Abstract
Biofilms are considered a basal resource with high nutritional quality in stream food webs, as periphytic algae are abundant of polyunsaturated fatty acids (PUFAs). PUFAs are essential for growth and reproduction of consumers who cannot or have very limited capacity to biosynthesize. Yet, how the nutritional quality based on PUFA of basal food sources changes with light intensity remains unclear. We conducted a manipulative experiment in mesocosms to explore the response and mechanisms of nutritional quality to shading, simulating riparian restoration. We found a significant increase in PUFA% (including arachidonic acid, ARA) under shading conditions. The increased PUFA is caused by the algal community succession from Cyanobacteria and Chlorophyta to Bacillariophyta which is abundant of PUFA (especially eicosapentaenoic acid, EPA; docosahexaenoic acid, DHA). On the other hand, shading increased PUFA via upregulating enzymes such as Δ12 desaturase (FAD2, EC:1.14.19.6) and 3-ketoacyl-CoA synthase (KCS, EC:2.3.1.199) in the biosynthesis of unsaturated fatty acid elongation pathways. Our findings imply that riparian reforestation by decreasing light intensity increases the nutritional quality of basal resources in streams, which may enhance transfer of good quality carbon to consumers in higher trophic levels through bottom-up effects.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Biofilms are microbial communities attached to various substrates (e.g. plants, wood piles, rocks) in submerged surfaces and are composed of autotrophic and heterotrophic microorganisms including algae, bacteria, fungi, and protozoa [1, 2]. The primary producers in biofilms, i.e., algae, therein play a fundamental role in primary production as basal resources for consumers in stream food webs [3,4,5]. Algae, in particular Bacillariophyta, usually containing abundant polyunsaturated fatty acid (PUFA), provide high-quality food for herbivores compared to allochthonous sources, e.g. terrestrial litter in river ecosystems [6,7,8]. The indispensability of PUFAs for growth, neural development, and reproduction in aquatic invertebrate consumers has been well-established [9, 10]. Nevertheless, PUFA are either incapable of being synthesized by animals or are synthesized at a negligible rate, which often choose direct sources from high nutritional quality basal resources—algae in biofilms [11, 12].
Algae are sensitive to changes in ambient environmental variables [13, 14]. As riparian canopy has been opened led by deforestation for agricultural practices, the amount of light that reaches the stream channel has been altered, consequently influencing the composition of benthic algae [14]. The composition of stream periphytic algal communities is commonly dominated by Bacillariophyta, Chlorophyta, and Cyanobacteria [15]. Cyanobacteria, which lack eicosapentaenoic acid (EPA, C20:5n3) and docosahexaenoic acid (DHA, C22:6n3), are abundant in α-linolenic acid (ALA; C18:3n-3), a precursor fatty acid that can be synthesized into EPA and DHA [16, 17]. Chlorophyta, usually required high light levels [18], are considered to be of medium dietary quality, containing short-chain PUFA such as ALA and linoleic acids (LIN, C18:2n6) but relatively deficient in EPA [17]. Bacillariophyta favour lower light than Chlorophyta [19], and are considered high-quality food sources for aquatic invertebrates due to their content of long-chain PUFA (LC-PUFA, including ARA, EPA, and DHA) [20]. Hence, shifts in community composition result in changes of fatty acid profiles in biofilms, subsequently influencing the availability of n-3 and n-6 PUFA [1, 21, 22].
Transcriptomic analysis could provide a molecular perspective on fatty acid synthesis. It has observed downregulation of genes in the photosynthetic apparatus that encode key proteins such as light-harvesting complexes (LHC) components under reduced light conditions [23]. Downregulation in fatty acid synthesis such as acetyl-CoA carboxylase (ACCase) and fatty acid synthase (FAS) could lead to a reduction in overall lipid content [24]. Moreover, key enzymes in the PUFA biosynthesis pathway, such as desaturases and elongases, exhibit altered expression levels under different light intensity [23, 25].
In pristine forested streams, biofilms are regulated directly from the bottom-up by light and nutrient availability, while in deforested streams, light limitation on biofilms is expected to be relaxed [26]. As human activities alter light regimes in many streams such as deforestation (increased light) [27], the alteration of biofilms nutritional quality is likely to cascade upward and influence organisms in higher trophic levels [28, 29]. To counteract these changes and promote instream ecosystem recovery, riparian revegetation is usually undertaken by focusing on the crucial functions that riparian zones provide for aquatic ecosystems [30]. While studies have conducted manipulative experiments in the field to examine the effects of abiotic factors on FA profiles [28, 29, 31], others have focused on exploring the transcriptional responses of specific algal species to different light intensities [23, 32, 33]. However, it remains unclear how the fatty acid composition is molecularly adjusted, coupled with community succession, with riparian revegetation.
In this study, we hypothesized that (i) shading, change in light intensity, would lead to alteration of PUFA in biofilms FA profiles and (ii) the alteration of FA profiles is attributable not only to the succession of algal community but also to the changes in differentially expressed genes in metabolic pathways. We intended to reveal change of nutritional quality of basal resources in food webs with multidimensional mechanisms at algal community and molecular levels, i.e., lipid metabolism and energy metabolism. Our study sheds light on the changes of the nutritional quality in biofilms with riparian deforestation.
Materials and Methods
Manipulative Experiment in Mesocosm
The manipulative experiment was performed in a greenhouse in Wuhan Botanical Garden, Chinese Academy of Sciences in 2021 for a period of 35 days (from June 18 to July 23). Six cylindrical polyethylene buckets (volume: 1000 L, diameter: 1.2 m, height: 0.8 m) were used to simulate stream ecosystems. A pump was installed in each mesocosm to allow the flowing of water (Fig. 1a). The bottom of the mesocosms was covered with a layer of cobbles. To closely simulate natural river biofilms, we collected cobbles already colonized by biofilms from the Chuka River (115°19′10″E, 31°5′50″N) in Huanggang City, Hubei Province (Fig. 1b), an area with minimal human disturbance. We collected the cobbles with colonized biofilms from the Chuka River between 10:00 and 14:00 in a day, during which the water temperature in unshaded areas ranged from 28.4 to 31.6 °C. These cobbles were subsequently placed in the mesocosms for 1 week, allowing the biofilms to acclimate to their new habitats before the experiments started.
Six mesocosms were divided into two groups. One group was covered with shade cloth to simulate riparian canopy conditions (shading groups), while the other group was exposed to ambient light and served as the control (ambient groups). Initial properties of the water were total dissolved nitrogen (TDN) of 1.34 ± 0.04 mg/L and total dissolved phosphorus (TDP) of 0.02 ± 0.00 mg/L. At noon, an illuminometer measured light intensity, revealing 141,000 ± 29,813 lx under ambient light and 827 ± 113 lx in shaded conditions. It should be noted that this significant reduction in light intensity is not uniform throughout the day, as light intensity naturally fluctuates with the time of day.
Sample Collection
Physical and Chemical Variables
In mesocosm, water temperature (WT), pH, dissolved oxygen (DO), electrical conductivity (EC), and ammonium (NH4+) were measured using a YSI EXO3 multi-parameter water quality meter (YSI Inc., Yellow Springs, OH, USA). The sensors were calibrated before measurements were taken. The samples of the surface water at each mesocosm in triplicates were collected and filtered using cellulose nitrate membrane filters (Whatman, 0.45 µm pore size) for the analysis of total dissolved nutrient, specifically total dissolved nitrogen (TDN), total carbon (TC), and total dissolved phosphorus (TDP). Water samples were measured and collected in pre- and post-treatment, as well as once a week throughout the shading treatment. Samples for analysis of nutrients were stored on ice in a cooler, and then frozen before analysis.
Biofilms
Five different cobbles were randomly collected, and an area with a 10-cm diameter on each rock was brushed. The biofilms from five rocks was rinsed with distilled water into one container forming a composite sample of around 50 mL as one replicate. The 5-mL samples were taken from 50 mL and preserved in 4% formaldehyde. The remaining samples (45 mL) were stored at − 80 ℃ and used for fatty acid analysis and transcriptome sequencing. Biofilms were filtered onto pre-ashed glass fibre filters (0.7 µm; Whatman GF/F filters) and stored at − 20 ℃ for FA analysis. Three replicates were made at each treatment.
Laboratory Analyses
Physical and Chemical Variables
Both TC and TDN were measured using a TOC/TN analyzer equipped with different modules for measuring these components (Elementar Corporation). Total dissolved phosphorus (TDP) was measured by inductively coupled plasma atomic emission spectrometer (ICP-OES) (Thermo Fisher, X Series 2, USA).
Biofilms Collected for Identification and FA Analysis
The biofilms containing samples (5 mL) for algal identification and counting were preserved in plastic bottles with Lugol’s solution. The samples were thoroughly mixed, and a 1-mL subsample was taken for identification and enumeration, conducted using a Sedgewick Rafter cell under a microscope (Olympus BX51, Olympus Corporation, Tokyo, Japan) with 400 × magnification [34]. Enumeration ceased when 40 squares were counted under the microscope. The slide was then scanned to 200 squares at 200 × magnification to identify any algae not previously sighted [35]. Taxa were combined into categories (Bacillariophyta, Cyanobacteria, and Chlorophyta) for analysis, and the relative proportions of each category were calculated based on the number of cells.
Prior to fatty acid analyses, samples were freeze dried for 48 h and homogenized. The three replicate samples from the same mesocosm were pooled before analyses to guarantee a sufficient amount of sample material. Lipids were extracted and methylated from the pooled samples (400 mg) using the following method [36]: A 15-mL mixture of 0.2 M KOH:MeOH (1:1) was maintained at 50 °C for 60 min, with the tubes vortexed every 10 min to lyse and saponify the cells. At the end of this process, 10 mL of n-hexane were added to extract the fatty acid methyl esters (FAMEs) from the acidic aqueous phase into the organic phase, and the upper organic layer was transferred. To ensure thorough extraction of the FAMEs, this process was repeated twice. To enhance the detection rate of FAMEs, the organic solvent was dried and concentrated under N2, and an internal standard solution (methyl undecanoate) was added.
FAMEs were analyzed using a gas chromatograph equipped with a mass detector (GC–MS, Agilent 7890B + 5975C, USA), which is equipped with a temperature programmable injector and an autosampler. Temperatures of the interface and ion source were 270 °C and 230 °C, respectively. Agilent J&W HP-88 column was used with the following temperature program: 100 ℃ was maintained for 5 min, then to 170 ℃ at a rate of 10 ℃/min for 8 min, then to 200 ℃ at a rate of 1 ℃/min for 20 min, and finally heated to 230 ℃ at a rate of 8 ℃/min for 10 min. Total program time was 84.75 min and solvent cut time 10 min. Helium was used as carrier gas, constant pressure mode is at 32 psi, injection volume is 1 µL, and split flow is 5:1. The identity of the FAMEs was determined by comparing the retention times of unknown sample peaks with those of known standards, including a 37-component FAME mix (Supelco No. 47885-U) and Bacterial Acid Methyl Ester Mix (Supelco No. 47080-U). Fatty acid concentrations were calculated using free fatty acid of C11:0 as internal standards. FA results were expressed as percentages relative to total FA (FA %).
Transcriptome Data Processing
RNA Extraction, Library Construction, and Sequencing
Total RNAs were extracted from the biofilm samples with four replicates using TRIzol reagent according the manufacturer’s instructions (Invitrogen). RNA quality was determined using 2100 Bioanalyzer (Agilent) and quantified using the ND-2000 (NanoDrop Technologies). High-quality RNA sample (OD260/280 ≥ 1.8, OD260/230 ≥ 1.0, RIN ≥ 6.5, 28S: 18S ≥ 1.0, > 1 µg) was used to construct sequencing library.
RNA purification, reverse transcription, library construction, and sequencing were conducted by Shanghai Majorbio Bio-Pharm Biotechnology Co., Ltd. (Shanghai, China). For the biofilms RNA-seq transcriptome library, 1 µg of total RNA was employed. Initially, messenger RNA was isolated through the poly(A) selection method using oligo (dT) beads and subsequently fragmented using a fragmentation buffer. Next, double-stranded cDNA was synthesized using the SuperScript double-stranded cDNA synthesis kit (Invitrogen, CA) with random hexamer primers (Illumina). Libraries were size selected for cDNA target fragments of 200–300 bp on 2% Low Range Ultra Agarose followed by PCR amplified using Phusion DNA polymerase for 15 PCR cycles. The PCR protocol included an initial denaturation step at 98 °C for 30 s, followed by 15 cycles of denaturation at 98 °C for 10 s, annealing at 60 °C for 15 s, and extension at 72 °C for 30 s. A final extension was performed at 72 °C for 5 min. After quantified by TBS380, paired-end libraries were sequenced by Illumina NovaSeq 6000 sequencing.
Transcriptome Assembly and Functional Annotation
For quality control, the raw reads were processed using SOAPnuke software (v2.1.0) to obtain high-quality clean reads. This involved steps such as image recognition, decontamination, removal of joints, removal of adaptor sequences, ambiguous reads (“N”), and low-quality reads (reads with more than 10% ambiguous bases or with bases below a quality score of 20) [37, 38]. Then, clean data from the biofilms (“shaded groups” and “ambient groups”) were used to do de novo assembly with Trinity software [39].
Gene function annotation utilized BLASTx with an E-value threshold of 1.0 × 10−5 against the following databases: NR (NCBI nonredundant protein sequences), KEGG (Kyoto Encyclopedia of Genes and Genomes), COG (Clusters of Orthologous Groups of proteins), Swiss-Prot, Pfam, and GO (Gene Ontology).
Differential Expression Genes (DEGs) Analysis and Functional Enrichment
To identify differential expression genes (DEGs) between two groups, we quantified transcript abundances using the transcripts per million reads (TPM) method and RSEM [38]. Differential expression analysis of two groups was conducted using the DEGseq R package. DEGs were identified based on a Poisson distribution with a false discovery rate (FDR) ≤ 0.001 and a fold change ≥ 2 (log2 ratio ≥ 1) between the two groups. In addition, functional enrichment analysis including GO and KEGG was performed to identify which DEGs were significantly enriched in GO terms at Bonferroni-corrected p-value ≤ 0.05 compared with the transcriptome background.
Data Analysis
All data were obtained by using at least three biological samples to ensure the fidelity of the results. The following FA were used for data analyses: LIN, ALA, ARA, EPA, DHA, the sum of saturated fatty acids (SAFA), the sum of monounsaturated fatty acids (MUFA), the sum of polyunsaturated fatty acids (PUFA), the sum of long-chain polyunsaturated fatty acids (LC-PUFA, typically containing 20 or more carbon atoms), and the sum of C18 PUFA. The independent samples T test was used to test the effects of shading on biofilms’ FA profiles and algal community structure after 5 weeks treatment. The statistical analyses were conducted in the statistical software IBM SPSS Statistics 22.
In transcriptome data, the DEGs output was further enriched through GO and KEGG annotation, with enrichment measured by rich factor, -log10 (p-value), and gene number. The results of gene set enrichment analysis (GSEA) are typically reported as enrichment scores, which indicate the level of enrichment of a specific gene set within the ranked list. Significantly enriched gene sets were selected based on the following: a significance level of p < 0.05 and an adjusted p-value (FDR) threshold of < 0.25. Statistical analyses were conducted using the statistical software R version 4.0.0 (R Core Team, 2020).
Result
Physicochemical Variables in the Mesocosms
At the end of the experiment, water temperatures (p = 0.100) and the concentration of dissolved nutrients, including total dissolved nitrogen (TDN) and total dissolved phosphorus (TDP), did not show significant changes in the shaded groups compared to the ambient groups (p = 0.381 and p = 0.944, respectively). Dissolved oxygen (DO) and pH exhibited significant decreases (p = 0.025 and p = 0.006, respectively), while electrical conductivity (EC) was significantly higher (p < 0.001) in the shaded groups. A relative increase in total carbon (TC) content was observed compared to the ambient groups (p = 0.101) (Table 1).
Algal Community Analysis
Analysis of the abundance of three primary algal groups—Bacillariophyta, Cyanobacteria, and Chlorophyta—showed that shading altered the periphyton algal community structure. Initially, 26 genera from three phyla of algae were identified. After 5 weeks of shading treatment, the number of genera within the phyla Bacillariophyta, Cyanobacteria, and Chlorophyta decreased in both shaded and ambient groups (Table 2). The average proportion of Bacillariophyta was significantly higher in the shaded groups (66.15%) compared to the ambient groups (52.66%) (p = 0.027). Conversely, Cyanobacteria were significantly lower in the shaded groups (1.87%) compared to the ambient groups (5.62%) (p = 0.035), while Chlorophyta showed borderline significance (p = 0.052) with a lower proportion in the shaded groups (Fig. 2, Table 2).
Fatty Acid Profiles of Biofilms
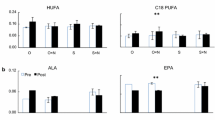
After 5 weeks of shading, total PUFA% and LC-PUFA% including ARA% significantly increased compared to the ambient groups (Fig. 3a, b). However, EPA% and DHA% did not change significantly (p = 0.986 and p = 0.904, respectively) (Fig. 3a). Additionally, SAFA% decreased significantly (p = 0.040), particularly C16:0% (p = 0.001). MUFA showed no significant change (p = 0.326) compared to the ambient groups, while the fatty acid biomarker C16:1n7 indicative of diatoms showed a significant increase (p = 0.041) (Fig. 3b, Table 3).
Fatty acid profiles in biofilms (% of total fatty acids, mean ± SD) in the ambient and shaded groups after 5-week shading treatment in mesocosms. a Five essential fatty acids: LIN, linoleic acid (C18:2n6); ALA, α-linoleic acid (C18:3n3); ARA, arachidonic acid (C20:4n6); EPA, eicosapentaenoic acid (C20:5n3); DHA, docosahexaenoic acid (C22:6n3). b Main fatty acid groups: PUFA, polyunsaturated fatty acids; C18 PUFA, C18 polyunsaturated fatty acids; LC-PUFA, long chain-polyunsaturated fatty acids; MUFA, monounsaturated fatty acid; SAFA, saturated fatty acids. *p < 0.05, **p < 0.01, ***p < 0.001
Transcriptome Analysis
Gene Function Annotation and Classification
The output of transcriptome sequencing of biofilms (shaded and ambient groups) was obtained (Table S1). A total of 832,836 unigenes which can be classified into three major GO categories: biological processes (BP), cell component (CC), and molecular function (MF) (Figure S1a). Genes of the BP group were divided into 25 subcategories with “cellular process” as the largest terms with 426,125 unigenes. The genes in the CC group were divided into 16 subcategories, and “cell part” as the largest terms. In the MF group, the genes were divided into 18 subcategories, where “catalytic activity” was the largest terms.
The metabolic pathway of KEGG was divided into five parts, “cellular processes”, “environmental information processing”, “genetic information processing”, “metabolism”, and “organismal systems” (Fig. S1b). Among these, the pathway represented by the most unigenes was “metabolism” (326,576, 68.80% of the total annotated transcripts). Within the “metabolism” category, carbohydrate metabolism (50,077, 15.33%) was the most represented, followed by energy metabolism (39,708, 12.16%). Our study specifically focuses on lipid metabolism, which ranked fifth at 24,479 unigenes (7.50%).
DEGs and Pathways Enrichment Analysis
Among the 53,908 DEGs, 27,397 genes were found to be significantly upregulated, while 26,511 genes were downregulated in the shaded group compared to the ambient group in response to shading treatment (Figure S2). The KEGG enriched pathways related to fatty acid metabolism, comprising photosynthesis of antenna proteins, photosynthesis, arachidonic acid metabolism, and carbon fixation in photosynthetic organisms, were found to be significant (Fig. 4).
The fatty acid biosynthesis pathway was downregulated, while the pathways for fatty acid elongation and the biosynthesis of unsaturated fatty acids were upregulated (Fig. 5). In the fatty acid biosynthesis pathway, the genes encoding the catalyzing enzymes acyl-ACP desaturase (FAB2, EC: 1.14.19.2) and fatty acyl-ACP thioesterase A (FATA, EC: 3.1.2.14) were significantly downregulated. In the fatty acid elongation pathway, the expression of genes trans-2-enoyl-CoA reductase (MECR, EC: 1.3.1.38) and palmitoyl protein thioesterase (PPT, EC: 3.1.2.22) in mitochondria, as well as 3-ketoacyl-CoA synthase (KCS, EC:2.3.1.199) in endoplasmic reticulum, was upregulated. In the biosynthesis of unsaturated FA pathway, Δ12 desaturase (FAD2, EC:1.14.19.6) actively participates and catalyzes the synthesis towards PUFA. Gene set enrichment analysis (GSEA) revealed significant enrichment in “Map00590” (arachidonic acid metabolism) (NES = 2.64, p < 0.001) and “Map00062” (fatty acid elongation) (NES = 1.51, p < 0.05) (Fig. 6a, b).
The effect of shading on the Calvin cycle, glycolysis, and fatty acid metabolism-related processes in biofilms. Key enzymes are presented as their names in red colours (upregulated) and in blue colours (downregulated). ACSL, long-chain acyl-CoA synthetase; ACP, transacylase; FAB2, acyl-ACP desaturase; FAD2, Δ12 desaturase; FATA, fatty acyl-ACP thioesterase A; KCS, 3-ketoacyl-CoA synthase; MECR, mitochondrial trans-2-enoyl-CoA reductase; PPT, palmitoyl protein thioesterase; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; HK, hexokinase; PK, pyruvate kinase; PDH, pyruvate dehydrogenase; Rubisco, ribulose-bisphosphate carboxylase; 1,3-bis-PG, 1,3-biphosphoglycerate; G-3-P, glyceraldehyde-3-phosphate; G-6-P, glucose-6-phosphate; PEP, phosphoenolpyruvate
In response to the shading, there was a downregulated key genes encoding the ferredoxin—NADP+ reductase (petH, EC:1.18.1.2) in the photosynthetic electron transport, which regulates NADPH synthesis (Fig. 7). Moreover, 11 out of the 12 genes (except LHCB7) encoding photosynthetic antenna proteins in the light-harvesting complex I (LHCA) and light-harvesting complex II (LHCB) families exhibited upregulation (Table S2). Most components of photosystem I (PSI), except for psaA and psaB, were upregulated. In contrast to PSI, the components associated with PSII, including PsbD, PsbK, and PsbS, were downregulated. Similarly, a substantial downregulated expressional level of three components of F-type H+ transporting ATPase (atpA, atpB, atpD, and atpH, Table S2) was observed. During the carbon fixation pathways, ribulose-1,5-bisphosphate (RuBP) was converted to glyceraldehyde-3-phosphate (G-3-P) by ribulose-bisphosphate carboxylase (Rubisco) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH, EC:1.2.1.12) with upregulated gene expression encoding GAPDH, while the Rubisco was downregulated (Fig. 7).
Transcriptional regulation on metabolic pathways involving photosynthesis in biofilms based on KEGG pathways. Key enzymes are presented as their names in red colours (upregulated) and in blue colours (downregulated). Cytb6, cytochrome b6/f complex subunit; petH, ferredoxin—NADP+ reductase; LHCA, light-harvesting complex I; LHCB, light-harvesting complex II; ATP synthase, F-type H+ transporting ATPase
Discussion
The advantage of manipulative experiments lies in their high controllability, whereas outdoor experiments are more closely natural environments. Compared to in situ experiments in rivers, this approach minimizes interference from human activity and extreme weather, thereby allowing for a more focused study on the mechanisms of shading on the fatty acid composition of biofilms. In our mesocosms, by manipulating light intensity and regulating water flow, we simulated shading and movement of river headwaters or upstream areas with dense canopy cover. We transported cobbles with colonized biofilms from natural rivers to ensure that the biofilms used in our experiments closely reflected the conditions and variation occurring in natural environments. Additionally, the water used in the mesocosms had total dissolved nitrogen (TDN) and total dissolved phosphorus (TDP) similar to those of the river from which the biofilms were collected, thereby ensuring that our findings are both relevant and applicable. To account for local variation in biofilm composition, we addressed it through random collection and composite sampling as previously described. Thus, our study provides a foundation that reveals the mechanisms underlying the impact of shading on basic resources.
Light availability, indirectly influenced by factors such as canopy cover, is a dominant abiotic factor affecting the community structure of benthic algae in biofilms [1, 40]. Lower light conditions can lead to a decrease in the concentration of short-chain PUFA such as LIN and ALA in biofilms, while EPA and ARA tend to increase [41, 42], and higher light intensity leads to excess carbon accumulation in storage lipids, such as SAFA and MUFA [43]. Several studies have debated the effect of light intensity on SAFA% [41, 42]. Even under open canopy conditions, light intensity may vary significantly and may account for the differing responses in SAFA. Our study observed a significant decrease in SAFA% in the shaded group compared to the ambient group, which aligns with the trend reported in a previous study [41]. In addition, SAFA accumulates under high temperature conditions, while PUFA tends to increase to maintain the fluidity of cell membranes under low temperature [44]. Although the water temperature in our mesocosms was higher than in the natural river, there was no significant difference between the shaded and ambient groups (Table 1).
The observed changes in PUFA may be attributed to physiological acclimation, specifically photoacclimation. In photoautotrophic eukaryotic microalgae, LC-PUFA primarily accumulates within complex polar lipids, such as glycolipids and phospholipids, which constitute the structural basis of cellular membranes [45, 46]. Photoautotrophic growth can be limited by light availability [47]. However, photoacclimation to low irradiance involves the development of associated thylakoid membranes, thereby enhancing the absorption of limited photons [41, 48]. Prior research suggests that photoacclimation likely increases PUFA content due to its abundance in thylakoid membranes [49]. In our study, increased PUFA% was consistent with a photoacclimation mechanism, involving the enhanced synthesis or desaturation of thylakoid membranes [41]. This suggests that under shading treatment, algae in biofilms adapt by modifying their membrane composition to optimize light absorption, thereby increasing PUFA accumulation. Studies on marine diatoms have shown a decline in PUFA as light levels rise, further indicating the involvement of photoacclimation [50, 51].
Diverse conditions not only modify the community structure of benthic algae in biofilms but also, to some extent, alter its biochemical composition [42, 52]. Our results support the second hypothesis that the changes in the nutritional quality of biofilms are linked to algal community succession, influenced by shading (Fig. 2, Table 2). Bacillariophyta are typically dominant in near-pristine streams [15], whereas Chlorophyta are more common in open streams exposed to high irradiance [53]. The increased C16:1n7 in biofilms under shading could be explained by the increased abundances of Bacillariophyta (Table 3, Fig. 2) [54, 55]. Shading diminished the competitive advantage of Cyanobacteria within the community (Fig. 2). However, it did not significantly affect the biomarker fatty acid ALA, despite an observed trend that might contribute to its decrease (Table 3). Additionally, although EPA and DHA did not show statistical significance, the increase in LC-PUFA implies an improvement in nutritional quality (Fig. 3a, b). The altered environmental condition provides growth opportunities for other algae, especially those well-adapted to low light, such as diatoms [15].
When algae are exposed to changing environmental conditions and stresses, they frequently adapt by modulating their lipid metabolism pathways, which encompass multiple metabolic routes [56]. While KEGG enrichment analysis showed no significant effects of shading on the overall fatty acid biosynthesis and elongation pathways (Fig. 4), it is essential to highlight the role of specific differentially expressed genes (DEGs) within these pathways. The absence of significant pathway level changes does not negate the importance of key regulatory enzymes, which can still play crucial roles in regulating fatty acid metabolism under shading treatment. During fatty acid biosynthesis, acetyl CoA is a precursor substance for fatty acid biosynthesis, which is converted to malonyl-CoA by acetyl-CoA carboxylase (ACACA, EC: 6.4.1.2, 6.3.4.14). Interestingly, ACACA catalyzing the initial step of de novo FA biosynthesis was upregulated in the shaded groups. However, 3-hydroxyacyl ACP dehydratase (FabZ, EC: 4.2.1.59) was downregulated, suggesting a repressed de novo FA biosynthesis. Although the expression level of long-chain acyl-CoA synthetase (ACSL, EC: 6.2.1.3) in the shaded group was higher, the product of ACSL, known as hexadecanoyl-CoA, could inhibit FA synthesis through allosteric negative feedback [57]. This inhibition may lead to the downregulation of key enzymes involved in de novo fatty acid synthesis. Concurrently, the downregulation of FAB2 and FATA responsible for SAFA (primarily C16:0 and C18:0) synthesis was observed, consistent with the reduction of SAFA (Table 3).
In the cytosol, free FAs can be ligated to CoA via ACSL producing acyl-CoA [58, 59], which could be assembled to the glycerol backbone to form TAG. Since endoplasmic reticulum (ER)-based FA elongation is involved in multiple biological processes providing precursors of membrane lipids and lipid mediators [25], it is important to maintain basal lipid supply to biological processes. The KCS catalyzing the first and rate limiting condensation step of elongating FAs in ER was upregulated significantly under shading (Fig. 5, Table S2), implying that KCS might be the key enzyme controlling PUFA biosynthesis. Under shading, the upregulation of FAD2 drives the conversion of saturated to unsaturated FA (Fig. 5), and such shifting carbon flux towards lipid synthesis may represent a response to stressful conditions [60]. The repression of the FA biosynthesis and enhancing FA elongation imply that the key regulation point of increased PUFA biosynthesis in shading might be not relying on de novo FAs synthesis [23, 61]. Furthermore, GSEA revealed significant activation of pathways involved in fatty acid elongation and arachidonic acid (ARA) metabolism (Fig. 6a, b). This suggests that shading induces the upregulation of PUFA-related synthesis and metabolic pathways, providing further evidence for the increased synthesis of PUFA, particularly ARA.
Photosynthesis also imposes a critical effect on the flux of acetyl CoA, NADPH, and ATP supply toward lipid biosynthesis [62]. However, under shading conditions, the gene expression of petH and ATP synthases were downregulated (Table S4), leading to reduced NADPH and ATP production, which ultimately lowers the energy required for FA synthesis. In contrast, the increased expression of GAPDH indicates the enhancement of the Calvin cycle pathway (Table S4), and the ultimate product G-3-P serves as a carbon source for lipid synthesis. LHC serving as a light receptor efficiently captures and transfers excitation energy to photosystems. The upregulation of LHC-related genes responsive to the shading may enable algae to flexibly make adjustments in photosynthetic efficiency under adverse abiotic stress [63]. Sustaining essential metabolic processes in the shading is imperative to enable rapid photosynthesis upon re-illumination [64].
The repression of glycolysis-related genes, such as pyruvate kinase (PK, EC: 2.7.1.40) (Table S2), suggests that biofilms tend to decrease the consumption of carbohydrates to save energy. This downregulation subsequently hinders the provision of additional substrates for fatty acid biosynthesis under shading conditions [23]. As the end product of glycolysis, pyruvate links glycolysis with TCA cycle and lipid biosynthesis, thus pyruvate is also considered a regulative metabolite for adjusting lipid biosynthesis [65, 66]. Pyruvate can also be converted to acetyl-CoA by a pyruvate dehydrogenase (PDH, EC: 1.2.4.1) bypass pathway, as an alternative pathway supplying acetyl-CoA for enhanced lipid biosynthesis [67]. The downregulation of PDH suggests a reduced conversion of pyruvate to acetyl-CoA and its incorporation into the tricarboxylic acid (TCA) cycle when compared to the ambient groups. Pyruvate might not provide a sufficient amount to replenish the levels of TCA intermediates, which are consistently depleted to meet the increased energy demands for lipid biosynthesis [67].
Conclusion
This study elaborates that nutritional quality indicated by LC-PUFA in stream food webs increases with riparian rehabilitation in a manipulative experiment. Result reveals mechanisms in two aspects contributing to increased nutritional quality: succession in algal community from Cyanobacteria and Chlorophyta to Bacillariophyta and upregulation of key enzyme such as FAD2 encoding genes in PUFA biosynthesis. Photosynthesis indirectly provides acetyl CoA, NADPH, and ATP which serve as precursors and energy for FA biosynthesis reactions. The increased LC-PUFA% at shading conditions suggests that photoacclimation influences the synthesis and desaturation of membranes. Our study provides a comprehensive understanding of stream food webs in terms of basal resource nutritional quality and offered empirical evidence supporting riparian revegetation as a vital strategy for watershed management.
Data Availability
No datasets were generated or analysed during the current study.
References
Honeyfield DC, Maloney KO (2015) Seasonal patterns in stream periphyton fatty acids and community benthic algal composition in six high-quality headwater streams. Hydrobiologia 744(1):35–47. https://doi.org/10.1007/s10750-014-2054-7
Battin TJ, Besemer K, Bengtsson MM, Romani AM, Packmann AI (2016) The ecology and biogeochemistry of stream biofilms. Nat Rev Microbiol 14(4):251–263. https://doi.org/10.1038/nrmicro.2016.15
Mallory MA, Richardson JS (2005) Complex interactions of light, nutrients and consumer density in a stream periphyton–grazer (tailed frog tadpoles) system. J Anim Ecol 74(6):1020–1028. https://doi.org/10.1111/j.1365-2656.2005.01000.x
Murdock JN, Shields FD, Lizotte RE (2013) Periphyton responses to nutrient and atrazine mixtures introduced through agricultural runoff. Ecotoxicology 22(2):215–230. https://doi.org/10.1007/s10646-012-1018-9
Nebgen EL, Herrman KS (2019) Effects of shading on stream ecosystem metabolism and water temperature in an agriculturally influenced stream in central Wisconsin, USA. Ecol Eng 126:16–24. https://doi.org/10.1016/j.ecoleng.2018.10.023
Frost PC, Elser JJ (2002) Effects of light and nutrients on the net accumulation and elemental composition of epilithon in boreal lakes. Freshw Biol 47(2):173–183. https://doi.org/10.1046/j.1365-2427.2002.00796.x
Torres-Ruiz M, Wehr JD, Perrone AA (2007) Trophic relations in a stream food web: importance of fatty acids for macroinvertebrate consumers. J N Am Benthol Soc 26(3):509–522. https://doi.org/10.1899/06-070.1
Ebm N, Guo F, Brett MT, Bunn SE, Kainz MJ (2021) Polyunsaturated fatty acids in fish tissues more closely resemble algal than terrestrial diet sources. Hydrobiologia 848(2):371–383. https://doi.org/10.1007/s10750-020-04445-1
Torres-Ruiz M, Wehr JD, Perrone AA (2010) Are net-spinning caddisflies what they eat? An investigation using controlled diets and fatty acids. J N Am Benthol Soc 29(3):803–813. https://doi.org/10.1899/09-162.1
Yeşilayer N, Kaymak IE (2020) Effect of partial replacement of dietary fish meal by soybean meal with betaine attractant supplementation on growth performance and fatty acid profiles of juvenile rainbow trout (Oncorhynchus mykiss). Aquac Res 51(4):1533–1541. https://doi.org/10.1111/are.14501
Twining CW, Brenna JT, Hairston NG, Flecker AS (2016) Highly unsaturated fatty acids in nature: what we know and what we need to learn. Oikos 125(6):749–760. https://doi.org/10.1111/oik.02910
Keva O, Tang P, Käkelä R, Hayden B, Taipale SJ, Harrod C, Kahilainen KK (2019) Seasonal changes in European whitefish muscle and invertebrate prey fatty acid composition in a subarctic lake. Freshw Biol 64(11):1908–1920. https://doi.org/10.1111/fwb.13381
Tan X, Ma P, Bunn SE, Zhang Q (2015) Development of a benthic diatom index of biotic integrity (BD-IBI) for ecosystem health assessment of human dominant subtropical rivers, China. J Environ Manage 151:286–294. https://doi.org/10.1016/j.jenvman.2014.12.048
Hill WR, Griffiths NA (2017) Nitrogen processing by grazers in a headwater stream: riparian connections. Freshw Biol 62(1):17–29. https://doi.org/10.1111/fwb.12833
Jyväsjärvi J, Rajakallio M, Brüsecke J, Huttunen K, Huusko A, Muotka T, Taipale SJ (2022) Dark matters: contrasting responses of stream biofilm to browning and loss of riparian shading. Glob Change Biol 28(17):5159–5171. https://doi.org/10.1111/gcb.16279
Taipale SJ, Hiltunen M, Vuorio K, Peltomaa E (2016) Suitability of phytosterols alongside fatty acids as chemotaxonomic biomarkers for phytoplankton. Front Plant Sci 7:212. https://doi.org/10.3389/fpls.2016.00212
Senar OE, Creed IF, Strandberg U, Arts MT (2019) Browning reduces the availability—but not the transfer—of essential fatty acids in temperate lakes. Freshw Biol 64(12):2107–2119. https://doi.org/10.1111/fwb.13399
Suzuki N, Koussevitzky S, Mittler R, Miller G (2012) ROS and redox signalling in the response of plants to abiotic stress. Plant, Cell Environ 35(2):259–270. https://doi.org/10.1111/j.1365-3040.2011.02336.x
Branco CCZ, Riolfi A, Crulha BP, Tonetto AF, Bautista AN, JúniorO N (2017) Tropical lotic primary producers: who has the most efficient photosynthesis in low-order stream ecosystems? Freshw Biol 62(9):1623–1636. https://doi.org/10.1111/fwb.12974
Lau DCP, Vrede T, Pickova J, Goedkoop W (2012) Fatty acid composition of consumers in boreal lakes – variation across species, space and time. Freshw Biol 57(1):24–38. https://doi.org/10.1111/j.1365-2427.2011.02690.x
Arts M T, Brett MT, Kainz MJ (2009) Lipids in aquatic ecosystems. Springer. https://doi.org/10.1007/978-0-387-89366-2
Galloway AWE, Winder M (2015) Partitioning the relative importance of phylogeny and environmental conditions on phytoplankton fatty acids. PLoS ONE 10(6):e0130053. https://doi.org/10.1371/journal.pone.0130053
He M, Song H, Chen W, Zhang Y, Wang T, Wang C, Du W (2019) Comparative transcriptome analysis of wild type and an oleaginous mutant strain of Desmodesmus sp. reveals a unique reprogramming of lipid metabolism under high light. J Appl Phycol 31(5):2895–2910. https://doi.org/10.1007/s10811-019-01821-w
Hu Q, Sommerfeld M, Jarvis E, Ghirardi M, Posewitz M, Seibert M, Darzins A (2008) Microalgal triacylglycerols as feedstocks for biofuel production: perspectives and advances. Plant J 54(4):621–639. https://doi.org/10.1111/j.1365-313X.2008.03492.x
Tan KWM, Lee YK (2016) The dilemma for lipid productivity in green microalgae: importance of substrate provision in improving oil yield without sacrificing growth. Biotechnol Biofuels 9(1):255. https://doi.org/10.1186/s13068-016-0671-2
Warren DR, Keeton WS, Kiffney PM, Kaylor MJ, Bechtold HA, Magee J (2016) Changing forests—changing streams: riparian forest stand development and ecosystem function in temperate headwaters. Ecosphere 7(8):e01435. https://doi.org/10.1002/ecs2.1435
Mulholland PJ, Fellows CS, Tank JL, Grimm NB, Webster JR, Hamilton SK, Peterson BJ (2001) Inter-biome comparison of factors controlling stream metabolism. Freshw Biol 46(11):1503–1517. https://doi.org/10.1046/j.1365-2427.2001.00773.x
Gjerløv C, Richardson JS (2010) Experimental increases and reductions of light to streams: effects on periphyton and macroinvertebrate assemblages in a coniferous forest landscape. Hydrobiologia 652(1):195–206. https://doi.org/10.1007/s10750-010-0331-7
Guo F, Kainz MJ, Sheldon F, Bunn SE (2016) Effects of light and nutrients on periphyton and the fatty acid composition and somatic growth of invertebrate grazers in subtropical streams. Oecologia 181(2):449–462. https://doi.org/10.1007/s00442-016-3573-x
Kaase CT, Katz GL (2012) Effects of stream restoration on woody riparian vegetation of Southern Appalachian Mountain streams North Carolina, U.S.A. Restor Ecol 20(5):647–655. https://doi.org/10.1111/j.1526-100X.2011.00807.x
McCall SJ, Hale MS, Smith JT, Read DS, Bowes MJ (2017) Impacts of phosphorus concentration and light intensity on river periphyton biomass and community structure. Hydrobiologia 792(1):315–330. https://doi.org/10.1007/s10750-016-3067-1
Li L, Zhang G, Wang Q (2016) De novo transcriptomic analysis of Chlorella sorokiniana reveals differential genes expression in photosynthetic carbon fixation and lipid production. BMC Microbiol 16(1):223. https://doi.org/10.1186/s12866-016-0839-8
Wohlrab S, Tillmann U, Cembella A, John U (2016) Trait changes induced by species interactions in two phenotypically distinct strains of a marine dinoflagellate. ISME J 10(11):2658–2668. https://doi.org/10.1038/ismej.2016.57
Tan X, Sheldon F, Bunn SE, Zhang Q (2013) Using diatom indices for water quality assessment in a subtropical river. China Environ Sci Pollut Res 20(6):4164–4175. https://doi.org/10.1007/s11356-012-1343-9
Entwisle TJ, Sonneman JA, Lewis SH (1997) Freshwater algae in Australia. A guide to conspicuous genera. Nordic J Botany 18(1):78–78. https://doi.org/10.1111/j.1756-1051.1998.tb01101.x
Schutter ME, Dick RP (2000) Comparison of fatty acid methyl ester (FAME) methods for characterizing microbial communities. Soil Sci Soc Am J 64(5):1659–1668. https://doi.org/10.2136/sssaj2000.6451659x
Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, Van Baren MJ, Pachter L (2010) Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol 28(5):511–515. https://doi.org/10.1038/nbt.1621
Li B, Dewey CN (2011) RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics 12(1):323. https://doi.org/10.1186/1471-2105-12-323
Grabherr MG, Haas BJ, Yassour M, Levin JZ, Thompson DA, Amit I, Regev A (2011) Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat Biotechnol 29(7):644–652. https://doi.org/10.1038/nbt.1883
Huggins K, Frenette J, Arts MT (2004) Nutritional quality of biofilms with respect to light regime in Lake Saint-Pierre (Québec, Canada). Freshw Biol 49(7):945–959. https://doi.org/10.1111/j.1365-2427.2004.01236.x
Hill WR, Rinchard J, Czesny S (2011) Light, nutrients and the fatty acid composition of stream periphyton: periphyton stoichiometry and fatty acids. Freshw Biol 56(9):1825–1836. https://doi.org/10.1111/j.1365-2427.2011.02622.x
Cashman MJ, Wehr JD, Truhn K (2013) Elevated light and nutrients alter the nutritional quality of stream periphyton. Freshw Biol 58(7):1447–1457. https://doi.org/10.1111/fwb.12142
Piepho M, Arts MT, Wacker A (2012) Species-specific variation in fatty acid concentrations of four phytoplankton species: does phosphorus supply influence the effect of light intensity or temperature? J Phycol 48(1):64–73. https://doi.org/10.1111/j.1529-8817.2011.01103.x
Guschina IA, Harwood JL (2006) Lipids and lipid metabolism in eukaryotic algae. Prog Lipid Res 45(2):160–186. https://doi.org/10.1016/j.plipres.2006.01.001
Tonon T, Harvey D, Larson TR, Graham IA (2002) Long chain polyunsaturated fatty acid production and partitioning to triacylglycerols in four microalgae. Phytochemistry 61(1):15–24. https://doi.org/10.1016/S0031-9422(02)00201-7
Guihéneuf F, Stengel D (2013) LC-PUFA enriched oil production by microalgae: accumulation of lipid and triacylglycerols containing n-3 LC-PUFA is triggered by nitrogen limitation and inorganic carbon availability in the marine haptophyte Pavlova lutheri. Mar Drugs 11(11):4246–4266. https://doi.org/10.3390/md11114246
Poerschmann J, Spijkerman E, Langer U (2004) Fatty acid patterns in Chlamydomonas sp. as a marker for nutritional regimes and temperature under extremely acidic conditions. Microb Ecol 48(1):78–89. https://doi.org/10.1007/s00248-003-0144-6
Falkowski PG, Raven JA (2007) Aquatic photosynthesis, 2nd edn. Princeton Univ. Press, Princeton, NJ
de Bianchi S, Ball’Ottari M, Dallosto L, Bassi R (2010) Regulation of plant light harvesting by thermal dissipation of excess energy. Biochem Soc Trans 38(2):651–660. https://doi.org/10.1042/BST0380651
Leu E, Wiktor J, Søreide J, Berge J, Falk-Petersen S (2010) Increased irradiance reduces food quality of sea ice algae. Mar Ecol Prog Ser 411:49–60. https://doi.org/10.3354/meps08647
Chen YC (2010) The biomass and total lipid content and composition of twelve species of marine diatoms cultured under various environments. Food Chem 131(1):211–219. https://doi.org/10.1016/j.foodchem.2011.08.062
Guo F, Ebm N, Bunn SE, Brett MT, Hager H, Kainz MJ (2021) Longitudinal variation in the nutritional quality of basal food sources and its effect on invertebrates and fish in subalpine rivers. J Anim Ecol 90(11):2678–2691. https://doi.org/10.1111/1365-2656.13574
Mosisch TD, Bunn SE, Davies PM (2001) The relative importance of shading and nutrients on algal production in subtropical streams. Freshw Biol 46(9):1269–1278. https://doi.org/10.1046/j.1365-2427.2001.00747.x
Taipale S, Strandberg U, Peltomaa E, Galloway A, Ojala A, Brett M (2013) Fatty acid composition as biomarkers of freshwater microalgae: analysis of 37 strains of microalgae in 22 genera and in seven classes. Aquat Microb Ecol 71(2):165–178. https://doi.org/10.3354/ame01671
Anderson CB, Tagliaferro M, Fisk A, Rosemond AD, Sanchez ML, Arts MT (2020) Fatty acids elucidate sub-Antarctic stream benthic food web dynamics invaded by the North American beaver (Castor canadensis). Polar Biol 43(5):423–433. https://doi.org/10.1007/s00300-020-02644-z
Zulu NN, Zienkiewicz K, Vollheyde K, Feussner I (2018) Current trends to comprehend lipid metabolism in diatoms. Prog Lipid Res 70:1–16. https://doi.org/10.1016/j.plipres.2018.03.001
Gavilanes JG, Lizarbe MA, Municio AM, Oñaderra M (1982) Effects of palmitoyl-CoA on the structure-function of the fatty acid synthetase complex from Ceratitis capitata. Int J Biochem 14(12):1061–1066. https://doi.org/10.1016/0020-711X(82)90162-8
Mashek DG, Li LO, Coleman RA (2007) Long-chain acyl-CoA synthetases and fatty acid channeling. Futur Lipidol 2(4):465–476. https://doi.org/10.2217/17460875.2.4.465
Fan J, Andre C, Xu C (2011) A chloroplast pathway for the de novo biosynthesis of triacylglycerol in Chlamydomonas reinhardtii. FEBS Lett 585(12):1985–1991. https://doi.org/10.1016/j.febslet.2011.05.018
Barneche DR, Hulatt CJ, Dossena M, Padfield D, Woodward G, Trimmer M, Yvon-Durocher G (2021) Warming impairs trophic transfer efficiency in a long-term field experiment. Nature 592(7852):76–79. https://doi.org/10.1038/s41586-021-03352-2
Li J, Han D, Wang D, Ning K, Jia J, Wei L, Xu J (2014) Choreography of transcriptomes and lipidomes of Nannochloropsis reveals the mechanisms of oil synthesis in microalgae. Plant Cell 26(4):1645–1665. https://doi.org/10.1105/tpc.113.121418
Lenka SK, Carbonaro N, Park R, Miller SM, Thorpe I, Li Y (2016) Current advances in molecular, biochemical, and computational modeling analysis of microalgal triacylglycerol biosynthesis. Biotechnol Adv 34(5):1046–1063. https://doi.org/10.1016/j.biotechadv.2016.06.004
Li H, Scheschonk L, Heinrich S, Valentin K, Harms L, Glöckner G, Bischof K (2020) Transcriptomic responses to darkness and the survival strategy of the kelp Saccharina latissima in the early polar night. Front Mar Sci 7:592033. https://doi.org/10.3389/fmars.2020.592033
Kennedy F, Martin A, Bowman JP, Wilson R, McMinn A (2019) Dark metabolism: a molecular insight into how the Antarctic sea-ice diatom Fragilariopsis cylindrus survives long-term darkness. New Phytol 223(2):675–691. https://doi.org/10.1111/nph.15843
Goodson C, Roth R, Wang ZT, Goodenough U (2011) Structural correlates of cytoplasmic and chloroplast lipid body synthesis in Chlamydomonas reinhardtii and stimulation of lipid body production with acetate boost. Eukaryot Cell 10(12):1592–1606. https://doi.org/10.1128/EC.05242-11
Ma YH, Wang X, Niu YF, Yang ZK, Zhang MH, Wang ZM, Li HY (2014) Antisense knockdown of pyruvate dehydrogenase kinase promotes the neutral lipid accumulation in the diatom Phaeodactylum tricornutum. Microb Cell Fact 13(1):100. https://doi.org/10.1186/s12934-014-0100-9
Avidan O, Pick U (2015) Acetyl-CoA synthetase is activated as part of the PDH-bypass in the oleaginous green alga Chlorella desiccata. J Exp Bot 66(22):7287–7298. https://doi.org/10.1093/jxb/erv424
Acknowledgements
We appreciate the considerable contribution of Ms. Qiaoling Zhao for identification of periphytic algae.
Funding
This study was supported by the National Natural Science Foundation of China (Nos. 32271665, 32030069).
Author information
Authors and Affiliations
Contributions
K.Z. wrote the main manuscript text and prepared all figures and tables. X.T. conceived the experiment and designed methodology, revised this manuscript. Q.Z. edited, supervised, and acquired funding. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, K., Tan, X. & Zhang, Q. Nutritional Quality of Basal Resource in Stream Food Webs Increased with Light Reduction—Implications for Riparian Revegetation. Microb Ecol 87, 114 (2024). https://doi.org/10.1007/s00248-024-02432-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00248-024-02432-w