Abstract
In this work the temperature dependence of the Soret band line shape in carbonmonoxy myoglobin is re-analyzed by using both the full correlator approach in the time domain and the frequency domain approach. The new analyses exploit the full density of vibrational states of carbonmonoxy myoglobin available from normal modes analysis, and avoid the artificial division of the entire set of vibrational modes coupled to the Soret transition into “high-frequency” and “low-frequency” subsets; the frequency domain analysis, however, makes use of the so-called short-times approximation, while the time domain one avoids it. Time domain and frequency domain analyses give very similar results, thus supporting the applicability of the short-times approximation to the analysis of hemeprotein spectra; in particular, they clearly indicate the presence of spectral heterogeneity in the Soret band of carbonmonoxy myoglobin. The analyses also show that a temperature dependence of the Gaussian width parameter steeper than the hyperbolic cotangent law predicted by the Einstein harmonic oscillator and/or a temperature dependence of inhomogeneous broadening are not sufficient to obtain quantitative information on the magnitude of anharmonic contributions to the iron–heme plane motion. However, the dependence of the previous two quantities may be used to obtain semiquantitative information on the overall coupling of the Soret transition to the low-frequency modes and therefore on the dynamic properties of the heme pocket in different states of the protein.









Similar content being viewed by others
Notes
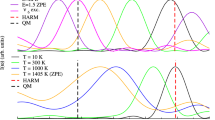
Use of the entire set of low-frequency modes reported in Fig. 2 with a single value for the coupling constant S could, in principle, be questioned. In fact, the density of vibrational states in Fig. 2 has to be “weighted” by the coupling of each mode to the Soret transition. With the Soret transition localized on the heme, only normal modes with a significant eigenvector contribution from the heme macrocycle and/or from atoms in close proximity of the heme should be considered; moreover, in view of the almost planar heme conformation in MbCO, in-plane vibrations are expected to couple much more strongly than out-of-plane vibrations. On the other hand, information on the coupling constants is not directly obtainable from low-frequency Raman spectra, even with Soret excitation, owing to their smallness (weak coupling with a large number of modes). In order to circumvent this difficulty and to check the influence of the previous assumptions on the results of the analyses, we used three different “weighted density of states”: (1) the one reported in Fig. 2; (2) a frequency-independent density of states, using the average value of the distribution of Fig. 2; (3) a third distribution of equal area but in which the maximum was shifted to 150 cm−1. The same three distributions were also used for the time domain analysis (see the “Time domain approach” section). For the three distributions the constant S value approximation was used. For all the distributions used the temperature dependence of relevant parameters (i.e., σ2 for the frequency domain analysis and σ2inh for the time domain analysis) remained essentially unaltered. We therefore conclude that the assumptions used, although crude, do not alter the main conclusions of the analyses.
References
Austin RH, Beeson KW, Eisenstein L, Frauenfelder H, Gunsalus IC (1975) Dynamics of ligand binding to myoglobin. Biochemistry 14:5355–5373
Bangcharoenpaurpong O, Schomacker KT, Champion PM (1984) A resonance Raman investigation of myoglobin and hemoglobin. J Am Chem Soc 106:5688–5698
Chan CK, Page JB (1983) Temperature effects in the time correlator theory of resonance Raman scattering. J Chem Phys 79:5234–5250
Chan CK, Page JB (1984) T≠0 K Multimode modeling of optical absorption spectra and resonance Raman profiles. Chem Phys Lett 104:609–614
Cupane A, Leone M, Vitrano E, Cordone L (1995) Low temperature optical absorption spectroscopy: an approach to the study of stereodynamic properties of hemeproteins. Eur Biophys J 23:385–398
Cupane A, Leone M, Cordone L, Gilch H, Dreybrodt W, Unger E, Schweitzer-Stenner R (1996) Conformational properties of Nickel(II)–Octaethylporphyrin in solution 2 A low temperature optical absorption spectroscopy study. J Chem Phys 100:14192–14197
Cupane A, Leone M, Unger E, Lemke C, Beck M, Dreybrodt W, Schweitzer-Stenner R (1998) Dynamics of various metal-octaethylporphyrins studied by resonance Raman and low-temperature optical absorption spectroscopies. Role of the central metal. J Phys Chem B102:6612–6620
Di Pace A, Cupane A, Leone M, Vitrano E, Cordone L (1992) Protein dynamics: vibrational coupling, spectral broadening mechanisms and anharmonicity effects in carbonmonoxy heme proteins studied by the temperature dependence of the Soret band lineshape. Biophys J 63:475–484
Doster W, Cusack S, Petry W (1989) Dynamical transition of myoglobin revealed by inelastic neutron scattering. Nature 337:754–756
Frauenfelder H, Parak F, Young RD (1988) Conformational substates in proteins. Annu Rev Biophys Biophys Chem 17:451–459
Leone M, Cupane A, Militello V, Cordone L (1994) Thermal broadening of the Soret band in heme complexes and in heme proteins: role of the iron dynamics. Eur Biophys J 23:349–352
Melchers B, Knapp EW, Parak F, Cordone L, Cupane A, Leone M (1996) Structural fluctuations of myoglobin from normal modes, Mössbauer, Raman and absorption spectroscopy. Biophys J 70:2092–2099
Parak F, Knapp EW, Kucheida D (1982) Protein dynamics. Mossbauer spectroscopy on deoxymyoglobin crystals. J Mol Biol 161:177–194
Schomacker KT, Champion PM (1986) Investigations of spectral broadening mechanisms in biomolecules: Cytochrome-c. J Chem Phys 84:5314–5325
Schweitzer-Stenner R, Bigman D (2001) Electronic and vibronic contributions to the band splitting in optical spectra of heme proteins. J Chem Phys B 105:7064–7073
Srajer V, Schomacker KT, Champion PM (1986) Spectral broadening in biomolecules. Phys Rev Lett 57:1267–1270
Acknowledgements
This work was supported by a grant (COFIN 2000) from the Italian Ministry of Education, University and Research and the Deutsche Forschungsgemeinschaft SFB 433.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cupane, A., Cammarata, M., Cordone, L. et al. Spectral broadening of the Soret band in myoglobin: an interpretation by the full spectrum of low-frequency modes from a normal modes analysis. Eur Biophys J 34, 881–889 (2005). https://doi.org/10.1007/s00249-004-0458-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00249-004-0458-4