Abstract
Biological soil crusts (biocrusts) are considered “desert ecosystem engineers” because they play a vital role in the restoration and stability maintenance of deserts, including those cold sandy land ecosystems at high latitudes, which are especially understudied. Microorganisms participate in the formation and succession of biocrusts, contributing to soil properties’ improvement and the stability of soil aggregates, and thus vegetation development. Accordingly, understanding the composition and successional characteristics of microorganisms is a prerequisite for analyzing the ecological functions of biocrusts and related applications. Here, the Hulun Buir Sandy Land region in northeastern China—lying at the highest latitude of any sandy land in the country—was selected for study. Through a field investigation and next-generation sequencing (Illumina MiSeq PE300 Platform), our goal was to assess the shifts in diversity and community composition of soil bacteria and fungi across different stages during the succession of biocrusts in this region, and to uncover the main factors involved in shaping their soil microbial community. The results revealed that the nutrient enrichment capacity of biocrusts for available nitrogen, total nitrogen, total phosphorus, total content of water-soluble salt, available potassium, soil organic matter, and available phosphorus was progressively enhanced by the succession of cyanobacterial crusts to lichen crusts and then to moss crusts. In tandem, soil bacterial diversity increased as biocrust succession proceeded but fungal diversity decreased. A total of 32 bacterial phyla and 11 fungal phyla were identified, these also known to occur in other desert ecosystems. Among those taxa, the relative abundance of Proteobacteria and Cyanobacteria significantly increased and decreased, respectively, along the cyanobacterial crust–lichen–moss crust successional gradient. However, for Actinobacteria, Chloroflexi, and Acidobacteria their changed relative abundance was significantly hump-shaped, increasing in the shift from cyanobacterial crust to lichen crust, and then decreasing as lichen crust shifted to moss crust. In this process, the improved soil properties effectively enhanced soil bacterial and fungal community composition. Altogether, these findings broaden our understanding about how soil microbial properties can change during the succession of biocrusts in high-latitude, cold sandy land ecosystems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Biological soil crusts (biocrusts) are complex communities formed by microorganisms (bacteria, fungi, archaea), cryptoflora (algae, lichens, moss), and other microscopic organisms bonded to soil surface particles via various secretions, such as mycelium, rhizoides, and polysaccharides [1,2,3]. As one of the oldest known life forms, biocrusts appeared in the fossil record as early as 2.6 billion years ago [4]. That initial formation of biocrusts long ago is linked to how terrestrial ecosystems originated, in that the widespread development of biocrusts and their improvement of local climate and soil conditions enabled vascular plants to emerge and strongly compete, thereby forming distinct vegetation communities [3]. In this respect, arid and semi-arid ecosystems are particularly noteworthy, since they collectively cover 30–40% of the world’s terrestrial area [5], but in these relatively dry regions, their limited water carrying capacity restricts the viability of large multidimensional vascular plants. Yet, biocrusts are still widely distributed in these ecosystems, constituting at least 70% of their biological cover in some areas, where they effectively enhance soil stability and perform key ecological functions (e.g., providing windbreaks, regulating hydrology, maintaining moisture), as well increasing the fertility and microbial activity of soil [6,7,8,9]. Accordingly, biocrusts have earned the moniker “desert ecosystem engineers”, being robust indicators for evaluating the health of desert ecosystems [3, 10, 11].
The morphology and structure of biocrusts is highly diverse, being composed of algae, lichens, and mosses, whose functional types and taxa are mixed together in varying degrees. Although biocrusts count among the planet’s major terrestrial communities, their scientific study started late and early progress was limited. Cyanobacteria, algae, archaea, bacteria, and microfungi are the basic substrates of biocrust formation [12,13,14,15,16,17,18,19], which together promote colonization by lichens, bryophytes, and microorganisms [20,21,22,23]. At both global and regional scales, the composition and biomass of particular biocrust communities strongly depends on climatic conditions [24]. For example, for regions whose evapotranspiration potential is relatively high, their biocrusts are mostly composed of low-biomass cyanobacteria, bacteria, and microfungi; i.e., cyanobacterial crusts, lacking mosses or lichens [25, 26]. With declining evapotranspiration, cyanobacteria increase in biomass, lichens and mosses appear, leading to the differentiation and diversification of biocrust types [27, 28]. Beside climate, the soil microhabitat and its characteristics—soil type, texture, nutrient content, salinity, pH, and moisture—can be critical factors shaping the composition and distribution of biocrusts on a regional scale [24, 29].
According to the dominant cryptogam present in them during their succession, biocrusts can be broadly classified into three stages: cyanobacterial crust, lichen crust, and moss crust [30]. As their main biological components, soil microbes (bacteria, fungi, archaea) are collectively responsible for essential ecological functions. Not surprisingly then, trends in the number of dominant species, α-diversity and richness, and community composition of microorganisms across biocrust stages are strongly correlated with biocrust succession [17, 19, 31,32,33]. Only recently, however, have we begun to explore how the biomass, species composition or ecological roles of these microbial organisms is changed under differing environmental conditions. Technical limitations precluded robust estimates of microbial diversity in previous studies of biocrusts. Fortunately, driven by technological advances in molecular biology within the last decade, microbiome techniques can now be readily applied to reveal the composition of microbial communities at different stages of biocrust succession.
Given the crucial ecological functions of biocrusts in arid and semi-arid ecosystems, in recent years the microbial community composition of biocrusts at different successional stages has been extensively studied in various deserts in distinct bioclimatic zones. This includes cold deserts (e.g., Colorado Plateau in the USA) typically found in temperate regions at high elevations, on plateaus, or in mountainous areas, though they also occur in polar regions (Antarctica and Arctic)—that is, generally where the regional mean annual temperature is close to 0 °C [33,34,35,36,37]; temperate deserts (e.g., Gurbantunggut Desert and Tengger desert in China, Kyzyl kum desert in Uzbekistan), these usually located at mid-high latitudes, where the regional mean annual temperature is under 18 °C [17, 19, 38,39,40,41]; and hot deserts (e.g., Atacama Desert in Chile, Namib Desert in Namibia, Negev Desert in Israel), these usually lying at mid-low latitudes and featuring a mean annual temperature above 18 °C, featuring hot summers, daytime temperatures regularly exceeding 30 °C, mild winter climates, and rainfall concentrated in summer (these three desert categories—cold, temperate, and hot—follow the usage at www.britannica.com) [42,43,44,45,46,47]. Yet, despite recent investigations in several regions, significant knowledge gaps remain concerning the composition of biocrust communities on soils in certain regions, particularly those in underrepresented areas [48, 49].
One of China’s largest sandy land areas is Hulun Buir Sandy Land, lying at the highest latitude among them. According to the above desert classification (www.britannica.com), the Hulun Buir Sandy Land is arguably a cold desert. Until now, however, no attempt has been made to investigate the soil microbial community composition of its biocrusts and the drivers of their succession process. Hence, the overarching goal of this study was to apply next-generation-sequencing (NGS) to reveal how the soil microbial community changes during biocrust succession in the Hulun Buir Sandy Land region. Furthermore, considering its high latitude and cold climate, we asked: Could the microbial community composition characteristics and pattern of biocrust succession in this region differ from those in other desert ecosystems? Therefore, our objectives were three-fold: (1) to uncover prominent trends in soil physical and chemical properties during biocrust development and succession; (2) to profile the α- and β-diversity of the soil microbial community vis-à-vis biocrust development and succession; and (3) to elucidate relationships between these complex microbial communities and aspects of their environment. The ecological findings will not only bolster our understanding of biocrusts and their community structure, but are also critical for expanding our knowledge of their diversity and functioning across terrestrial ecosystems.
Materials and Methods
Study Site and Soil Sampling
The field research was carried out in the New Barag Left Banner of Hulun Buir Sandy Land (Fig. 1), which consists of three large sand belts in northeastern China. Our study site was in the eastern end of the biggest sand belt, which lies along the southern bank of the Hailar River in the northern part of Hulun Buir Sandy Land (118°4′9.7356″ E, 49°19′9.9732″ N, Fig. 1). This region has a temperate continental climate, with a mean annual precipitation (MAP) of 290 to 400 mm and a mean annual temperature (MAT) of − 5 to 1.5 °C. The zonal vegetation type is typical temperate grassland dominated by annual herbaceous plants, such as Leymus chinensis, Stipa grandis, Agropyron cristatum, and Carex duriuscula [50].
Location of the study site in northeastern China where different stages of biocrust succession in the Hulun Buir Sandy Land were sampled (n = 12 interspersed plots). Along the bottom are images of stage 1, bare sand (i.e., control, n = 3); stage 2, cyanobacterial crust (n = 3); stage 3, lichen crust (n = 3); and stage 4, moss crust (n = 3), which formed the successional gradient investigated (using a ‘space-for-time’ substitution approach). Red arrow and red circle mean lichen crust and moss crust, respectively
In this study, we used a “space-for-time substitution” method to uncover changes in microbial composition during the biocrusts succession in the Hulun Buir Sandy Land in China. At the research site, a total of 12 plots (each 20 m × 20 m) were established, corresponding to four soil cover types: bare sand (no visible crusts, as the control; stage 1), cyanobacterial crust (stage 2), lichen crust (stage 3), and moss crust (stage 4) (Fig. 1). Plots having the same biocrust successional stage were separated by more than 1 km. Within each plot, we established four representatives’ subplots (each 5 m × 5 m, at least 10 m apart), in which five soil samples were randomly taken at a 0–5 cm depth under the biological layer, using a sterile cutting ring (9.0-cm diam.). These subplot-level samples were mixed to form one composite sample per plot for each soil cover type, for a total of 12 composite plot-level replicate samples (three × four soil cover types). These were sieved (through 2.0 mm mesh) to remove any visible roots and stones, and then each sample was divided into three portions for further analyses. The first was simply air-dried; the second was stored at 4 °C, to analyze various soil physiochemical properties; immediately after its collection, the third portion was transported on ice to the laboratory where it was freeze-dried at –80 °C for subsequent DNA extractions.
Soil Physiochemical Properties
For each sample per soil cover type, measurements of its soil material (second portion) were made. Soil particle size composition was determined using a Laser Diffraction Particle Size Analyzer (Mastersizer 2000, Malvern, England). Soil pH of a suspension of soil: water in a 1:5 ratio was recorded with a calibrated pH meter (Mettler Toledo, Giessen, Germany) [51]. To measure the total content of water-soluble salt (WST) and soil organic matter (SOM), we respectively used the residue drying-quality and K2Cr2O7 methods [52]. Total nitrogen (TN) was measured using a Kjeldahl analysis system (Kjeltec 8400, Foss, Hillerød, Denmark) [53]. Total phosphorus (TP) was determined via colorimetry using sulfuric acid-perchloric acid digestion [54]. Flame photometry was used to quantify total potassium (TK), using a perchloric acid-hydrofluoric acid digestion [55]. The soil available nitrogen (AN), available phosphorus (AP), and available potassium (AK) were determined by alkaline hydrolysis diffusion method, molybdenum antimony colorimetry, and flame photometry method, respectively [56].
Soil DNA Extraction and High-throughput Sequencing
To extract soil DNA from the biocrust samples, the E.Z.N.A. DNA Kit (Omega Bio-Tek, Norcross, GA, USA) was used, by following the manufacturer’s protocols. The obtained nine DNA extracts (three plot-level replicates for each biocrust type (i.e., stages 2, 3, 4) and control (stage 1)) were PCR-amplified and then underwent sequencing analyses. The bacterial 16S ribosomal RNA gene and fungal ITS rRNA genes were respectively amplified, using the primer pairs of 338F_806R [57] and ITS1F_ITS2R [58], under these thermocycling parameters: 95 °C for 3 min, followed by 25 cycles at 95 °C for 30 s, 55 °C for 30 s, and 72 °C for 45 s, with a final extension at 72 °C for 10 min. Both PCRs were performed in triplicate, each using a 20-μL reaction mixture containing 2 μL of 5 × FastPfu buffer, 2 μL of 2.5 mM dNTPs, 0.8 μL of each F/R primer (5 μM), 0.2 μL of FastPfu polymerase, and 10 ng of template DNA.
Amplicons were first extracted from 2% agarose gels and purified, using an AxyPrepDNA gel extraction kit (Axygen Biosciences, Union City, CA, USA) according to the manufacturer’s instructions, and then quantified with a QuantiFluor-ST fluorometer (Promega, Madison, WI, USA). Purified amplicons were pooled in equimolar quantities and paired-end sequenced (2 × 300 bp) on the Illumina MiSeq platform PE300. All obtained raw reads have been deposited into the database of the NCBI Sequence Read Archive (SRA) (accession number: PRJNA1026485).
Bioinformatics Analysis
The paired-end reads from the original DNA fragments were merged using FLASH software [59], a tool designed to combine them when reads 1 and 2 overlap. The resulting paired-end reads were then assigned to each sample according to their unique barcodes. Next, the raw sequencing data were quality-filtered according to these criteria: (i) the reads were truncated at any site that received an average quality score < 20 over a 50-bp sliding window, with those truncated reads < 50 bp removed; and (ii) any reads that had exact barcode matches, or two nucleotide mismatches during primer matching, and which contained ambiguous characters were discarded. Only sequences with overlaps > 10 bp were assembled, this according to their overlap sequence; those reads that could not be assembled were discarded. Operational taxonomic units (OTUs) were clustered with a 97% similarity cutoff using UPARSE [60] and chimeric sequences were identified and removed using UCHIME [61]. Singleton OTUs was removed from the dataset. The taxonomic status of the 16S and ITS rRNA were identified using the RDP Classifier (http://rdp.cme.msu.edu/) against the SILVA (v132) (https://www.arb-silva.de/) or Unite (v8.0) (https://unite.ut.ee/) [62] database, respectively, at a 0.7-confidence threshold. Importantly, to account for differences in their sequencing depth, all samples were normalized in QIIME software (v1.8.0) [63]. The ensuing OTUs were used to calculate the α-diversity and β-diversity metrics.
Statistical Analysis
The α-diversity (http://www.mothur.org/wiki/Calculators) indexes were calculated using the diversity function of the “vegan” package (https://CRAN.R-project.org/package=vegan) for the R computing platform (v3.2.1) (www.r-project.org) [64]. To examine differences in the relative abundance of dominant groups of the bacterial (genus level) or fungal (family level) community among stages 1–4, the Kruskal–Wallis test followed by Tukey’s HSD (honest significant difference) post hoc test was used, both implemented in R with the “agricolae” package (https://CRAN.R-project.org/package=agricolae).
To gauge the relevance of soil physiochemical properties (i.e., D1, D2, D3, pH, AN, TN, TK, TP, WST, AK, SOM, and AP) and assess their ability to explain variation in the distribution patterns of microbial community members in the different biocrusts samples, distance-based redundancy analysis (db-RDA) and Monte Carlo permutations were used. Mantel tests were implemented to evaluate how bacterial or fungal community composition was related to the measured site-level soil variables. Pearson’s r coefficient was used to test for a positive (R > 0) or negative (R < 0) linear correlation between two variables, carried out in R using its cor () function. The “vegan” package was used to run the db-RDA and Mantel tests in R, and also to build the matrices for the pairwise taxonomic distances for bacterial or fungal communities (Bray–Curtis dissimilarity) vis-à-vis the environmental variables (Euclidean distance).
Results and Discussion
Response of Soil Physicochemical Properties to Biocrust Succession
With the succession of biocrusts, significant differences in soil properties emerged in moss crust vis-à-vis the other two crust types and the bare sand (i.e., the control) (Table 1). Regarding the three soil particle diameter (D) properties—0.002 < D1 ≤ 0.02 mm; 0.02 < D2 ≤ 2 mm; D3 < 0.002 mm—in comparison to bare sand (stage 1), there was a lower proportion of D1 in all three biocrust types, which declined through their succession trajectory (stage 2 > stage 3 > stage 4. Conversely, the D3 proportion rose in all three biocrusts, but their D2 proportion remained similar along the successional gradient. Of the nine soil chemical properties, the contents of seven (AN, TN, TP, WST, AK, SOM, and AP) tended to increase in the three biocrust types relative to bare sand, being significantly higher in moss crust (stage 4) than either cyanobacterial crust (stage 2) or lichen crust (stage 3). Impressively, in moss crust, these AN, TN, TP, WST, AK, SOM, and AP contents were respectively 3.26, 10, 2.71, 3.38, 5.51, 13.31, and 4.33 times greater than in bare sand, respectively (Table 1). Evidently, the development and succession of biocrusts resulted in the enrichment of the shallow soil layer with carbon (C), nitrogen (N), and phosphorus (P), the most common limiting elements in terrestrial ecosystems. In contrast, both soil pH and TK were negligibly affected by the succession of biocrusts in Hulun Buir Sandy Land.
These results can be explained by the powerful ecological enhancement function of biocrusts, which mediate most of the input, transport, and output of matter and energy at the surface boundaries of desert soils. Soil aggregates produced by biocrusts have been shown stabilize soil particles and soil structure [65, 66], thereby altering the ecohydrological processes of desert ecosystems [3] in addition to capturing and retaining resources (e.g., soil, organic matter, seeds, and nutrient-rich dust) [67,68,69,70]. Further, biocrusts can bolster soil fertility by fixing atmospheric C and N [71, 72] and releasing it into the subsoil layer, thus contributing to the global C and N cycling [73]. Moreover, biocrusts were also identified as a key component of biogeochemical phosphorus cycling during the pedogenesis of sandy substrates [74, 75]. Overall, our findings suggest biocrust development markedly improves the soil properties of bare sand, with a well-developed crust (i.e., moss crust, end of succession: stage 4) having a stronger ameliorating effect than less-developed crusts (cyanobacterial or lichen crusts). Actually, our findings largely agreed with those recently reported for European dunes [76].
Structure and Succession of the Biocrust Microbial Community
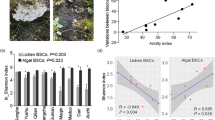
Microbial α-diversity was estimated by the Chao1 index (Fig. 2). Whereas its mean value for the bacterial community ranged from 1988.7 ± 235.07 (stage 1) to 2529.8 ± 358.53 (stage 4) (Fig. 2A), it was much lower for the fungal community, ranging from 722.24 ± 196.56 (stage 4) to 943.01 ± 114.2 (stage 2) across the successional gradient. In general, while the bacterial community’s α-diversity continually increased, the fungal community’s first increased and then decreased through succession, but these changes were not significant (P > 0.05). Other calculated indexes for species richness and diversity of the bacterial and fungal communities are summarized in Table S1.
Microbial α-diversity (Chao1 index) of the four biocrust successional types and their comparison. Shown are boxplots for the Chao1 index values of the bacterial (A) and fungal (B) community in the four stages of biocrust succession. The differences in means between any two were compared statistically by the t test. The horizontal line is the median value; an asterisk between two boxplots indicates their significant difference at * P < 0.05; stage 1, bare sand (n = 3); Stage 2, cyanobacterial crust (n = 3); stage 3, lichen crust (n = 3); stage 4, moss crust (n = 3)
That bacterial α-diversity increased with biocrust succession—from bare sandy soil to lichen crusts or moss crusts—is consistent with successional theory of low-to-high level shifts in diversity; hence, in this respect, the dynamics of Hulun Buir Sandy Land are much like other desert ecosystems [17, 30, 31, 33, 77,78,79]. Interestingly, fungal α-diversity was similar across the different stages, conflicting with the view that it continually increases across biocrust successional stages [78, 80]. In general, fungal diversity has been found to vary with both the age and type of biocrusts, being higher in their late than early succession [81]. We suggest this discrepancy is most probably due to habitat specificity effects on a regional scale. Again, our results show that mossy crusts could provide more pivotal resources and protection for soil bacterial communities, mainly because of their higher dust capture, water-holding, and nutrient retention capacities [45].
Overall, 32 bacterial and 11 fungal phyla were detected across all 12 plot-level samples based on NGS sequencing. For bacteria, the six dominant phyla (i.e., with a relative abundance > 5%) in all samples of stages 1–4 were Cyanobacteria and Actinobacteriota, respectively constituting 23.83% and 23.09% of all sequences, on average; followed by Proteobacteria (17.68%), Chloroflexi (9.71%), Bacteroidota (8.39%), and Acidobacteriota (5.53%) (Fig. 3A; Table S2). For fungi, the dominant phylum in all soil samples was Ascomycota, on average constituting 72.03% of all sequences, followed far behind by Basidiomycota (18.70%), along with Fungi_unclassified (4.73%) and Chytridiomycota (4.32%) (Fig. 3B; Table S2). Globally, the bacterial phyla Actinobacteria, Cyanobacteria, Proteobacteria, Firmicutes, Chloroflexi, Bacteroidetes, Acidobacteria, Verrucomicrobia, Gemmatimonadetes, Planctomycetes, and Deinococcus-Thermus [21, 38, 42, 82] and the fungal phyla Ascomycota, Basidiomycota, and Chytridiomycota [17, 42, 83] have been reported as the most abundant taxa across all biocrusts developmental stages in various desert ecosystems. Hence, our findings strongly agreed with those reported assessments.
Not surprisingly, in our study, the relative abundances of those phyla changed with the successional stage of biocrusts. For bacteria, in shifting from cyanobacterial crust to lichen crust and then to moss crust, the corresponding relative abundance of Proteobacteria increased significantly (from 15.33 to 16.08% and then to 23.34%), while that of Cyanobacteria decreased significantly (from 35.16 to 17.00% and then 20.23%) (Fig. 3A; Table S2). Meanwhile, a hump-shaped response whereby relative abundance rose then fell was found for Actinobacteria (from 18.50 to 24.68% and then 21.98%), Chloroflexi (from 8.26 to 14.04% and then 8.06%), and Acidobacteria (from 4.89 to 8.78% and then 4.92%). Of them, Proteobacteria are dominant in a wide range of harsh conditions, especially oligotrophic habitats, with Actinobacteria also described as a dominant group in desert soils given their ability for filamentous growth, which may effectively mitigate damage from drought, high temperatures, and UV radiation [84,85,86]. Moreover, as the oldest known photosynthetic autotrophic component of biocrusts, Cyanobacteria can survive and rapidly grow in water and nutrient-poor desert soils; the fossilized soil structure of a 2.6-billion-year-old biocrust indicates that it was most likely composed of Cyanobacteria members [4, 6]. We found that Firmicutes usually attained their highest relative abundance in desert topsoil, but then gradually declined in the course of biocrust succession. Similarly, many other studies have shown that, during the succession of biocrusts, the Cyanobacteria initially dominant in the cyanobacterial crust undergo a predictable reduction in abundance as Actinobacteria, Proteobacteria, Chloroflexi, Acidobacteria, Gemmatimonadetes, Bacteroidetes, Planctomycetes, Verrucomicrobia, and Deinococcus-Thermus become more common [12,13,14,15,16,17, 19, 21, 38, 42]. Furthermore, a total of 154 bacterial genera displayed significant differences in their relative abundance across the successional gradient (i.e., stages 1–4) (Fig. 4; Table S3). Among those, the 15 most abundant (in descending rank) were Microcoleus_PCC-7113, norank_Coleofasciculaceae, Crinalium_SAG_22.89, norank_Acetobacteraceae, norank_Frankiales unclassified_Micromonosporaceae, Deinococcus, Roseisolibacter, Acidiphilium, Microvirga, Haliangium, Arthrobacter, norank_Vicinamibacterales, norank_Spirosomaceae, and Candidatus_Alysiosphaera (Fig. 4A; Table S3). Both Microcoleus_PCC-7113 and norank_Coleofasciculaceae, which were always affiliated with Cyanobacteria, each reached a significantly higher relative abundance in cyanobacterial crust than in either lichen crust or moss crust and especially vis-à-vis bare sand (stage 1) in the Hulun Buir Sandy Land. Although more than 320 cyanobacterial species from 70 genera have been identified in biocrusts so far, few actually participate in biocrust formation [3]. Among these, Microcoleus is the most dominant cyanobacterial genus in biocrusts found in most arid and semi-arid regions, such as the Colorado Plateau in the USA [34], the Negev Desert in Israel [46, 47], and both the Gurbantunggut Desert and Tengger Desert in China, as well as the Kyzyl-Kum desert in Uzbekistan [41, 87]; its species are typical filamentous nonheterocystous cyanobacteria. Notably, M. vaginatus and M. steenstrupii are often affiliated with Microcoleus, albeit harboring different adaptations to temperature, and both species appear dominant in cyanobacterial biocrust communities worldwide; the former being more abundant in cooler environments, while the latter dominates warmer ones [3, 41, 88]. Also belonging to the Cyanobacteria is the Coleofasciculaceae family, whose members reach substantially higher relative abundances in cyanobacterial crust than other biocrust types, being widely found in the Tengger Desert and Kyzyl-Kum Desert, as well as the Tabernas Desert in Spain [39, 41].
Differences in the relative abundance (%) of the top-15 bacterial genera (A) and the top-15 fungal families (B) in the microbial communities among the four biocrust successional types. For each taxon, the asterisk on the right indicates a significant difference among stages (P < 0.05). Stage 1, bare sand (n = 3); stage 2, cyanobacterial crust (n = 3); stage 3, lichen crust (n = 3); stage 4, moss crust (n = 3)
Unlike bacteria, for fungi its community composition has been reported to change negligibly during biocrust succession, with Chytridiomycota found at lower relative abundance in bare sandy soils [42] whereas Ascomycota reached maximal abundances (over 60%) in different successional stages [17]. In our study, however, the relative abundance of Ascomycota in the lichen crust (stage 2) was below 60% (ca. 52%), while that of Basidiomycota reached as high as 40% (Fig. 3B). Conversely, Chytridiomycota was greatly reduced in abundance, from 11.08% (bare sand) to 0.79% (moss crust), across the biocrust successional gradient of Hulun Buir Sandy Land. In fact, Chytridiomycota has only been detected at very tiny abundances in the Oman and Chihuahuan deserts up to date worldwide [89, 90]. These results are consistent with those of previous studies, which together suggest that Chytridiomycota dominate the early stage of biocrust development, hinting at their tolerance of stressful environments [42]. Collectively, these phyla showed no site-specificity and were ubiquitous in previous research in various desert soil and biocrusts. We found a total of 25 fungal families whose relative abundance differed significantly among stages 1–4 that formed the successional gradient (Table S3). Of those, the 15 most abundant (in descending order) were Atheliaceae, Trichomeriaceae, Didymellaceae, Pleosporaceae, Aspergillaceae, Camarosporidiellaceae, unclassified_Agaricomycetes, Verrucariaceae, Taphrinaceae, Magnaporthaceae, Sclerotiniaceae, Periconiaceae, Cantharellales_fam_Incertae_sedis, Trimorphomycetaceae, and Cephalothecaceae (Fig. 4B; Table S3). This provides compelling evidence that fungal community composition varies more considerably at the family than phylum level through the succession of biocrusts.
Environmental Factors Influencing the Community Composition of Biocrust Types
The Mantel test results revealed that variation in bacterial and fungal community composition (weighted UniFrac distance matrix-based) responded to the 12 soil parameters examined (Fig. 5; Table S4). Notably, bacterial community composition was positively and strongly correlated with both D1 (r = 0.657, p = 0.001) and AK (r = 0.534, p = 0.004), moderately so with SOM (r = 0.454, p = 0.003), TN (r = 0.439, p = 0.007), AN (r = 0.437, p = 0.007), and WST (r = 0.399, p = 0.01), and likewise, but to a lesser degree, with TP (r = 0.375, p = 0.016) and AP (r = 0.335, p = 0.023) (Fig. 5; Table S4). The fungal community composition also had positive correlations of similar magnitude with D1 (r = 0.715, p = 0.001), AK (r = 0.507, p = 0.001), SOM (r = 0.449, p = 0.002), TN (r = 0.430, p = 0.001), AN (r = 0.394, p = 0.006), and WST (r = 0.389, p = 0.01), along with TP (r = 0.390, p = 0.012) as well as AP (r = 0.342, p = 0.043) (Fig. 5; Table S4).
Pairwise comparisons of environmental factors with soil bacterial or fungal community composition of the four biocrusts (pooled, n = 12). In the heat map, the magnitude of positive and negative correlations is respectively indicated by the depth of blue and red coloring; Pearson’s r > 0 is a positive correlation and r < 0 is a negative correlation. The correlation strength (Mantel’s r) between bacterial or fungal community composition and a particular environmental factor is indicated by the thickness of their connecting curved line; its color denotes the degree of statistical significance (Mantel’s p). The size of each inner box is proportional to Mantel’s r statistic for the corresponding distance correlations, whose color denotes the degree of statistical significance (based on 9999 permutations). D, soil particle diameter: 0.002 mm < D1 ≤ 0.02 mm; 0.02 mm < D2 ≤ 2 mm; D3 ≤ 0.002 mm; AN, available nitrogen; TN, total nitrogen; AK, available potassium; TK, total potassium; TP, total phosphorus; WST, total content of water-soluble salt; SOM, soil organic matter
Furthermore, we used db-RDA to evaluate the effects of five VIFs (variance inflation factors) on soil bacterial and fungal community composition (Fig. 6; Table S5). These results showed that about 31.51% of the variance in bacterial community composition could be explained by the selected edaphic properties (Fig. 6A; CAP1 and CAP2 explained 19.66% and 11.85% of the variance, respectively). Crucially, three variables alone were mainly responsible for successional shifts in the bacterial community composition of biocrusts: WST (r2 = 0.825, p = 0.002), D1 (r2 = 0.770, p = 0.002), and pH (r2 = 0.726, p = 0.004) (Fig. 6A; Table S5). Likewise, for fungal community composition, edaphic properties accounted for about 27.86% of its variance (Fig. 6B; CAP1 and CAP2 explained 17.10% and 10.76% of the variance, respectively). In this respect, the observed shifts in fungal community composition were driven by four variables: D1 (r2 = 0.868, p = 0.001), WST (r2 = 0.751, p = 0.003), pH (r2 = 0.521, p = 0.028), and TK (r2 = 0.525, p = 0.037) (Fig. 6B; Table S5). Nonnegligible, only a small proportion of their community-level variation could be explained by all variables we examined, especially for fungal taxa, for which a high proportion of variation was unexplained. It is largely ascribed to the unmeasured environmental variables. So much unexplained variation in the communities of bacteria and fungi belowground suggested potential effects of neutral or stochastic processes upon community assembly during the succession of biological soil crusts, especially for the fungi [91, 92]. Therefore, more environmental variables, especially availability of soil nutrients (i.e., Ca2+, Mg2+, and Al−), should be incorporated into coupling analysis in the future.
Distance-based redundancy analysis (db-RDA) ordination plots of the relation between five environmental factors and the soil (A) bacterial or (B) fungal communities of the four successional biocrust types. D, soil particle diameter: 0.002 mm < D1 ≤ 0.02 mm; 0.02 mm < D2 ≤ 2 mm; TK, total potassium; WST, total content of water-soluble salt; stage 1, bare sand (n = 3); stage 2, cyanobacterial crust (n = 3); stage 3, lichen crust (n = 3); stage 4, moss crust (n = 3). Each circular symbol is a plot-level sample
Previous studies have demonstrated that certain soil properties, namely pH, soil organic carbon, and salinity, can variously play an instrumental role in shaping soil microbial diversity and community composition. Thus, as our results suggest, biocrusts may indirectly affect the microbial community in their underlying soil via their modulation of chemical soil properties. Importantly, the impact of environmental factors on soil bacterial and fungal communities depends on the spatial scale considered. Globally, soil pH is deemed the paramount determinant of bacterial community composition [93, 94]. Regionally, however, the soil type, texture, nutrient content, salinity, and moisture are all critical factors governing the bacterial structure and composition of biocrusts [24, 29, 76]. Despite this new knowledge of changing microbial characteristics through the succession of biocrusts, the responsible mechanisms remain unclear. Therefore, distinguishing the fundamental ecological processes (deterministic versus stochastic) shaping soil microbial community composition in the Hulun Buir Sandy Land is a future research priority of ours. Moreover, we used a “space-for-time substitution” sampling approach to reflect the changes in microbial composition along the cyanobacterial crust–lichen–moss crust successional gradient in Hulun Buir Sandy Land. Admittedly, in our analysis, only three samples from each stage included. Consequently, such small sample size may lead to a bias in the analysis of the results. Thus, it is necessary to collect further samples for a complementary analysis in the future.
Over all, our findings thus lend support to this emerging view, and point to complex, possibly divergent mechanisms at work in shaping the successional microbial dynamics of biocrusts in cold desert ecosystems.
Conclusion
This study employed a “space-for-time substitution” to infer changes in soil properties and microbial dynamics during the succession of biocrusts in the Hulun Buir Sandy Land of Northeast China. Our results revealed significant improvement in the aggregated structure and nutrient status of the shallow soil layer in the course of biocrust succession (i.e., going from bare sandy surface to cyanobacterial crust, then to lichen crust, and eventually to moss crust). Meanwhile, soil bacteria and fungi exhibited contrasting trends during succession, with the former increasing but the latter decreasing. As biocrust succession progressed, soil bacterial and fungal communities at various taxonomic levels (phylum and genus) underwent predictable shifts, albeit to varying degrees, this largely driven by altered soil properties. Although more in-depth studies are needed for the present study, these results still provide guidance for analyzing the ecological functions of biocrusts and related applications.
Data availability
The dataset generated during and/or analyzed during the current study is available at NCBI Sequence Read Archive (SRA) (accession number: PRJNA1026485).
Abbreviations
- D :
-
Soil particle diameter, 0.002 mm < D1 ≤ 0.02 mm, 0.02 mm < D2 ≤ 2 mm; D3 < 0.002 mm
- AN:
-
Available nitrogen
- TN:
-
Total nitrogen
- AK:
-
Available potassium
- TK:
-
Total potassium
- TP:
-
Total phosphorus
- WST:
-
Total content of water-soluble salt
- SOM:
-
Soil organic matter
- NGS:
-
Next-generation sequencing
References
Belnap J, Eldridge D (2003) Disturbance and recovery of biological soil crusts. In: Belnap J, Lange OL (eds) Biological soil crusts: Structure, function, and management. Springer, Berlin Heidelberg, Berlin, Heidelberg, pp 363–383
Li XR, Jia RL, Zhang ZS et al (2018) Hydrological response of biological soil crusts to global warming: A ten-year simulative study. Glob Change Biol 24(10):4960–4971
Weber B, Büdel B, Belnap J (2016) Biological soil crusts: An organizing principle in drylands. In: Belnap J, Weber B, Büdel B (eds) Chapter 1 Biological Soil Crusts as an Organizing Principle in Drylands. Springer, Berlin, pp 3–13
Beraldi-Campesi H, Retallack GJ (2016) Terrestrial ecosystems in the Precambrian. In: Weber B, Büdel B, Belnap J (eds) Biological Soil Crusts: An Organizing Principle in Drylands. Springer-Verlag, Switzerland, pp 37–54
Rodriguez-Caballero E, Belnap J, Büdel B et al (2018) Dryland photoautotrophic soil surface communities endangered by global change. Nat Geosci 11(3):185–189
Beraldi-Campesi H, Hartnett HE, Anbar A et al (2009) Effect of biological soil crusts on soil elemental concentrations: Implications for biogeochemistry and as traceable biosignatures of ancient life on land. Geobiology 7(3):348–359
Knelman JE, Schmidt SK, Graham EB (2021) Cyanobacteria in early soil development of deglaciated forefields: Dominance of non-heterocytous filamentous cyanobacteria and phosphorus limitation of N-fixing Nostocales. Soil Biol Biochem 154:108127
Nevins CJ, Inglett PW, Reardon CL et al (2022) Seasonality drives microbiome composition and nitrogen cycling in soil below biocrusts. Soil Biol Biochem 166:108551
Ding YA, Geng YC, Zhou WC et al (2023) Habitat-specific environmental factors regulate the spatial variability of biological soil crust microbial communities on the Qinghai-Tibet Plateau. Sci Total Environ 901:165937
Aslam SN, Dumbrell AJ, Sabir JS et al (2016) Soil compartment is a major determinant of the impact of simulated rainfall on desert microbiota. Environ Microbiol 18(12):5048–5062
Bowker MA (2007) Biological soil crust rehabilitation in theory and practice: An underexploited opportunity. Restor Ecol 15(1):13–23
Beraldi-Campesi H (2013) Early life on land and the first terrestrial ecosystems. Ecol Process 2(1):1
Muñoz-Martín MÁ, Becerra-Absalón I, Perona E et al (2019) Cyanobacterial biocrust diversity in Mediterranean ecosystems along a latitudinal and climatic gradient. New Phytol 221(1):123–141
Ferrenberg S, Tucker CL, Reed SC (2017) Biological soil crusts: diminutive communities of potential global importance. Front Ecol Environ 15(3):160–167
Rossi F, Li H, Liu Y et al (2017) Cyanobacterial inoculation (cyanobacterisation): Perspectives for the development of a standardized multifunctional technology for soil fertilization and desertification reversal. Earth Sci Rev 171:28–43
Blay ES, Schwabedissen SG, Magnuson TS et al (2017) Variation in biological soil crust bacterial abundance and diversity as a function of climate in cold steppe ecosystems in the Intermountain West, USA. Microb Ecol 74(3):691–700
Liu LC, Liu YB, Hui R et al (2017) Recovery of microbial community structure of biological soil crusts in successional stages of Shapotou desert revegetation, northwest China. Soil Biol Biochem 107:125–128
Steven B, Gallegos-Graves LV, Belnap J et al (2013) Dryland soil microbial communities display spatial biogeographic patterns associated with soil depth and soil parent material. FEMS Microbiol Ecol 86(1):101–113
Zhang BC, Zhang YQ, Li XZ et al (2018) Successional changes of fungal communities along the biocrust development stages. Biol Fertil Soils 54(2):285–294
Concostrina-Zubiri L, Martínez I, Escudero A (2018) Lichen-biocrust diversity in a fragmented dryland: Fine scale factors are better predictors than landscape structure. Sci Total Environ 628–629:882–892
Xiao B, Veste M (2017) Moss-dominated biocrusts increase soil microbial abundance and community diversity and improve soil fertility in semi-arid climates on the Loess Plateau of China. Appl Soil Ecol 117–118:165–177
Elbert W, Weber B, Burrows S et al (2012) Contribution of cryptogamic covers to the global cycles of carbon and nitrogen. Nat Geosci 5(7):459–462
Weber B, Wu D, Tamm A et al (2015) Biological soil crusts accelerate the nitrogen cycle through large NO and HONO emissions in drylands. PNAS 112(50):15384–15389
Bowker MA, Belnap J, Büdel B et al (2016) Controls on distribution patterns of biological soil crusts at micro- to global scales. In: Weber B, Büdel B, Belnap J (eds) Soil Crusts: An Organizing Principle in Drylands. Springer-Verlag, Switzerland, pp 173–197
Williams WJ, Chilton AM, Schneemilch M et al (2019) Microbial biobanking-cyanobacteria-rich topsoil facilitates mine rehabilitation. Biogeosciences 16(10):2189–2204
Galun M, Garty J (2003) Biological Soil Crusts of the Middle East. In: Belnap J, Lange OL (eds) Biological soil crusts: Structure, function, and management. Springer, Berlin Heidelberg, Berlin, Heidelberg, pp 95–106
Machado de Lima NM, Fernandes VMC, Roush D et al (2019) The compositionally distinct cyanobacterial biocrusts from Brazilian savanna and their environmental drivers of community diversity. Front Microbiol 10:2798
Belnap J, Miller DM, Bedford DR et al (2014) Pedological and geological relationships with soil lichen and moss distribution in the eastern Mojave Desert, CA, USA. J Arid Environ 106:45–57
Ochoa-Hueso R, Delgado-Baquerizo M, Gallardo A et al (2016) Climatic conditions, soil fertility and atmospheric nitrogen deposition largely determine the structure and functioning of microbial communities in biocrust-dominated Mediterranean drylands. Plant Soil 399(1):271–282
Liu YB, Wang ZR, Gao TP (2020) Succession of microbial community structure and their functions of biological soil crusts in temperate desert: A review. Microbiology China 47(9):2974–2983 (in Chinese)
Lan SB, Ouyang HL, Wu L et al (2017) Biological soil crust community types differ in photosynthetic pigment composition, fluorescence and carbon fixation in Shapotou region of China. Appl Soil Ecol 111:9–16
Wang ZR, Liu YB, Zhao LN (2019) Development of fungal community is a potential indicator for evaluating the stability of biological soil crusts in temperate desert revegetation. Appl Soil Ecol 147:103404
Zhao LN, Liu YB, Yuan SW et al (2020) Development of archaeal communities in biological soil crusts along a revegetation chronosequence in the Tengger Desert, north central China. Soil and Tillage Research 196:104443
Gundlapally SR, Garcia-Pichel F (2006) The Community and phylogenetic diversity of biological soil crusts in the Colorado Plateau studied by molecular fingerprinting and intensive cultivation. Microb Ecol 52(2):345–357
Pushkareva E, Pessi IS, Wilmotte A et al (2015) Cyanobacterial community composition in Arctic soil crusts at different stages of development. FEMS Microbiol Ecol 91(12):143
Kuske CR, Yeager CM, Johnson S et al (2012) Response and resilience of soil biocrust bacterial communities to chronic physical disturbance in arid shrublands. ISME J 6(4):886–897
Johnson SL, Kuske CR, Carney TD et al (2012) Increased temperature and altered summer precipitation have differential effects on biological soil crusts in a dryland ecosystem. Glob Change Biol 18(8):2583–2593
Maier S, Schmidt TSB, Zheng L et al (2014) Analyses of dryland biological soil crusts highlight lichens as an important regulator of microbial communities. Biodivers Conserv 23(7):1735–1755
Miralles I, Lázaro R, Sánchez-Marañón M et al (2020) Biocrust cover and successional stages influence soil bacterial composition and diversity in semiarid ecosystems. Sci Total Environ 709:134654
Wang J, Bao JT, Su JQ et al (2015) Impact of inorganic nitrogen additions on microbes in biological soil crusts. Soil Biol Biochem 88:303–313
Wang J, Zhang P, Bao JT et al (2020) Comparison of cyanobacterial communities in temperate deserts: A cue for artificial inoculation of biological soil crusts. Sci Total Environ 745:140970
Abed RMM, Tamm A, Hassenrück C et al (2019) Habitat-dependent composition of bacterial and fungal communities in biological soil crusts from Oman. Sci Rep 9(1):6468
Moreira-Grez B, Tam K, Cross AT et al (2019) The bacterial microbiome associated with arid biocrusts and the biogeochemical influence of biocrusts upon the underlying soil. Front Microbiol 10:2143
Pombubpa N, Pietrasiak N, De Ley P et al (2020) Insights into dryland biocrust microbiome: geography, soil depth and crust type affect biocrust microbial communities and networks in Mojave Desert, USA. FEMS Microbiol Ecol 96(9):125
Maier S, Tamm A, Wu D et al (2018) Photoautotrophic organisms control microbial abundance, diversity, and physiology in different types of biological soil crusts. ISME J 12(4):1032–1046
Hagemann M, Henneberg M, Felde VJ et al (2015) Cyanobacterial diversity in biological soil crusts along a precipitation gradient, Northwest Negev Desert, Israel. Microb Ecol 70:219–230
Hagemann M, Henneberg M, Felde VJ et al (2017) Cyanobacterial populations in biological soil crusts of the northwest Negev Desert, Israel-effects of local conditions and disturbance. FEMS Microbiol Ecol 93(6):fiw228
Machado de Lima NM, Muñoz-Rojas M, Vázquez-Campos X et al (2021) Biocrust cyanobacterial composition, diversity, and environmental drivers in two contrasting climatic regions in Brazil. Geoderma 386:114914
Bowker MA, Mau RL, Maestre FT et al (2011) Functional profiles reveal unique ecological roles of various biological soil crust organisms. Funct Ecol 25(4):787–795
Chen Y, Li YQ, Cao WJ et al (2023) Response of the plant–soil system to desertification in the Hulun Buir Sandy Land, China. Land Degrad Dev 34(7):2024–2037
Duan YL, Wang XY, Wang LL et al (2022) Biogeographic patterns of soil microbe communities in the deserts of the Hexi Corridor, northern China. CATENA 211:106026
Agriculture Chemistry Specialty Council, Soil Science Society of China (1983) General analysis methods of soil agriculture chemistry Science Press Beijing. pp 146–226 (in Chinese)
Bremner J (1996) Nitrogen-total. In: Sparks DL, Page AL, Helmke PA, Loeppert RH, Soltanpour PN, Tabatabai MA, Johnston CT, Sumner ME (eds) Methods of soil analysis, Part 3. Soil Science Society of America, Madison, WI, USA, pp 1085–1121
Olsen SR, Sommers LE (1983) Phosphorus. In: Page AL, Miller RH, Keeney DR (eds) Methods of soil analysis, Part 2, Chemical and microbial properties. Agronomy Monograph, vol 9. American Society of Agronomy, Madison, Wisconsin, pp 403–430
Pratt PF (1965) Potassium. In: Norman AG (ed) Methods of soil analysis, Part 2, Chemical and microbial properties. Agronomy Monograph, vol 9. American Society of Agronomy, Madison Wisconsin, pp 1022–1030
Li Y, Fang F, Wei JL et al (2019) Humic acid fertilizer improved soil properties and soil microbial diversity of continuous cropping peanut: A three-year experiment. Sci Rep 9(1):12014
Xu N, Tan GC, Wang HY et al (2016) Effect of biochar additions to soil on nitrogen leaching, microbial biomass and bacterial community structure. Eur J Soil Biol 74:1–8
Kusstatscher P, Zachow C, Harms K et al (2019) Microbiome-driven identification of microbial indicators for postharvest diseases of sugar beets. Microbiome 7(1):112
Magoč T, Salzberg SL (2011) FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics 27(21):2957–2963
Edgar RC (2013) UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Methods 10(10):996–998
Edgar RC, Haas BJ, Clemente JC et al (2011) UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 27(16):2194–2200
Wang Q, Garrity GM, Tiedje JM et al (2007) Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol 73(16):5261–5267
Caporaso JG, Kuczynski J, Stombaugh J et al (2010) QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7(5):335–336
Oksanen FJ et al (2012) Vegan: Community Ecology Package. R package Version 2.6–4. https://CRAN.R-project.org/package=vegan
Deng SQ, Zhang DY, Wang GH et al (2020) Biological soil crust succession in deserts through a 59-year-long case study in China: How induced biological soil crust strategy accelerates desertification reversal from decades to years. Soil Biol Biochem 141:107665
Cania B, Vestergaard G, Kublik S et al (2020) Biological soil crusts from different soil substrates harbor distinct bacterial groups with the potential to produce exopolysaccharides and lipopolysaccharides. Microb Ecol 79(2):326–341
Belnap J, Wilcox BP, Van Scoyoc MW et al (2013) Successional stage of biological soil crusts: an accurate indicator of ecohydrological condition. Ecohydrology 6(3):474–482
Shi W, Wang XP, Zhang YF et al (2018) The effect of biological soil crusts on soil moisture dynamics under different rainfall conditions in the Tengger Desert, China. Hydrological Processes 32(10):1363–1374
Mallen-Cooper M, Eldridge DJ (2016) Laboratory-based techniques for assessing the functional traits of biocrusts. Plant Soil 406(1):131–143
Pietrasiak N, Regus JU, Johansen JR et al (2013) Biological soil crust community types differ in key ecological functions. Soil Biol Biochem 65:168–171
Swenson TL, Karaoz U, Swenson JM et al (2018) Linking soil biology and chemistry in biological soil crust using isolate exometabolomics. Nat Commun 9(1):19
Colesie C, Allan Green TG, Haferkamp I et al (2014) Habitat stress initiates changes in composition, CO2 gas exchange and C-allocation as life traits in biological soil crusts. ISME J 8(10):2104–2115
Li XR, Song G, Hui R et al (2017) Precipitation and topsoil attributes determine the species diversity and distribution patterns of crustal communities in desert ecosystems. Plant Soil 420(1):163–175
Baumann K, Siebers M, Kruse J et al (2019) Biological soil crusts as key player in biogeochemical P cycling during pedogenesis of sandy substrate. Geoderma 338:145–158
Baumann K, Jung P, Samolov E et al (2018) Biological soil crusts along a climatic gradient in Chile: Richness and imprints of phototrophic microorganisms in phosphorus biogeochemical cycling. Soil Biol Biochem 127:286–300
Kammann S, Schiefelbein U, Dolnik C et al (2023) Successional development of the phototrophic community in biological soil crusts on Coastal and Inland Dunes. Biology 12:58
Liu YB, Wang ZR, Zhao LN et al (2019) Differences in bacterial community structure between three types of biological soil crusts and soil below crusts from the Gurbantunggut Desert, China. Eur J Soil Sci 70(3):630–643
Liu YB, Zhao LN, Wang ZR et al (2018) Changes in functional gene structure and metabolic potential of the microbial community in biological soil crusts along a revegetation chronosequence in the Tengger Desert. Soil Biol Biochem 126:40–48
Tian C, Xi J, Ju MC et al (2021) Biocrust microbiomes influence ecosystem structure and function in the Mu Us Sandland, northwest China. Eco Inform 66:101441
Liu LC, Liu YB, Zhang P et al (2017) Development of bacterial communities in biological soil crusts along a revegetation chronosequence in the Tengger Desert, northwest China. Biogeosciences 14(16):3801–3814
Bates ST, Nash TH, Garcia-Pichel F (2012) Patterns of diversity for fungal assemblages of biological soil crusts from the southwestern United States. Mycologia 104(2):353–361
Nagy ML, Pérez A, Garcia-Pichel F (2005) The prokaryotic diversity of biological soil crusts in the Sonoran Desert (Organ Pipe Cactus National Monument, AZ). FEMS Microbiol Ecol 54(2):233–245
Bates ST, Garcia-Pichel F (2009) A culture-independent study of free-living fungi in biological soil crusts of the Colorado Plateau: their diversity and relative contribution to microbial biomass. Environ Microbiol 11(1):56–67
Wang Y, Hong Y, Tian YL et al (2022) Changes in bacterial community composition and soil properties altered the response of soil respiration to rain addition in desert biological soil crusts. Geoderma 409:115635
Manzoni S, Schimel JP, Porporato A (2012) Responses of soil microbial communities to water stress: results from a meta-analysis. Ecology 93(4):930–938
Fierer N, Lauber CL, Ramirez KS et al (2012) Comparative metagenomic, phylogenetic and physiological analyses of soil microbial communities across nitrogen gradients. ISME J 6(5):1007–1017
Zhang BC, Li RH, Xiao P et al (2016) Cyanobacterial composition and spatial distribution based on pyrosequencing data in the Gurbantunggut Desert, Northwestern China. J Basic Microbiol 56(3):308–320
Garcia-Pichel F, Loza V, Marusenko Y et al (2013) Temperature drives the continental-scale distribution of key microbes in topsoil communities. Science 340(6140):1574–1577
Green LE, Porras-Alfaro A, Sinsabaugh RL (2008) Translocation of nitrogen and carbon integrates biotic crust and grass production in desert grassland. J Ecol 96(5):1076–1085
Abed RMM, Al-Sadi AM, Al-Shehi M et al (2013) Diversity of free-living and lichenized fungal communities in biological soil crusts of the Sultanate of Oman and their role in improving soil properties. Soil Biol Biochem 57:695–705
Chen WD, Ren KX, Isabwe A et al (2019) Stochastic processes shape microeukaryotic community assembly in a subtropical river across wet and dry seasons. Microbiome 7:138
Jiao S, Yang YF, Xu YQ et al (2020) Balance between community assembly processes mediates species coexistence in agricultural soil microbiomes across eastern China. ISME J 14:202–216
Fierer N, Jackson RB (2006) The diversity and biogeography of soil bacterial communities. PNAS 103(3):626–631
Lauber CL, Hamady M, Knight R et al (2009) Pyrosequencing-based assessment of soil pH as a predictor of soil bacterial community structure at the continental scale. Appl Environ Microbiol 75(15):5111–5120
Funding
This research was supported by Strategic Priority Research Program of the Chinese Academy of Sciences (grant no. XDA26020104); the National Key R&D Program of China (grant no. 2017YFA0604803); the Second Tibetan Plateau Scientific Expedition and Research (STEP) program (grant no. 2019QZKK0305); the National Natural Science Foundation of China (grant no. 31971466, 41971142); and the Science and Technology Research Project of Inner Mongolia Autonomous Region (2022YFDZ0036).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Yulong Duan, Yuqiang Li, Jianhua Zhao, Chun Luo, and Junbiao Zhang. Financial support for the study came from grants to Xinping Liu, Yuqiang Li, and Rongliang Jia. The first draft of the manuscript was written by Yulong Duan and Yuqiang Li, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics Approval and Consent to Participate
No ethics approvals or consents were required.
Competing Interests
The authors declare no competing interests.
Additional information
Highlights
• Through biocrust succession, bacterial diversity increased yet fungal diversity decreased.
• Overall, 32 bacterial phyla (154 genera) and 11 fungal phyla (25 families) were identified in the soil samples.
• The relative abundance of Proteobacteria and Cyanobacteria respectively increased and decreased significantly during biocrust succession.
• For Actinobacteria, Chloroflexi, and Acidobacteria, their relative abundance rose then fell with biocrust succession.
• Enhanced soil properties caused by the succession of biocrusts enhanced soil bacterial and fungal community composition.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Duan, Y., Li, Y., Zhao, J. et al. Changes in Microbial Composition During the Succession of Biological Soil Crusts in Alpine Hulun Buir Sandy Land, China. Microb Ecol 87, 43 (2024). https://doi.org/10.1007/s00248-024-02359-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00248-024-02359-2