteoarthritis knee pain. Pain Rep 3, e696 (2018).
Khalid, S. et al. Transient receptor potential vanilloid 1 (TRPV1) antagonism in patients with refractory chronic cough: a double-blind randomized controlled trial. J. Allergy Clin. Immunol. 134, 56–62 (2014).
Round, P., Priestley, A. & Robinson, J. An investigation of the safety and pharmacokinetics of the novel TRPV1 antagonist XEN-D0501 in healthy subjects. Br. J. Clin. Pharm. 72, 921–931 (2011).
Privitera, R. & Anand, P. Capsaicin 8% patch Qutenza and other current treatments for neuropathic pain in chemotherapy-induced peripheral neuropathy (CIPN). Curr. Opin. Support Palliat. Care 15, 125–131 (2021).
Lee, Y. W. et al. Efficacy and safety of PAC-14028 cream - a novel, topical, nonsteroidal, selective TRPV1 antagonist in patients with mild-to-moderate atopic dermatitis: a phase IIb randomized trial. Br. J. Dermatol. 180, 1030–1038 (2019).
Borbiro, I. et al. Activation of transient receptor potential vanilloid-3 inhibits human hair growth. J. Invest. Dermatol. 131, 1605–1614 (2011).
Othman, A. A., Nothaft, W., Awni, W. M. & Dutta, S. Pharmacokinetics of the TRPV1 antagonist ABT-102 in healthy human volunteers: population analysis of data from 3 phase 1 trials. J. Clin. Pharm. 52, 1028–1041 (2012).
Othman, A. A., Nothaft, W., Awni, W. M. & Dutta, S. Effects of the TRPV1 antagonist ABT-102 on body temperature in healthy volunteers: pharmacokinetic/ pharmacodynamic analysis of three phase 1 trials. Br. J. Clin. Pharm. 75, 1029–1040 (2013).
Moreno-Montanes, J., Bleau, A. M. & Jimenez, A. I. Tivanisiran, a novel siRNA for the treatment of dry eye disease. Expert Opin. Investig. Drugs 27, 421–426 (2018).
Heber, S. et al. TRPV1 antagonist BCTC inhibits pH 6.0-induced pain in human skin. Pain 161, 1532–1541 (2020).
Abdulqawi, R. et al. P2X3 receptor antagonist (AF-219) in refractory chronic cough: a randomised, double-blind, placebo-controlled phase 2 study. Lancet 385, 1198–1205 (2015).
Kuebler, W. M., Jordt, S. E. & Liedtke, W. B. Urgent reconsideration of lung edema as a preventable outcome in COVID-19: inhibition of TRPV4 represents a promising and feasible approach. Am. J. Physiol. Lung Cell Mol. Physiol. 318, L1239–L1243 (2020).
Klein, A. H., Carstens, M. I. & Carstens, E. Eugenol and carvacrol induce temporally desensitizing patterns of oral irritation and enhance innocuous warmth and noxious heat sensation on the tongue. Pain 154, 2078–2087 (2013).
Kim, H. O. & Jin Cheol, K. Yu Gyeong, K. & In Suk, K. Itching caused by TRPV3 (transient receptor potential vanilloid-3) activator application to skin of burn patients. Medicina (Kaunas) 56, 560 (2020).
Duchatelet, S. et al. A new TRPV3 missense mutation in a patient with Olmsted syndrome and erythromelalgia. JAMA Dermatol 150, 303–306 (2014).
Matsumura, T. et al. A pilot study of tranilast for cardiomyopathy of muscular dystrophy. Intern. Med. 57, 311–318 (2018).
Robbins, N. et al. Probenecid improves cardiac function in patients with heart failure with reduced ejection fraction in vivo and cardiomyocyte calcium sensitivity in vitro. J. Am. Heart Assoc. 7, https://doi.org/10.1161/JAHA.117.007148 (2018).
Chan, P. et al. Translational and pharmacokinetic-pharmacodynamic application for the clinical development of GDC-0334, a novel TRPA1 inhibitor. Clin. Transl. Sci. 14, 1945–1954 (2021).
Wetzels, S. et al. Methylglyoxal-derived advanced glycation endproducts accumulate in multiple sclerosis lesions. Front. Immunol. 10, 855 (2019).
Dull, M. M. et al. Methylglyoxal causes pain and hyperalgesia in human through C-fiber activation. Pain 160, 2497–2507 (2019).
Gyamfi, O. A. et al. Analysis of TRPA1 antagonist, A-967079, in plasma using high-performance liquid chromatography tandem mass-spectrometry. J. Pharm. Anal. 10, 157–163 (2020).
Behringer, M., Nowak, S., Leyendecker, J. & Mester, J. Effects of TRPV1 and TRPA1 activators on the cramp threshold frequency: a randomized, double-blind placebo-controlled trial. Eur. J. Appl. Physiol. 117, 1641–1647 (2017).
Kanezaki, M., Terada, K. & Ebihara, S. Effect of olfactory stimulation by L-menthol on laboratory-induced dyspnea in COPD. Chest 157, 1455–1465 (2020).
Colvin, L. A. et al. From bench to bedside: a case of rapid reversal of bortezomib-induced neuropathic pain by the TRPM8 activator, menthol. J. Clin. Oncol. 26, 4519–4520 (2008).
Mukerji, G. et al. Cool and menthol receptor TRPM8 in human urinary bladder disorders and clinical correlations. BMC Urol 6, 6 (2006).
Weyer, A. D. & Lehto, S. G. Development of TRPM8 antagonists to treat chronic pain and migraine. Pharmaceuticals 10, 37 (2017).
Horne, D. B. et al. Discovery of TRPM8 Antagonist (S)-6-(((3-Fluoro-4-(trifluoromethoxy)phenyl)(3-fluoropyridin-2-yl)methyl)carbamoyl)nicotinic Acid (AMG 333), a Clinical Candidate for the Treatment of Migraine. J. Med. Chem. 61, 8186–8201 (2018).
Amato, A. et al. TRPM8 channel activation reduces the spontaneous contractions in human distal colon. Int. J. Mol. Sci. 21, 5403 (2020).
Sugino, S. et al. Association between the cool temperature-dependent suppression of colonic peristalsis and transient receptor potential melastatin 8 activation in both a randomized clinical trial and an animal model. J. Neurogastroenterol. Motil. 28, 693–705 (2022).
Yang, J. M. et al. A novel TRPM8 agonist relieves dry eye discomfort. BMC Ophthalmol 17, 101 (2017).
Xu, C. et al. Association of the putative susceptibility gene, transient receptor potential protein melastatin type 2, with bipolar disorder. Am. J. Med. Genet. B Neuropsychiatr. Genet. 141B, 36–43 (2006).
Dyment, D. A. et al. De novo substitutions of TRPM3 cause intellectual disability and epilepsy. Eur. J. Hum. Genet. 27, 1611–1618 (2019).
Cabanas, H., Muraki, K., Staines, D. & Marshall-Gradisnik, S. Naltrexone restores impaired transient receptor potential melastatin 3 ion channel function in natural killer cells from myalgic encephalomyelitis/chronic fatigue syndrome patients. Front. Immunol. 10, 2545 (2019).
Norton, N. et al. Association of genetic variants at TRPC6 with chemotherapy-related heart failure. Front. Cardiovasc. Med. 7, 142 (2020).
Acknowledgements
The authors are grateful to Dr. Y Jiang of Lingang Laboratory for his help with the preparation of the structural diagram in this paper. This work was supported by grants from the National Key Research and Development Program of China (2022YFC2303504), the Program of the Shanghai Committee of Science and Technology, China (21ZR1475200 and 22ZR1469900), the National Natural Science Foundation of China (92157106, 32270917), the Strategic Priority Research Program of the Chinese Academy of Sciences (XDPB16), Shanghai Municipal Science and Technology Major Project (2019SHZDZX02).
Author information
Authors and Affiliations
Contributions
B.W., L.P., and N.Z. contributed to the conception of the review, M.Z. contributed significantly to the complete manuscript preparation, and Y.M.M. and X.L.Y. contributed to the constructive discussions. All authors have read and approved the article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, M., Ma, Y., Ye, X. et al. TRP (transient receptor potential) ion channel family: structures, biological functions and therapeutic interventions for diseases. Sig Transduct Target Ther 8, 261 (2023). https://doi.org/10.1038/s41392-023-01464-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41392-023-01464-x
- Springer Nature Limited
This article is cited by
-
Matrix stiffness affects tumor-associated macrophage functional polarization and its potential in tumor therapy
Journal of Translational Medicine (2024)
-
Magnetogenetics as a promising tool for controlling cellular signaling pathways
Journal of Nanobiotechnology (2024)
-
Calcium signalling and transport in the kidney
Nature Reviews Nephrology (2024)