Abstract
Intensive crop production leads to the disruption of the symbiosis between plants and their associated microorganisms, resulting in suboptimal plant productivity and lower yield quality. Therefore, it is necessary to improve existing methods and explore modern, environmentally friendly approaches to crop production. One of these methods is biotization, which involves the inoculation of plants with appropriately selected symbiotic microorganisms which play a beneficial role in plant adaptation to the environment. In this study, we tested the possibility of using a multi-microorganismal inoculum composed of arbuscular mycorrhizal fungi (AMF) and AMF spore-associated bacteria for biotization of the red raspberry. Bacteria were isolated from the spores of AMF, and their plant growth-promoting properties were tested. AMF inocula were supplemented with selected bacterial strains to investigate their effect on the growth and vitality of the raspberry. The investigations were carried out in the laboratory and on a semi-industrial scale in a polytunnel where commercial production of seedlings is carried out. In the semi-industrial experiment, we tested the growth parameters of plants and physiological response of the plant to temporary water shortage. We isolated over fifty strains of bacteria associated with spores of AMF. Only part of them showed plant growth-promoting properties, and six of these (belonging to the Paenibacillus genus) were used for the inoculum. AMF inoculation and co-inoculation of AMF and bacteria isolated from AMF spores improved plant growth and vitality in both experimental setups. Plant dry weight was improved by 70%, and selected chlorophyll fluorescence parameters (the contribution of light to primary photochemistry and fraction of reaction centre chlorophyll per chlorophyll of the antennae) were increased. The inoculum improved carbon assimilation, photosynthetic rate, stomatal conductance and transpiration after temporary water shortage. Raspberry biotization with AMF and bacteria associated with spores has potential applications in horticulture where ecological methods based on plant microorganism interaction are in demand.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plant-associated microorganisms play a decisive role in plant growth (biomass production) and stress tolerance [15, 17, 58]. In the last decades, intensive agriculture has significantly impacted the community structure of soil microorganisms which forms a natural reservoir of microorganisms for the plant. Thus, in modern agriculture, more attention is being paid to plant biotization with microorganisms that are capable of increasing mineral uptake by plants (as biofertilisers), fine-tunning plant metabolism (as bioregulators) and resistance to abiotic and biotic stress (as bioprotectors) and thus play a beneficial role in plant adaptation to the environment [11, 22, 25].
Arbuscular mycorrhizal fungi (AMF) are commonly known to provide many benefits to the plant. AMF improve growth and nutritional quality of crops [4, 48] and protect plants against environmental stress such as pathogens and abiotic environmental conditions [40, 56]. Widespread occurrence of mycorrhizal symbiosis and the obligatory nature of this plant (fungus association for many plant species) have long directed the attention of the scientific community to the practical use of mycorrhiza in protection of endangered plants, in phytostabilization of toxic wastes and in sustainable agriculture to improve food security [8, 39, 54].
In recent years, more attention has been paid to the use of multi-microorganismal inocula that include AMF and other groups of symbiotic organisms [7]. This approach provides the plant with microorganisms possessing a variety of plant growth-promoting traits. It has been demonstrated that AMF can be used effectively in co-inoculation mainly with endophytic fungi, endophytic bacteria and soil bacteria [7, 18, 56]. The effects of such practises differ in between studies. Plant growth was affected either positively or negatively compared to the inoculation with a single microorganism. This is quite understandable since successful biotization relies on appropriately selected microorganisms used for inoculation of a given plant cultivar. Mutual interactions between the microorganisms and the plant host seem to determine the efficiency of plant growth promotion [51].
Bacteria associated with AMF spores have been recently investigated on several occasions [1, 3, 9, 16, 29, 41, 53] and represent different taxa including Proteobacteria (Achromobacter, Agrobacterium, Aquitalea, Bosea, Burkholderia, Cellvibrio, Cupriavidus, Desulfovibrio, Duganella, Ensifer, Enterobacter, Herbaspirillum, Ideonella, Lysobacter, Massilia, Methylibium, Mitsuaria, Proteus, Pseudomonas, Ralstonia, Rheinheimera, Rhizobium, Sinorhizobium), Actinobacteria (Amycolatopsis, Arthrobacter, Curtobacterium, Gordonia, Leifsonia, Mycobacterium, Nocardia, Propionibacterium, Streptomyces) Firmicutes (Bacillus, Brevibacillus, Paenibacillus) and Bacteroidetes (Flexibacter). Although the first reports of bacteria in AMF spores date back to over 50 years ago [31, 32], their role in plant-microorganism interactions is still poorly recognised. Bacteria associated with AMF spores, Bacillus subtilis, Pseudomonas diminuta, Enterobacter hormaechei, Bacillus sp., Bacillus thuringiensis and Paenibacillus rhizosphaerae have been shown to be able to inhibit the growth of pathogens and to activate the development of hyphae of Gigaspora [9, 16]. Functional analysis of 43 bacterial strains isolated from spores of Gigaspora margarita revealed that about 30% of them stimulated spore germination, nearly 60% solubilised phosphorus, 15% degraded chitin and three taxa, Curtobacterium, Ensifer and Bacillus, improved growth of alfalfa [29]. According to some authors, certain plant growth-promoting functions provided by AMF may be related to mycorrhiza-associated bacteria [16, 53]. Keeping in mind the multiple benefits provided to plants by AMF or AMF spore-associated bacteria, inocula based on both groups of microorganisms can be used for sustainable crop production.
The aims of this study were to test the possibility of improving the efficiency of AMF-based inocula for biotization of the red raspberry by supplementing different compositions of AMF with bacteria isolated from AMF spores. We investigated how these multi-microorganismal inocula affect (1) plant growth in laboratory and greenhouse conditions and (2) the physiological response of plant to temporary water shortage. As a model plant, the red raspberry (Rubus idaeus L.) was selected. This is an important crop species with growing consumer interest. Its fruits are desirable for their taste and nutritional properties, [10]. These properties result in a high demand for raspberry fruit worldwide.
Materials and Methods
Experimental Design
The investigations were carried out in three steps. In the first step, the bacterial components for the inoculum were isolated and selected based on the plant growth-promoting properties. In the second step, AMF inoculum supplemented with selected bacteria was used to inoculate raspberries in laboratory experiments. In the third step, the efficiency of the selected AMF and bacterial supplement for AMF was verified in semi-industrial scale raspberry production in a polytunnel.
Isolation and Identification of Bacteria from Spores
Bacteria were isolated from the spores of arbuscular mycorrhizal fungi. Roots of plants were collected from raspberry (five plants) and blackberry (one plant) plantations and used for inoculum preparation in the pot (16 pots) culture system (in association with Plantago lanceolata) as described in Orłowska et al. [36]. Sixteen samples of air-dried rhizosphere (5 g) soil from trap cultures were used for AMF identification. The obtained spores were divided into groups according to their morphological features and subsequently identified according to their SSU rRNA sequence. AML1 (5′-ATCAACTTTGATGGTAGGATAGA-3′) and AML2 (5′-GAACCCAAACACTTTGGTTTC C-3′) [28] primers were used for nested PCR followed by a Strata Clone PCR Cloning Kit (Agilent, USA). Amplicons were purified and then sequenced (Sanger sequencing) bidirectionally by Macrogen Europe (The Netherlands). Sequences were compared with sequences available in the NCBI (National Centre for Biotechnology Information).
Spore-associated bacteria isolation was carried out from approximately 200 AMF spores. Spores were suspended in 1 mL sterile 0.9% NaCl in a 1.5-mL tube and shaken for 1 min using a vortex. Subsequently, spores were washed aseptically in 0.9% NaCl 15 times (centrifugation at 1500 × g for 1 min) [5] and crushed with a micro pestle. The suspension was heated at 80 °C for 10 min in order to isolate spore-forming bacteria. Then, 100 µL of suspension was plated in five replicates on five different media: TSB and NA (Tryptic Soy Broth and Nutrient Agar—for heterotrophic bacteria isolation), WAM (Waksman’s Agar Medium—aimed at actinobacteria), Winogradsky Culture Agar (N-free medium aimed at nitrogen-fixing bacteria) and minimal medium with chitin as carbon source (for chitinolytic bacteria). Plates were incubated at 28 °C in darkness for 7 days. Emerging bacteria were transferred into new media. Bacteria selected for the inoculum were identified based on the sequence of 16S rDNA region. DNA was isolated with a DNA Mini Kit (Syngen). 27F (5′-GAGTTTGATCCTGGCTCAG-3′) and 1492R (5′-GGTTACCTTGTTACGACTT-3′) primers were used for PCR [52]. Bacterial amplicons were sequenced and analysed as described for fungi.
Plant Growth-Promoting Properties of Isolated Bacteria Selection for the Inoculum
Phosphate Solubilisation
Bacteria were examined to test their inorganic phosphate solubilising potential. Bacteria were cultivated on NA (Nutrient Agar) medium at 30 °C in darkness for 2 days. Subsequently, bacteria were cultured on Pikovskaya Agar medium [38] for 7 days at 30 °C in darkness (N = 3). Tri-calcium phosphate was the source of insoluble phosphate. Phosphate solubilising activity was indicated as a clearance around the microorganism colony.
Phytate Solubilisation
The ability of bacteria to solubilise organic phosphate was examined on Phytate Screening Medium (PSM; 10 g · L−1 d-glucose, 4 g · L−1 C6H18P6O24·12Na·H2O, 2 g · L−1 CaCl2, 5 g · L−1 NH4NO3, 0.5 g · L−1 KCl, 0.5 g · L−1 MgSO4·7H2O, 0.01 g · L−1 FeSO4·7H2O, 0.01 g · L−1 MnSO4·H2O, 15 g · L−1 agar, pH 7) for 7 days at 30 °C in darkness (N = 3). Organic phosphate solubilising activity was indicated as a clearance around the microorganism colony [5].
Production of Indole Acetic Acid (IAA)
Bacteria were cultured in Luria–Bertani Broth (LBB) supplemented with 1 mg · L−1 L-tryptophan (Sigma-Aldrich) at 20 °C at 200 rpm for 24 h and then centrifuged at 7500 × g for 10 min. The supernatant (1 mL) was mixed with 2 mL Salkowski reagent (1.2% FeCl3 in 37% sulphuric acid) in a well plate and incubated for 30 min in darkness (N = 3) [23]. The production of IAA was assessed based on colour development (‘-’ no colour development, no production,‘ ± ’ pink pale, low production; ‘ + ’ light pale, production; ‘ + + ’ bright purple, moderate production; ‘ + + + ’ dark purple, high production).
Siderophore Production
To assess siderophore production, the modified blue agar chromeazurol S (CAS) method by Schwyn and Neilands [44] was used. Four different solutions (1–4) were prepared, mixed in the following order: 2, 3, 4, 1 and aseptically poured onto plates. Solution 1: 100 mL dd H2O, 2.7 g FeCl3 · 6H2O, 180 µL HCl (0.56 mM), 60.5 g Chromeazurol S (CAS) and 72.8 mg HDTMA bromide, autoclave; Solution 2: 800 mL dd H2O, 0.3 g KH2PO4, 0.5 g NaCl, 1 g NH4Cl, 30.24 g PIPES (to dissolve PIPES pH was adjusted to 6.8) and 15 g agar, autoclave; Solution 3: 70 mL dd H2O, 2 g glucose, 2 g mannitol, 0.493 g MgSO4 · 7H2O, 11 mg CaCl2 · 2H2O, 1.17 mg MnSO4 · H2O, 1.4 mg H3BO4, 0.04 mg CuSO4 · 5H2O, 1.2 mg ZnSO4 · 7H2O, 1 mg Na2MoO4 · 2H2O, autoclave; Solution 4: 3 g hydrate of casein was dissolved in 30 mL dd H2O and filter sterilised. Bacteria were cultured on CAS blue agar for 14 days (N = 3). Bacteria that possessed the ability to produce siderophores removed iron from the dye complex, and the medium colour changed from blue to orange.
Plant Growth Response Tests
Laboratory Experiment
Raspberry plant cuttings were cultured in vitro in Murashige and Skoog Medium supplemented with 1.2 mg · L−1 α naphthalene acetic acid (NAA) and 0.3 mg · L−1 indole-3-butyric acid (IBA) for 4 weeks and subsequently transferred to a mixture of garden soil (supplied by ARO, Poland), sand and clay (in equal volumes) supplemented with 40 mL·L−1 rock phosphate (Siarkopol, Poland) in pot cultures [56]. Prior to planting, the soil was sterilised at 100 °C for 1 h for 3 consecutive days and sprayed with sterile water for 2 weeks. During transfer, plants were inoculated by adding 5 mL of AMF inoculum and 2 mL of bacterial inoculum to the planting hole. Plants were grown in a greenhouse under natural day/night conditions with additional light during the day (12 h), approximately 500 µmol m−2 · s−1, and were watered alternately with tap water and nutrient solution (Long Ashton) every 2 to 3 days to keep the substrate humidity at the level of approximately 60%. Plants were subject to four different treatments (with 20 replicates per treatment): (1) inoculation with AMF, (2) inoculation with bacteria, (3) inoculation with AMF + bacteria and (4) no-inoculation (control, without any supplementation). Three-month-old plants were transferred to bigger pots (volume 1 L). Plants were harvested after 5 months.
The AMF inoculum was a mixture of Entrophospora lamellosa (Dalpé, Koske & Tews) Błaszk., Niezgoda, B.T. Goto & Magurno, Entrophospora sp. R.N. Ames & R.W. Schneid. 1979 and Rhizophagus irregularis (Błaszk., Wubet, Renker & Buscot) C. Walker & A. Schüßler prepared separately in pot cultures of Plantago lanceolata L and mixed (v:v:v; 1:3:3). Approximately 5 mL of the inoculum, containing spores, mycelium and colonised root fragments was mixed with the upper layer of substrates.
The bacterial inoculum was a mixture of bacteria isolated from spores of AMF: Paenibacillus amylolyticus (Nakamura 1984) Ash et al. 1994, P. contaminans Chou et al. 2009, P. alginolyticus (Nakamura 1987) Shida et al. 1997, Paenibacillus soli Park et al. 2007, Paenibacillus sp. 1 Ash et al. 1994, Paenibacillus sp. 2 and one bacterial strain not associated with AMF spores, Stenotrophomonas sp. Palleroni and Bradbury 1993, from culture collection in the Institute of Environmental Sciences of the Jagiellonian University. Bacteria were cultured in TSB (Tryptone Soy Broth) medium in natural day/night cycles at 120 rpm at 30 °C for 96 h. Cultures were washed twice with sterile 0.9% NaCl (5000 g, 5 min) suspended in 50 mL 0.9% NaCl and mixed.
Tunnel Experiment
Raspberry plants were cultured in vitro for 4 weeks and transferred to garden soil (Novarbo) in pot culture (pot volume 40 mL). Plants were grown in a phytotron room for 6 weeks and then transferred to bigger pots (pot volume 200 mL) with a new substrate. The substrate was a mixture of garden soil (supplied by ARO, Poland), sand and clay (in equal volumes) supplemented with 40 mL·L−1 powdered rock phosphate (Siarkopol, Poland) [56]. The substrate was sterilised in 100 °C for 1 h for 3 consecutive days and sprayed with sterile water for 2 weeks. During transfer, plants were inoculated by adding 5 mL of AMF inoculum and 2 mL of liquid bacterial inoculum to the planting hole. Plants were grown in greenhouse under natural day/night conditions and were watered with tap water every 2 days. Plants were inoculated in June (N = 70) and harvested in September.
The inoculum used for raspberry biotization was a mixture of mycorrhizal fungi and bacteria associated with AMF spores. Mycorrhizal inoculum was a combination of four different AMF inocula from the collection of Institute of Environmental Sciences at Jagiellonian University in Kraków:
-
1)
Rhizoglomus intraradices,
-
2)
Funneliformis mosseae (T.H. Nicolson & Gerd.) C. Walker & A. Schüßler,
-
3)
Mix3 (was composed of R. intraradices, F. mosseae, F. constrictus (Trappe) C. Walker & A. Schüßler, F. geosporus (T.H. Nicolson & Gerd.) C. Walker & A. Schüßler (1:1:1:1)) and
-
4)
Mix4 (composed of R. intraradices, F. mosseae, F. constrictus, F. geosporus (4:3:1:1)).
Each of the four inocula was prepared separately in pot cultures of Plantago lanceolata L. and mixed (v:v:v:v, 2:2:1:1). Approximately 5 mL of the inoculum, containing spores, mycelium and colonised root fragments was mixed with the upper layer of the soil. The same bacterial inoculum as described in the “Laboratory Experiment” section was used.
Photosynthetic Efficiency
Photosynthetic efficiency was determined as described in Strasser et al. [46]. Briefly, chlorophyll fluorescence measurements were performed with a Handy Pea fluorimeter (Hansatech Instruments, UK). One mature leaf from each plant (10 replicates) was dark-adapted for 20 min in special clips before the measurement. Data were processed with BIOLYZER software (Laboratory of Bioenergetics, Geneva, Switzerland).
Gas Exchange in Response to Temporary Water Shortage
To apply water deficit, irrigation of cultures was discontinued for 7 days until the appearance of first water deficit symptoms in control plants (decrease in leaf turgor). Subsequently, plants were watered every 2 days. Two weeks later, the photosynthetic rate, stomatal conductance of H2O and transpiration rate were measured using LCpro-SD (ADC BioScientific Ltd., Hoddesdon, UK). All measurements were performed on the second leaf of randomly selected plants using a 6.25 cm2 chamber equipped with a mixed Red/Blue LED Light Source Head. The measurements were carried out under the following conditions: CO2 saturated conditions (600 µmol · mol−1 air), irradiance of 100 µmol (photons) · m−2 · s−1 red light intensity and a leaf temperature of 24 °C ± 0.5 °C in five biological replications for each group.
Statistical Analysis
Statistical analysis was performed using Statistica 13 (Tibco). Differences between experimental groups were considered significant at p ≤ 0.05. Data normality and variance homogeneity were assessed with Shapiro–Wilk’s and Levene’s tests, respectively. Differences were tested using a t-test or analysis of variance (ANOVA) followed by Tukey’s post hoc tests.
Results
Selection of Bacteria for Inoculum Components
Fifty-six bacterial strains were isolated from AMF spores collected from the roots and rhizosphere of raspberry and blackberry plants. AMF spore morphotypes were identified as three different taxa: Entrophospora lamellosa, Entrophospora sp. and Rhizophagus irregularis (Table 1). Seventeen bacterial strains were isolated on TSA, 16 – on NA, 9 – on N-free medium, 9 – on WAM and 5 – on MM. 64% of the strains exhibited phytate solubilisation, whereas the ability to solubilise phosphate was shown in only 7% of the strains. Twenty-nine percent of strains were able to synthesise IAA and 20% to produce siderophores (Table 2). The strains that exhibited the highest rate of IAA production, phosphate and phytate solubilisation and siderophore production were selected for the inoculum (Table 1). Additionally, the bacterial strain Stenotrophomonas sp. from the culture collection at the Institute of Environmental Sciences of Jagiellonian University were included in the inoculum.
Bacteria Isolated from AMF Spores Improve AMF-Inoculated Raspberry Growth
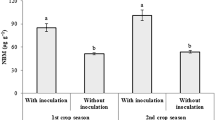
Non-inoculated raspberry yielded 16.7 cm in height after 5 months of growth in the laboratory (Fig. 1a). Plants inoculated with AMF spore-associated bacteria were not significantly higher (18.2 cm) than control plants. Inoculation with AMF significantly increased plant height. Plants inoculated with the fungi yielded 22.1 cm. Co-inoculation with bacteria and AMF had the best effect on plant height. The plants yielded 28.4 cm which was significantly better in relation to control plants and plants inoculated with single: AMF or bacteria (Fig. 1a, c).
Height (a) and PSII efficiency of plants cultured in the greenhouse—laboratory experiment (JIP-test parameters: PIabs—absorbance performance index, PItotal—total performance index, Φ0/(1-Φ0)—contribution of light reactions for primary photochemistry, RC/ABS—fraction of reaction centre chlorophyll per chlorophyll of the antennae, Ψ0/(1-Ψ0)—electron transport beyond primary quinone acceptor and RE/ABS—contribution of the reduction of end equivalents are presented relative to entirely non-inoculated plants; statistically significant differences between particular treatments and those entirely non-inoculated plants are indicated by asterisk (t-test, P ≤ 0.05, N = 10) (b) and photographs (c) of red raspberry inoculated with AMF, AMF spore-associated bacteria. For each treatment, 25 seedlings were inoculated. Plants were grown in peat and perlite (5:1, v:v) and irrigated with water. Plants were harvested for analysis at the end of the growing season
Plant vitality was assessed based on fluorescence of chlorophyll a. Out of the analysed parameters describing the efficiency of electron transport in PSII (and energy production), only two were significantly changed in inoculated plants in comparison to control. The contribution of light to primary photochemistry (Φ0/(1-Φ0)) was significantly higher in AMF-inoculated and AMF-bacteria co-inoculated plants compared to non-inoculated plants (Fig. 1b). However, electron transport beyond primary quinone acceptor (Ψ0/(1-Ψ0)) in these plants was significantly lower than in non-inoculated plants (Fig. 1b).
Multi-microorganismal Inoculum Improves Plant Growth and Response to Temporary Water Shortage on a Semi-industrial Scale
The experimental setup in the semi-industrial scale experiment was simplified compared to the initial screening in the laboratory. In this experiment, we compared the growth of plants either supplemented with AMF and bacteria or not inoculated. At the end of the growing season, non-inoculated plants reached 14.4 cm in height. Inoculated plants were significantly higher than control plants yielding 20.6 cm (40% increase) (Fig. 2a). Plant inoculation increased plant fresh biomass (28% increase) and plant dry biomass (76% increase) (Fig. 2b–d).
Parameters of plants from semi-industrial scale experiment: a height, b fresh weight, c dry weight, d photographic visualisation, e PSII efficiency (JIP-test parameters are described in Fig. 1) of red raspberry co-inoculated with AMF and AMF spore-associated bacteria. For each treatment, 70 seedlings were grown in greenhouse from June to September. Statistically significant differences between inoculated and non-inoculated plants are indicated by asterisk (t-test, P ≤ 0.05, N = 20)
Inoculation was beneficial for plant vitality. Two parameters describing fluorescence of chlorophyll, the contribution of light to primary photochemistry and fraction of reaction centre chlorophyll per chlorophyll of the antennae were significantly higher in AMF-bacteria co-inoculated plants than for non-inoculated plants (Fig. 2e).
Two weeks after a temporary water shortage, gas exchange in plant leaves was examined to verify if inoculation improved C assimilation. Photosynthetic rate, stomatal conductance of H2O and transpiration rate were improved in inoculated plants. CO2 assimilation (photosynthetic rate) was increased by 70%, stomatal conductance was improved over two-fold and the rate of transpiration was increased by 80% (Fig. 3).
Physiological parameters of plants from semi-industrial scale experiment measured 2 weeks after a temporary water shortage: A photosynthetic rate (PN, mmol CO2·m−2·s−1), b stomatal conductance (GS, mmol H2O·m−2·s−1) and c transpiration rate (E, mmol H2O·m−2·s.−1) of red raspberry co-inoculated with AMF and AMF spore-associated bacteria. Statistically significant differences between inoculated and non-inoculated plants are indicated by asterisk (t-test, P ≤ 0.05, N = 5)
Discussion
Biotization with microorganisms may result in changes in plant development and physiology facilitating adaptation to the environment [20]. The appropriate selection of microorganisms for this purpose is of utmost importance. The development of sustainable horticulture requires reducing usage of chemical pesticides and fertilisers and improvement of plant productivity. One symbiotic microorganism may not fully cover the needs that crops require for optimal growth [21]. On the other hand, multi-microbial inocula may not be effective for different crop species and even for different cultivars of the same species [24, 34]. Therefore, better understanding of the compatibility between symbiotic microorganisms and plants is required for targeted biotization of crop plants [55]. In the vast majority of previously published studies, plants were inoculated with single AMF species [49], and their response varied from inhibition to activation of plant growth, depending on AMF species. The synergistic effect of AMF and other factors (soil organic matter, insect pollination, nutrient availability) on the production of raspberry has also been examined on a few occasions [12, 13]. Gianinazzi et al. [21] showed that synergistic effect of AMF and soil-borne strain of Paenibacillus protected tomato against pathogenic Phytophthora parasitica. However, the synergistic effect of AMF and AMF spore-associated bacteria has not been investigated.
We isolated over fifty strains of bacteria from spores of AMF. It has to be emphasised that these bacteria most probably resided either within the cell wall or on the outside of the spore, thus being accessible for isolation and cultivation. Up until now only a limited number of reports describe the community structure of bacteria residing inside spores. According to Bianciotto et al. [6] and Naito et al. [33], only two bacterial taxa: ‘Candidatus Glomeribacter gigasporarum’ and ‘Candidatus Moeniiplasma glomeromycotorum’ were shown to inhabit spore interior However, Lastovetsky et al. [27], in a recently published paper, have shown a larger diversity of bacteria inhabiting AMF spores. Only some of them showed plant growth-promoting properties, and the best strains were selected for plant inoculation. Six bacterial strains, which were selected, belonged to Paenibacillus genus. Representatives of this genus have been previously shown to associate with AMF spores [1, 29] and additionally to inhibit the growth of soil-borne pathogenic fungi [16]. The results of our laboratory experiment showed that bacteria isolated from AMF spores alone did not improve plant growth or vitality. Co-culture with AMF was required for plant growth activation. AMF alone had a beneficial impact on plant yield, whereas supplementation with AMF and bacteria from AMF spores had a synergistic effect on raspberry growth. It was assumed that the bacteria may improve root colonisation by AMF,plant biomass yield was often related with high root colonisation by AMF [14, 45]. Other reports, however, did not show such a relationship [19, 30]. Here, supplementation with bacteria did not affect root colonisation by AMF (data not shown). It should be noted though that the majority of mycorrhizal colonisation parameters reached over 90% in AMF inoculated plants not supplemented with bacteria.
In the tunnel experiment, the beneficial effect of co-inoculation with AMF and bacteria on raspberry growth and vitality was verified positively. Plant growth parameters (high, fresh weight, dry weight) were improved by 28–76%, and selected parameters of chlorophyll a fluorescence were increased. These results indicate that biotization of raspberry with AMF and AMF spore-associated bacteria may be an alternative to conventional methods in large-scale raspberry production.
One of the main barriers to crop production is drought. Therefore, methods to increase plant resistance against drought are of particular interest for farmers and scientists. Arbuscular mycorrhizal fungi, endophytic fungi and endophytic bacteria have been documented as biological agents capable of improving drought resistance in crop plants [2, 35, 37, 47, 57]. Plant inoculation with symbiotic microorganisms often improves the absorptive surface of the roots and the activity of stress protective agents, osmoregulation, and antioxidant capacity [42, 43]. Thus, we investigated the effect of temporary water shortage (7 days) on basic physiological parameters of biotized raspberry. Two weeks after the treatment, the tested physiological parameters of inoculated plants were clearly improved. Plant inoculation with AMF and spore-associated bacteria improved stomatal conductance. Increased aperture of stomata promotes more efficient diffusion of CO2 and H2O; hence, in our study, a significant increase in transpiration rate was observed in inoculated plants. Better diffusion of CO2 through the stomata allows its efficient distribution in the stroma of chloroplasts increasing the intercellular carbon dioxide concentration and thus reducing the likelihood of photorespiration [26]. Inoculated plants were characterised by increased concentrations of intercellular CO2 (unpublished data). At the same time, we observed an increased proportion of active reaction centres and more efficient transport of electrons out of PSII in plants supplemented with the tested inoculum. Such functional remodelling of the photosynthetic apparatus increases the efficiency of capturing incoming radiation and the efficiency of linear transport of electrons [50]. The observed changes resulted in a significant increase in the rate of carbon dioxide assimilation in inoculated plants.
In conclusion, our results indicate that biotization of raspberry with arbuscular mycorrhizal fungi and selected bacteria isolated from spores significantly improved plant growth and biometric photosynthetic activity. Moreover, physiological performance of inoculated plants was improved compared to non-inoculated plants after temporary water shortage, suggesting improved resistance to drought. This shows that biotization with AMF and bacteria isolated from spores has potential application in raspberry production. This is particularly important due to the increasing demand for horticultural methods that rely on plant-microorganisms interaction [20].
Data Availability
No datasets were generated or analysed during the current study.
References
Agnolucci M, Battini F, Cristani C, Giovannetti M (2015) Diverse bacterial communities are recruited on spores of different arbuscular mycorrhizal fungal isolates. Biol Fertil Soils 51:379–389. https://doi.org/10.1007/s00374-014-0989-5
Azcón R, Gómez M, Tobar R (1996) Physiological and nutritional responses by Lactuca sativa L. to nitrogen sources and mycorrhizal fungi under drought conditions. Biol Fertil Soils 22:156–161. https://doi.org/10.1007/s003740050091
Basiru S, Ait Si Mhand K, Hijri M (2023) Disentangling arbuscular mycorrhizal fungi and bacteria at the soil-root interface. Mycorrhiza 33:119–137
Baslam M, Esteban R, García-Plazaola JI, Goicoechea N (2013) Effectiveness of arbuscular mycorrhizal fungi (AMF) for inducing the accumulation of major carotenoids, chlorophylls and tocopherol in green and red leaf lettuces. Appl Microbiol Biotechnol 97:3119–3128. https://doi.org/10.1007/s00253-012-4526-x
Battini F, Cristani C, Giovannetti M, Agnolucci M (2016) Multifunctionality and diversity of culturable bacterial communities strictly associated with spores of the plant beneficial symbiont Rhizophagus intraradices. Microbiol Res 183:68–79. https://doi.org/10.1016/j.micres.2015.11.012
Bianciotto V, Lumini E, Bonfante P, Vandamme P (2003) “Candidatus Glomeribacter gigasporarum” gen. nov., sp. nov., an endosymbiont of arbuscular mycorrhizal fungi. Int J Syst Evol Microbiol 53:121–124. https://doi.org/10.1099/ijs.0.02382-0
Bona E, Cantamessa S, Massa N, Manassero P, Marsano F, Copetta A, Lingua G, D’Agostino G, Gamalero E, Berta G (2017) Arbuscular mycorrhizal fungi and plant growth-promoting pseudomonads improve yield, quality and nutritional value of tomato: a field study. Mycorrhiza, 27:1–11. https://doi.org/10.1007/s00572-016-0727-y
Bothe H, Turnau K, Regvar M (2010) The potential role of arbuscular mycorrhizal fungi in protecting endangered plants and habitats. Mycorrhiza 20(7):445–457. https://doi.org/10.1007/s00572-010-0332-4
Budi SW, May NL (2013) Bacteria from arbuscular mycorrhizal fungi spores Gigaspora sp. and Glomus sp.: their antagonistic effects towards soilborne fungal pathogens and growth stimulation of Gigaspora sp in vitro. Biotropia (Bogor) 20:38–49. https://doi.org/10.11598/btb.2013.20.1.38
Burton-Freeman BM, Sandhu AK, Edirisinghe I (2016) Red raspberries and their bioactive polyphenols: cardiometabolic and neuronal health links. Adv Nutr 7:44–65. https://doi.org/10.3945/an.115.009639
Chaudhary P, Singh S, Chaudhary A, Sharma A, Kumar G (2022) Overview of biofertilizers in crop production and stress management for sustainable agriculture. Front Plant Sci 13:1–21. https://doi.org/10.3389/fpls.2022.930340
Chen K, Kleijn D, Scheper J, Fijen TPM (2022) Additive and synergistic effects of arbuscular mycorrhizal fungi, insect pollination and nutrient availability in a perennial fruit crop. Agric Ecosyst Environ 325:107742. https://doi.org/10.1016/j.agee.2021.107742
Chen K, Scheper J, Fijen TPM, Kleijn D (2022) Potential tradeoffs between effects of arbuscular mycorrhizal fungi inoculation, soil organic matter content and fertilizer application in raspberry production. PLoS ONE 17:1–11. https://doi.org/10.1371/journal.pone.0269751
Chen Q, Deng X, Elzenga JTM, van Elsas JD (2022) Effect of soil bacteriomes on mycorrhizal colonization by Rhizophagus irregularis—interactive effects on maize (Zea mays L.) growth under salt stress. Biol Fertil Soils 58:515–525. https://doi.org/10.1007/s00374-022-01636-x
Compant S, Samad A, Faist H, Sessitsch A (2019) A review on the plant microbiome: ecology, functions, and emerging trends in microbial application. J Adv Res 19:29–37. https://doi.org/10.1016/j.jare.2019.03.004
Cruz AF, Ishii T (2008) Arbuscular mycorrhizal fungal spores host bacteria that affect nutrient biodynamics and biocontrol of soil- borne plant pathogens. https://doi.org/10.1242/bio.2011014
Delitte M, Caulier S, Bragard C, Desoignies N (2021) Plant microbiota beyond farming practices: a review. Front Sustain Food Syst 5:624203. https://doi.org/10.3389/fsufs.2021.624203
Flor-Peregrín E, Azcón R, Martos V, Verdejo-Lucas S, Talavera M (2014) Effects of dual inoculation of mycorrhiza and endophytic, rhizospheric or parasitic bacteria on the root-knot nematode disease of tomato. Biocontrol Sci Technol 24:1122–1136. https://doi.org/10.1080/09583157.2014.925091
Formenti L, Iwanycki Ahlstrand N, Hassemer G, Glauser G, van den Hoogen J, Rønsted N, van der Heijden M, Crowther TW, Rasmann S (2023) Macroevolutionary decline in mycorrhizal colonization and chemical defense responsiveness to mycorrhization. iScience 26:106632. https://doi.org/10.1016/j.isci.2023.106632
Gianinazzi S, Gollotte A, Binet M-N, van Tuinen D, Redecker D, Wipf D (2010) Agroecology: the key role of arbuscular mycorrhizas in ecosystem services. Mycorrhiza 20:519–530. https://doi.org/10.1007/s00572-010-0333-3
Gianinazzi S, Oubaha L, Chahbandar M, Blal B, Lemoine M-C (2003) Biotization of microplants for improved performance. Acta Hortic 625:165–172. https://doi.org/10.17660/ActaHortic.2003.625.17
Gianinazzi S, Schuepp H, Barea JM, Haselwandter K (2002) Mycorrhizal technology in agriculture. From Genes to Bioproducts. Springer Basel AG, pp 1-296
Gordon SA, Weber RP (1951) Colorimetric estimation of indoleacetic acid. Plant Physiol 26:192–195
Gustab M, Ważny R, Jędrzejczyk RJ, Kalisz A, Domka A, Nosek M, Tokarz K, Rozpądek P (2024) Beneficial impact of multi-bacterial inoculation on growth of selected Brassicaceae seedlings in a greenhouse culture. Sci Hortic 324:112575. https://doi.org/10.1016/j.scienta.2023.112575
Jeffries P, Gianinazzi S, Perotto S, Turnau K, Barea JM (2003) The contribution of arbuscular mycorrhizal fungi in sustainable maintenance of plant health and soil fertility. Biol Fertil Soils 37:1–16
Kocurek M, Kornas A, Pilarski J, Tokarz K, Lüttge U, Miszalski Z (2015) Photosynthetic activity of stems in two Clusia species. Trees - Structure and Function 29:1029–1040. https://doi.org/10.1007/s00468-015-1182-7
Lastovetsky OA, Caruso T, Brennan FP, Wall D, Pylni S, Doyle E (2024) Spores of arbuscular mycorrhizal fungi host surprisingly diverse communities of endobacteria. New Phytol. https://doi.org/10.1111/nph.19605
Lee J, Lee S, Young JPW (2008) Improved PCR primers for the detection and identification of arbuscular mycorrhizal fungi. FEMS Microbiol Ecol 65:339–349. https://doi.org/10.1111/j.1574-6941.2008.00531.x
Long L, Lin Q, Yao Q, Zhu H (2017) Population and function analysis of cultivable bacteria associated with spores of arbuscular mycorrhizal fungus Gigaspora margarita. 3 Biotech 7:8. https://doi.org/10.1007/s13205-017-0612-1
Lutz S, Bodenhausen N, Hess J, Valzano-Held A, Waelchli J, Deslandes-Hérold G, Schlaeppi K, van der Heijden MGA (2023) Soil microbiome indicators can predict crop growth response to large-scale inoculation with arbuscular mycorrhizal fungi. Nat Microbiol 8:2277–2289. https://doi.org/10.1038/s41564-023-01520-w
MacDonald RM, Chandler MR (1981) Bacterium-like organelles in the vesicular-arbuscular mycorrhizal fungus Glomus Caledonius. New Phytol 89:241–246. https://doi.org/10.1111/j.1469-8137.1981.tb07486.x
Mosse B (1970) Honey-coloured sessile Endogone spores. II. Changes in fine structure during spore development. Arch Mikrobiol 74:146–159
Naito M, Desirò A, González JB, Tao G, Morton JB, Bonfante P, Pawlowska TE (2017) ‘Candidatus Moeniiplasma glomeromycotorum’, an endobacterium of arbuscular mycorrhizal fungi. Int J Syst Evol Microbiol 67:1177–1184. https://doi.org/10.1099/ijsem.0.001785
Nanjundappa A, Bagyaraj DJ, Saxena AK, Kumar M, Chakdar H (2019) Interaction between arbuscular mycorrhizal fungi and Bacillus spp. In soil enhancing growth of crop plants. Fungal Biol Biotechnol 6:23. https://doi.org/10.1186/s40694-019-0086-5
Odokonyero K, Andres J, Arango C, De J, Jimenez C, Rao IM, Acuña TB (2015) Influence of fungal endophyte on plant water status, non-structural carbo- hydrate content and biomass partitioning in Brachiaria grasses grown under drought stress. “Building Productive, Diverse and Sustainable Landscapes“ Proceedings of the 17th ASA Conference, 20 – 24 September 2015, Hobart, Australia. Web site www.agronomy2015.com.au
Orłowska E, Przybyłowicz W, Orłowski D, Mongwaketsi NP, Turnau K, Mesjasz-Przybyłowicz J (2013) Mycorrhizal colonization affects the elemental distribution in roots of Ni-hyperaccumulator Berkheya coddii Roessler. Environ Pollut 175:100–109. https://doi.org/10.1016/j.envpol.2012.12.028
Ortiz N, Armada E, Duque E, Roldán A, Azcón R (2015) Contribution of arbuscular mycorrhizal fungi and/or bacteria to enhancing plant drought tolerance under natural soil conditions: effectiveness of autochthonous or allochthonous strains. J Plant Physiol 174:87–96. https://doi.org/10.1016/j.jplph.2014.08.019
Pikovskaya RI (1948) Mobilization of phosphorus in soil connection with the vital activity of some microbial species. Microbiology (N Y) 17:362–370
Rodriguez A, Sanders IR (2014) The role of community and population ecology in applying mycorrhizal fungi for improved food security. ISME J 9(5):1053–1061. https://doi.org/10.1038/ismej.2014.207
Rodriguez RJ, Henson J, Van Volkenburgh E, Hoy M, Wright L, Beckwith F, Kim Y-O, Redman RS (2008) Stress tolerance in plants via habitat-adapted symbiosis. ISME J 2:404–416. https://doi.org/10.1038/ismej.2007.106
Roesti D, Ineichen K, Braissant O, Redecker D, Wiemken A, Aragno M (2005) Bacteria associated with spores of the arbuscular mycorrhizal fungi Glomus geosporum and Glomus constrictum. Appl Environ Microbiol 71:6673–6679. https://doi.org/10.1128/AEM.71.11.6673-6679.2005
Rozpądek P, Domka A, Ważny R, Nosek M, Jędrzejczyk R, Tokarz K, Turnau K (2018) How does the endophytic fungus Mucor sp. improve Arabidopsis arenosa vegetation in the degraded environment of a mine dump? Environ Exp Bot 147:31–42. https://doi.org/10.1016/j.envexpbot.2017.11.009
Rozpądek P, Wężowicz K, Stojakowska A, Malarz J, Surówka E, Sobczyk Ł, Anielska T, Ważny R, Miszalski Z, Turnau K (2014) Mycorrhizal fungi modulate phytochemical production and antioxidant activity of Cichorium intybus L. (Asteraceae) under metal toxicity. Chemosphere 112:217–224. https://doi.org/10.1016/j.chemosphere.2014.04.023
Schwyn B, Neilands JB (1987) Universal chemical assay for the detection and determination of siderophores. Anal Biochem 160:47–56. https://doi.org/10.1016/0003-2697(87)90612-9
Selvakumar G, Shagol CC, Kim K, Han S, Sa T (2018) Spore associated bacteria regulates maize root K+/Na+ ion homeostasis to promote salinity tolerance during arbuscular mycorrhizal symbiosis. BMC Plant Biol 18:109. https://doi.org/10.1186/s12870-018-1317-2
Strasser R, Srivastava A, Tsimilli-Michael M (2000) The fluorescence transient as a tool to characterize and screen photosynthetic samples. In: Yunus M, Pathre U, Mohanty P (eds). Probing photosynthesis: mechanism, regulation & adaptation. CRC Press, London, 25:445–483
Tahiri A, ilah, Meddich A, Raklami A, Alahmad A, Bechtaoui N, Anli M, Göttfert M, Heulin T, Achouak W, Oufdou K, (2022) Assessing the potential role of compost, PGPR, and AMF in improving tomato plant growth, yield, fruit quality, and water stress tolerance. J Soil Sci Plant Nutr 22:743–764. https://doi.org/10.1007/s42729-021-00684-w
Tang B, Man J, Lehmann A, Rillig MC (2023) Arbuscular mycorrhizal fungi benefit plants in response to major global change factors. Ecol Lett. https://doi.org/10.1111/ele.14320
Taylor J, Harrier L (2000) A comparison of nine species of arbuscular mycorrhizal fungi on the development and nutrition of micropropagated Rubus idaeus L. cv. Glen Prosen (Red Raspberry). Plant Soil 225:53–61. https://doi.org/10.1023/A:1026519431096
Tokarz KM, Makowski W, Tokarz B, Hanula M, Sitek E, Muszyńska E, Jędrzejczyk R, Banasiuk R, Chajec Ł, Mazur S (2020) Can ceylon leadwort (Plumbago zeylanica L.) acclimate to lead toxicity?—studies of photosynthetic apparatus efficiency. Int J Mol Sci 21:1866. https://doi.org/10.3390/ijms21051866
Trivedi P, Leach JE, Tringe SG, Sa T, Singh BK (2020) Plant–microbiome interactions: from community assembly to plant health. Nat Rev Microbiol 18:607–621
Turner S, Pryer KM, Miao VP, Palmer JD (1999) Investigating deep phylogenetic relationships among cyanobacteria and plastids by small subunit rRNA sequence analysis. J Eukaryot Microbiol 46:327–338. https://doi.org/10.1111/j.1550-7408.1999.tb04612.x
Ujvári G, Turrini A, Avio L, Agnolucci M (2021) Possible role of arbuscular mycorrhizal fungi and associated bacteria in the recruitment of endophytic bacterial communities by plant roots. Mycorrhiza 31:527–544
van der Heijden MGA, Martin FM, Selosse MA, Sanders IR (2015) Mycorrhizal ecology and evolution: The past, the present, and the future. New Phytologist 205(4):1406–1423. https://doi.org/10.1111/nph.13288
Ważny R, Jędrzejczyk RJ, Rozpądek P, Domka A, Turnau K (2022) Biotization of highbush blueberry with ericoid mycorrhizal and endophytic fungi improves plant growth and vitality. Appl Microbiol Biotechnol 106:4775–4786. https://doi.org/10.1007/s00253-022-12019-5
Ważny R, Rozpądek P, Jędrzejczyk RJ, Śliwa M, Stojakowska A, Anielska T, Turnau K (2018) Does co-inoculation of Lactuca serriola with endophytic and arbuscular mycorrhizal fungi improve plant growth in a polluted environment? Mycorrhiza 28:235–246. https://doi.org/10.1007/s00572-018-0819-y
Yin B, Wang Y, Liu P, Hu J, Zhen W (2010) Effects of vesicular-arbuscular mycorrhiza on the protective system in strawberry leaves under drought stress. Front Agric China 4:165–169. https://doi.org/10.1007/s11703-010-0109-8
Yue H, Yue W, Jiao S, Kim H, Lee YH, Wei G, Song W, Shu D (2023) Plant domestication shapes rhizosphere microbiome assembly and metabolic functions. Microbiome 11:70. https://doi.org/10.1186/s40168-023-01513-1
Acknowledgements
The authors would like to acknowledge Teresa Anielska and Weronika Janas (Jagiellonian University in Kraków, PL) for technical support and Niwa Hodowla Roślin Jagodowych (PL company) for cooperation in the project.
Funding
This work was supported by The National Science Centre and The National Centre for Research and Development, Poland, project TANGO (contract No. TANGO1/269101/NCBR/2015). The open‐access publication of this article was funded by the Priority Research Area BioS under the programme ‘Excellence Initiative—Research University’ at the Jagiellonian University in Kraków.
Author information
Authors and Affiliations
Contributions
RW and KT conceived and designed the research. RW, AD, RJJ, PR, KMT MJ and KT performed research. RW analysed data and wrote the original draft. PR, RJJ, AD, KMT and KT edited the manuscript. KT acquired funding. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of Interest
The authors declare no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ważny, R., Jędrzejczyk, R.J., Rozpądek, P. et al. Bacteria Associated with Spores of Arbuscular Mycorrhizal Fungi Improve the Effectiveness of Fungal Inocula for Red Raspberry Biotization. Microb Ecol 87, 50 (2024). https://doi.org/10.1007/s00248-024-02364-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00248-024-02364-5