Abstract
Numerous insect species and their associated microbial pathogens are exposed to elevated CO2 concentrations in both artificial and natural environments. However, the impacts of elevated CO2 on the fitness of these pathogens and the susceptibility of insects to pathogen infections are not well understood. The yellow mealworm, Tenebrio molitor, is commonly produced for food and feed purposes in mass-rearing systems, which increases risk of pathogen infections. Additionally, entomopathogens are used to control T. molitor, which is also a pest of stored grains. It is therefore important to understand how elevated CO2 may affect both the pathogen directly and impact on host-pathogen interactions. We demonstrate that elevated CO2 concentrations reduced the viability and persistence of the spores of the bacterial pathogen Bacillus thuringiensis. In contrast, conidia of the fungal pathogen Metarhizium brunneum germinated faster under elevated CO2. Pre-exposure of the two pathogens to elevated CO2 prior to host infection did not affect the survival probability of T. molitor larvae. However, larvae reared at elevated CO2 concentrations were less susceptible to both pathogens compared to larvae reared at ambient CO2 concentrations. Our findings indicate that whilst elevated CO2 concentrations may be beneficial in reducing host susceptibility in mass-rearing systems, they may potentially reduce the efficacy of the tested entomopathogens when used as biological control agents of T. molitor larvae. We conclude that CO2 concentrations should be carefully selected and monitored as an additional environmental factor in laboratory experiments investigating insect-pathogen interactions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
CO2 (carbon dioxide) has the potential to affect host-pathogen interactions if either the host, pathogen, or both are affected by changes in CO2 concentrations. Numerous insect species are constantly exposed to CO2 concentrations above the atmospheric level, which is currently recorded as approximately 420 ppm (parts per million) [1]. Elevated CO2 concentrations can be a result of the respiration of insects [2, 3] or a product of increased microbial activity and subsequent accumulation in enclosed areas [4]. The CO2 concentration in soil air (inside soil pores), for example, is typically higher than the atmospheric CO2 concentration due to decreased gas exchange [4], hence soil-dwelling insect species are exposed to elevated CO2 concentrations in their environment. Furthermore, it is known that CO2 can accumulate in colonies of social insects reaching up to 60,000 ppm in leaf-cutting ant colonies [5], and 92,000 ppm in termite mounds [6]. Insects that are mass-reared for food and feed purposes can also be exposed to elevated CO2 concentrations because they are typically kept at high densities in closed systems [7], which facilitates the accumulation of CO2 [8].
The yellow mealworm, Tenebrio molitor, is an insect species that is increasingly being mass-reared to produce proteins and fats to feed livestock and for use in aquaculture [9, 10]. Respiration of T. molitor larvae produces approximately 60 g CO2 per kg of body mass per day or approximately 1,000 g CO2 per kg body mass gain [2]. Despite the utilisation of appropriate ventilations, CO2 is still likely to accumulate in production facilities of T. molitor [8, 11]; for example, in a closed experimental T. molitor rearing, CO2 concentrations reached up to 6,000 ppm [12]. The maximum permitted CO2 concentrations in production facilities are regulated by law in most countries to ensure the health and safety of employees [13]. For example, the long-term (8 h) exposure limit of CO2 concentration in the workplace is 5,000 ppm in many countries including the UK [14], the US [15], and countries belonging to the EU [16], which is more than tenfold higher than atmospheric concentrations.
Besides the use of T. molitor to produce feed, the yellow mealworm is also a global pest of stored grains and grain by-products [17]. The CO2 concentrations inside stored grains can exceed atmospheric CO2 concentration [18] and when there is microbial or insect activity, CO2 concentrations may increase even further [19, 20]. Various organisms (entomopathogens) such as bacteria, fungi, protists, nematodes, and viruses can infect T. molitor [21, 22]. Some of these entomopathogens are used as biological control agents against T. molitor in stored grains [23, 24] but at the same time, entomopathogens can also cause lethal or sublethal diseases in insects mass-reared for food and feed leading to economic losses in production systems [21]. Currently, there is a dearth of knowledge on how CO2 concentrations affect host-pathogen interactions in both mass-reared and wild insects [25]. Improving our understanding of the effects of CO2 on entomopathogens and their interactions with insect hosts will help to guide decisions of whether CO2 should be considered a relevant factor to include for insect-pathogen interaction experiments and in the design of insect mass rearing facilities.
CO2 is known to affect entomopathogenic organisms; for example, Pseudoxylaria spp., an entomopathogenic fungus infecting termites (Odontotermes obesus), showed reduced growth when exposed to elevated CO2 concentrations [6]. Furthermore, the number of conidia produced by different strains of the entomopathogenic fungal species Metarhizium anisopliae, Isaria farinosa, and Beauveria bassiana were generally decreased at 1,000 ppm CO2 compared to 350 ppm CO2 [26]. CO2 has also been found to affect the virulence of pathogenic organisms of humans [27]; in the human-pathogenic bacterium Bacillus cereus, for example, the expression of virulence genes was higher at elevated CO2 concentrations [28] and Candida albicans, a fungal pathogen of humans, switches from the monocellular to the more virulent filamentous growth at elevated CO2 concentrations [27]. Nevertheless, the impact of CO2 on the virulence of entomopathogenic organisms that can infect economically important insects remains unknown.
In this study, we examined the effects of CO2 on two entomopathogens, the bacterium Bacillus thuringiensis, and the fungus Metarhizium brunneum, which both naturally infect T. molitor [21, 22]. We used in vitro experiments and full-factorial bioassays to study interactions between CO2, insects, and pathogens. The pathogens were selected because both B. thuringiensis and M. brunneum can be found in stored grains [29,30,31]; grain products are both an important habitat of T. molitor and often used to feed T. molitor larvae in production systems [11]. Species of the genus Metarhizium are facultative entomopathogens, as these fungi can also colonize the rhizosphere of plants or live as saprotrophs [32, 33]. The impact of CO2 on fungal germination and growth in the external insect host environment is therefore highly relevant. On the other hand, B. thuringiensis is thought to only multiply inside the insect host while the environment (external to the insect host) constitutes a transition compartment for the spores and crystals without reproduction [34]. Therefore, the effects of CO2 on the viability and virulence of spores and crystals in the environment (e.g., soil or stored grains) are relevant to evaluate. The aims of this study were to assess the effects of elevated CO2 (4,500 ± 500 ppm) on: (1) the in vitro germination of conidia and mycelial growth of M. brunneum, (2) the in vitro viability and persistence of B. thuringiensis spores, and (3) the in vivo interactions between M. brunneum or B. thuringiensis and the larvae of T. molitor.
Methods
All insect rearing and experiments took place in two separate 50-litre LEEC Culture Safe CO2 incubators adjacent to each other, one used for low [450 ppm (± 50 ppm)] CO2, and one used for high [4,500 ppm (± 500 ppm)] CO2 concentrations (see Supplementary methods). The low CO2 concentration corresponds approximately with ambient CO2 concentration, whereas the choice of the high CO2 concentration was based on maximum permitted concentrations for human safe working [13,14,15,16] and data from experimental setups [12]. To allow for maximum gas exchange in the Petri dishes in which the microorganisms were grown, the lids of all Petri dishes (unless otherwise stated) were elevated by adding 2 cm wide plastic strips between the lids and the lower dish. Metarhizium brunneum isolate KVL12-30 (culture collection of the Department of Plant and Environmental Sciences, University of Copenhagen, Denmark) and Bacillus thuringiensis serovar morrisoni tenebrionis 4AA1 (Bacillus genetic stock center, Ohio State University, USA) were used in experiments. The in vitro and the in vivo experiments were performed on three and two independent occasions, respectively.
Germination and Growth of M. brunneum
The germination of M. brunneum conidia was assessed by adding 100 μl of 106 conidia/ml (see Supplementary methods) on each of three replicate (per condition and time point) 10 ml SDAY/4 (16.25 g Sabouraud dextrose agar, 2.5 g yeast extract, and 11.25 g agar in 1 l dH2O) Petri dishes. The suspensions were spread using a Drigalski spatula and the Petri dishes were incubated at either low or high CO2 for 6, 8, 10, 12, 14, 18, or 24 h. Thereafter, 100 conidia were counted at three different locations on each Petri dish (300 conidia per Petri dish) and the numbers of germinated and un-germinated conidia were noted. A conidium was considered as germinated when it had a germ tube at least as long as the smallest diameter of the conidium.
The colony growth rates of M. brunneum at different CO2 concentrations were assessed by adding 2 μl of 106 conidia/ml on the centre of each of ten replicate 30 ml SDAY/4 Petri dishes and subsequent incubation at either low or high CO2. The area of each colony was measured using a digital calliper on two perpendicular diameters, every second day for eight days, starting two days after the preparation of the Petri dishes. The average of the two diameters per colony was used as one data point for calculating the growth rate (mm/day) between days two and eight. Petri dishes that dried out before the end of the experiment were excluded from the analysis.
Viability and Persistence of B. thuringiensis
The in vitro viability of B. thuringiensis spores was assessed by adding 100 μl of 103 spores/ml (see Supplementary methods) to each of ten replicate 10 ml LB-Agar (lysogeny broth agar; 10 g tryptone, 5 g yeast extract, 10 g NaCl, and 15 g bacteriological agar in 1 l dH2O) Petri dishes. The suspensions were spread using a Drigalski spatula and the Petri dishes were incubated at either low or high CO2. At both CO2 concentrations, 100 μl of sterile dH2O was spread on each of three replicate 10 ml LB-Agar Petri dishes as controls (in the case of contamination this would be apparent on these Petri dishes). The numbers of colonies per Petri dish were counted after 24 h to calculate cfu/ml (colony forming units/ml; see Supplementary methods).
To measure in vitro persistence of B. thuringiensis spores, the method of Wood et al. [35] was adapted. Nine replicate autoclaved glass coverslips (22 × 22 mm) were placed inside an empty sterile Petri dish (three coverslips per Petri dish). On each coverslip, 100 μl of 6 × 105 spores/ml (see Supplementary methods) were added and the Petri dishes containing the coverslips were incubated at either low or high CO2. Additionally, 100 μl of sterile dH2O was added on a separate coverslip in each Petri dish as a control (in the case of contamination this would be apparent on these coverslips). After two days the coverslips were transferred individually to 50 ml Falcon tubes containing 15 ml PBS (phosphate buffered saline) with Triton X-100 (0.1% v/v) and the tubes were put on an orbital shaker at 200 rpm at 25 °C for 15 min. Thereafter, 10 μl of the resulting suspensions were pipetted onto LB-Agar plates. By tilting the Petri dish on one side, the diluted suspensions ran down the media forming straight lines (three technical replicates on different Petri dishes were prepared). The average of the three technical replicates was used as one data point to calculate cfu/ml.
In Vivo Bioassays
Tenebrio molitor larvae were reared at either low or high CO2 concentrations for 18 days. Bacillus thuringiensis spores and crystals mixed in diet were exposed to either low or high CO2 concentrations for two days. Metarhizium brunneum was grown at either low or high CO2 concentrations for 14 days. The pathogens (100 μl of 4 × 109 spores/ml per 100 mg diet for B. thuringiensis and 100 μl of 108 conidia/ml per 100 mg diet for M. brunneum) were mixed into the larval diet [wheat bran (96% w/w) and dried egg white (4% w/w)] as described in the Supplementary methods. The larvae were exposed to lethal concentrations (previously determined in pre-experimental bioassays) of each pathogen separately in a full-factorial bioassay (n = 5, 30 larvae per cup). Furthermore, two groups of unexposed larvae (one at low and one at high CO2; n = 5, 30 larvae per cup) were prepared as control treatments (Fig. 1). The larvae per cup were weighed as a group the day before, and on the day of exposure to the pathogens. Two days after the start of the exposure to the pathogens, the larvae were transferred to fresh cups. The larvae and the remaining diet in each cup were separated from frass by using a sieve (0.5 mm) 2, 4, 6, 8, 10, 12 and 14 days after exposure. Larval mortality was also assessed on the same days after exposure and dead larvae were removed. The remaining diet and live larvae were weighed individually and new diet (0.6 × weight of live larvae) and water agar (1% w/v; 0.6 × weight of live larvae) were added on the same days as larval mortality was assessed (the value 0.6 was established in a pre-experimental bioassay to ensure that the larvae did not starve between feeding time points). The larvae from one cup treated with B. thuringiensis in the second experimental repetition were excluded from analysis because the cup was tipped over during the experiment.
Schematic representation of the experimental design. A Larvae reared at either low or high CO2 for 18 days were exposed to B. thuringiensis previously exposed to either low or high CO2 for two days or to water as a control. B Larvae reared at either low or high CO2 for 18 days were exposed to M. brunneum grown at low or high CO2 for 14 days. The lids of the Petri dishes were elevated by adding a 2 cm wide plastic strip between the lower dish and the lid. A, B Each arrow represents one treatment (12 treatments in total, n = 5, 30 larvae per cup, two experimental repetitions). The survival, feed intake and weight of the larvae were assessed every second day for a period of 14 days after pathogen exposure. Figure created with BioRender (www.biorender.com)
Statistical Analysis
Differences were considered as significant at p < 0.05 and data was only subjected to one-, two- or three-way ANOVAs (analysis of variances) when normality (QQ-plots) and homogeneity of variances (Levene test, p > 0.05) assumptions were satisfied. Tukey’s HSD (Honestly Significant Difference) tests were used to separate the means. All statistical analyses were performed using R v. 4.1.0 [36].
The effect of CO2 on M. brunneum conidia germination was described using a three-parameter log-logistic model (\(y=\frac{d}{(1+{e}^{(b\left(\text{ln}\left(x\right)-\text{ln}\left(i\right)\right)})}\); where y = germinated conidia (%), i = inflection point (i.e., hours to 50% germination), b = slope, d = upper limit, x = time in hours) using the drc package [37]. The times to 50% germination at different CO2 concentrations were compared using the compParm function [37]. Metarhizium brunneum growth rates at different CO2 concentrations were analysed using a one-way ANOVA. Experimental repetitions were combined, as no interactive effect of repetition and CO2 was found in a previous two-way ANOVA. Bacillus thuringiensis spore persistence, spore viability, and density of larvae before the start of the in vivo assays at different CO2 concentrations were compared by implementing generalized linear mixed models with a negative binomial error distribution (used for over dispersed count data) using the lme4 package [38] with experimental repetition included as a random effect.
Mixed effects cox proportional hazards models were used to analyse the survival of the larvae in the in vivo assays (fixed effects: pathogen exposure, CO2 exposure of larvae, CO2 exposure of pathogens; random effects: experimental repetition, cup) using the coxme package [39]. Only significant fixed effects were retained in the final models and pairwise comparisons of treatments were performed using Tukey contrasts with single-step adjustment for multiple testing using the multcomp package [40]. The effect of CO2 on larval weight at the start of the experiment was analysed using a generalized linear mixed model with a gamma error distribution using the lme4 package [38] with experimental repetition included as a random effect. Weight gain per larva for the duration of the experiments and feed intake during exposure data were analysed separately for both experimental repetitions (Exp. 1 and 2) using two-way ANOVAs because interactive effects of experimental repetition and exposure of larvae or pathogens to CO2 were found in previous three-way ANOVAs.
Results
First, the effects of CO2 on different pathogen traits outside of the host were tested. The time to 50% germination of M. brunneum conidia was significantly lower at high CO2 compared to low CO2 (t = 26.07; p < 0.001). At both CO2 levels, the germination of conidia was > 99% after 24 h (Fig. 2).
Three-parameter log-logistic models for germination of M. brunneum conidia over time (hours) at either low (e = 9.58 (confidence limits = 9.49 and 9.66); b = -12.74; d = 99.83) or high (e = 7.77 (confidence limits = 7.66 and 7.87); b = -9.83; d = 99.90) CO2 concentrations. The shaded areas represent the 95% confidence intervals
Metarhizium brunneum colony growth rate was not affected by CO2 (Table 1). In contrast, B. thuringiensis spores incubated at high CO2 showed significantly lower viability than spores incubated at low CO2 (Table 1). Similarly, B. thuringiensis spore persistence was significantly decreased at high compared to low CO2 concentration (Table 1).
To investigate host-pathogen interactions, full-factorial bioassays were performed in which the pathogens and the host were exposed to either low or high CO2 (Fig. 1). We tested the larval density in the two CO2 conditions before the start of the experiments to ensure that it did not affect our results. The larval density in the rearing containers at low and high CO2 was indeed not affected by CO2 (p = 0.311, χ2 = 1.026, d.f. = 1,6). Likewise, CO2 did not affect the weight of the larvae at the start of the experiment (p = 0.387, χ2 = 0.748, d.f. = 1,97). The germination rates of M. brunneum conidia were > 99% in all treatments and experimental repetitions. Larvae reared at high CO2 were significantly less susceptible (i.e., less likely to die) to B. thuringiensis (Fig. 3A; Table 2) and M. brunneum (Fig. 3B; Table 2) than larvae reared at low CO2 resulting in approximately 12 and 8% higher survival after 14 days, respectively. There was no effect of CO2 on survival of control larvae (p = 0.771, Fig. 3A, B). Moreover, exposure of the pathogens to different CO2 concentrations before exposure of the larvae did not affect the virulence of B. thuringiensis or M. brunneum (Table 2).
Survival of T. molitor larvae reared at either low (blue) or high (red) CO2 concentrations after exposure to pathogens for a period of 14 days. A Cumulative survival probability of larvae exposed to either low or high CO2 without (dotted survival curves) and with B. thuringiensis exposure (continuous survival curves). B Cumulative survival probabilities of larvae exposed to either low or high CO2 without (dotted survival curves) and with M. brunneum exposure (continuous survival curves). A, B Different letters to the right of the survival curves indicate statistical differences among treatments at p < 0.05. The shaded areas represent the 95% confidence intervals. Hazard ratios and p-values of fixed and random effects of the mixed effects cox proportional hazards models are displayed in Table 2. Figure created with GraphPad Prism version 9.3.1
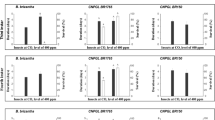
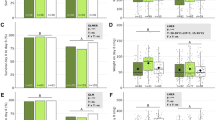
The effect of CO2 concentration and pathogen exposure on feed intake was measured during pathogen exposure. The feed intake per larva was reduced by B. thuringiensis exposure (Fig. 4A), but CO2 did not affect feed intake in either the control or B. thuringiensis exposed larvae (Fig. 4A; Table 3). Similarly, feed intake was reduced by M. brunneum exposure in the second iteration of the experiment, and in certain treatments of the first iteration (Fig. 4B). CO2 did not affect the feed intake during M. brunneum or control exposure (Fig. 4B; Table 3).
Feed intake per 30 larvae during exposure (two days) to the pathogens. A Feed intake during exposure to B. thuringiensis in Exp. (experiment) 1 and 2: control (no exposure to B. thuringiensis), lowCO2-larv (larvae exposed to low CO2), highCO2-larv (larvae exposed to high CO2), lowCO2-Bt (B. thuringiensis exposed to low CO2), highCO2-Bt (B. thuringiensis exposed to high CO2). B Feed intake during exposure to M. brunneum in Exp. (experiment) 1 and 2: control (no exposure to M. brunneum), lowCO2-larv (larvae exposed to low CO2), highCO2-larv (larvae exposed to high CO2), lowCO2-Mb (M. brunneum grown at low CO2), highCO2-Mb (M. brunneum grown at high CO2). A, B Boxplots show median, interquartile range, and minimum and maximum. Different letters above boxplots indicate statistical differences among treatments at p < 0.05 for each experiment separately. Degrees of freedom, F-values and p-values of the two-way ANOVAs are displayed in Table 3. Figure created with GraphPad Prism version 9.3.1
Exposure of larvae to B. thuringiensis significantly reduced weight gain of the larvae over the course (14 days) of the experiments (Fig. 5A). However, weight gain was not affected by exposure of either the larvae or B. thuringiensis to different CO2 concentrations (Fig. 5A; Table 3). Exposure of larvae to M. brunneum did not affect the weight gain over the course of the experiment except for one treatment in the second iteration of the experiment (Fig. 5B). Furthermore, weight gain was not affected by exposure of either the larvae or M. brunneum to different CO2 concentrations (Fig. 5B; Table 3).
Weight gain per larva (mg) during 14 days after exposure to the pathogens. A Weight gain after exposure to B. thuringiensis in Exp. (experiment) 1 and 2: control (no exposure to B. thuringiensis), lowCO2-larv (larvae exposed to low CO2), highCO2-larv (larvae exposed to high CO2), lowCO2-Bt (B. thuringiensis exposed to low CO2), highCO2-Bt (B. thuringiensis exposed to high CO2). B Weight gain after exposure to M. brunneum in Exp. (experiment) 1 and 2: control (no exposure to M. brunneum), lowCO2-larv (larvae exposed to low CO2), highCO2-larv (larvae exposed to high CO2), lowCO2-Mb (M. brunneum grown at low CO2), highCO2-Mb (M. brunneum grown at high CO2). A, B Boxplots show median, interquartile range, and minimum and maximum. Different letters above boxplots indicate statistical differences among treatments at p < 0.05 for each experiment separately. Degrees of freedom, F-values and p-values of the two-way ANOVAs are displayed in Table 3. Figure created with GraphPad Prism version 9.3.1
Discussion
In this study, elevated CO2 concentrations were found to decrease the viability and persistence of B. thuringiensis spores in vitro, whilst decreasing the duration of conidial germination of M. brunneum. Interestingly, exposure of the pathogens to different CO2 concentrations before infection did not affect the virulence of these entomopathogens toward T. molitor larvae, but larvae reared at elevated CO2 were less susceptible (i.e., less likely to die) to the pathogens than larvae reared at ambient CO2. These findings are important because T. molitor larvae are often exposed to CO2 concentrations above ambient conditions [8, 11, 18]. Here we show that CO2 levels affect the susceptibility of T. molitor to entomopathogens, which has implications for both mass-rearing of mealworms for food and feed purposes, and for biocontrol of this insect species. In addition to our main findings, we also found that CO2 did not affect the feed intake of the larvae during exposure to the pathogens and overall, did not affect the individual weight gain of the larvae. Investigating sub-lethal effects such as these is crucial, especially for the production of insects because a reduction in weight gain leads to economic losses, as the overall mass of insects produced is reduced.
It is challenging to put our study in context with other studies on CO2 because the few other studies that have been published investigating the effects of CO2 on insect-pathogen interactions either use lower (< 1,000 ppm) or significantly higher (> 50,000 ppm) CO2 concentrations than in this present study. To our knowledge, this is the first study to measure the effect of industrially relevant CO2 concentrations for the mass-rearing of T. molitor and other reared insect species. Elevated CO2 concentrations have been suggested to act as a cue promoting the germination of an entomophthoralean fungus (Entomophaga maimaiga) as CO2 concentrations might be elevated near the insect cuticle [41]. This increased germination of fungal conidia is in accordance with our study. However, decreased germination and mycelial growth of a hypocrealean fungus (B. bassiana) were reported as a result of a very high CO2 concentration (400,000 ppm) [42]. Similarly, 50,000 ppm CO2 decreased the mycelial growth and sporulation of M. brunneum, Aspergillus sp., and B. bassiana in vitro [43]. Moreover, it was proposed (without statistical analyses) that the growth rates of different M. anisopliae strains are either positively or negatively affected by elevated CO2 (650 and 1,000 ppm) [26]. We, in contrast, did not find an effect of CO2 at industrially relevant concentrations on the growth rate of M. brunneum in vitro.
This is, to our knowledge, the first study that measures the direct effects of elevated environmental CO2 on the persistence and viability of a bacterial entomopathogen. However, it is known from other species that CO2 can reduce bacterial growth [44]. We found that the persistence of B. thuringiensis spores was almost three times lower at elevated CO2. Surprisingly, there was no effect of exposure of B. thuringiensis to elevated CO2 on the subsequent virulence in the insect host. This could be because the crystals of B. thuringiensis that are essential for the infection process might not be affected by CO2. Moreover, we speculate that the spores kept at elevated CO2 could have been only temporarily inactivated (dormant) and might be reactivated in the host. It has been shown for other species of the Bacillus genus that suboptimal thermal and pH conditions during incubation can increase the time to germination of spores [45].
Interestingly, we could not detect any sublethal effects of elevated CO2 on the larvae. In contrast, in a study by Li et al. [12], T. molitor larvae reared in a closed system had a lower weight gain compared to larvae reared in an open system, which was argued to be due to higher CO2 concentrations in the closed system [12]. However, these differences could also have been due to other factors such as different relative humidity or different concentrations of other gases in the two systems. It is important to note that elevated CO2 concentrations may be more detrimental to insects when the relative humidity is low, because elevated CO2 forces the insects to keep their spiracles open, which can result in water loss [46].
Our study supports prior findings by Borisade & Magan [26] who exposed desert locusts (Schistocerca gregaria) and house crickets (Acheta domesticus) to elevated CO2 concentrations (1,000 ppm). The authors suggested that S. gregaria and A. domesticus kept at elevated CO2 showed increased survival and lethal times, respectively, when exposed to B. bassiana, although this was not statistically validated [26]. In contrast to these findings, the survival of red flour beetles (Tribolium castaneum) exposed to B. bassiana was significantly decreased at very high CO2 concentrations (440,000 ppm) [42]. Due to our experimental design, we can disentangle the effects of CO2 on the interactions between the pathogens and T. molitor, demonstrating that previous exposure of the pathogens to elevated CO2 did not affect the virulence of the pathogens, but that rearing the larvae at elevated CO2 decreases the susceptibility of the larvae to the pathogens. One possible explanation is that CO2 may affect the insect immune response. For example, in Drosophila melanogaster the production of antimicrobial peptides was inhibited by CO2 (130,000 ppm) correlating with increased susceptibility to bacterial infections [47]. Moreover, in T. castaneum CO2 increased the production of benzoquinones [48] (a quinone that is also produced by T. molitor [49]), which inhibit B. bassiana [50]. The mechanism underlying the decreased susceptibility of T. molitor to pathogens at elevated CO2 concentrations remains to be investigated. Moreover, it would be beneficial for the production of T. molitor and other mass-reared insect species to investigate the CO2 concentrations T. molitor is evolutionarily adapted to in order to optimise rearing conditions. Tenebrio molitor might be adapted to elevated CO2 concentrations whereas other species may be adapted to different CO2 concentrations.
Here, we demonstrate that CO2 directly affects a bacterial and a fungal entomopathogen in vitro and their in vivo interactions with an insect host. Based on these results, we conclude that the tested elevated CO2 concentration (4,500 ± 500 ppm) in T. molitor mass-rearing systems is beneficial for larvae exposed to the tested pathogens by increasing larval survival. Furthermore, we did not find any sublethal effects of CO2 on T. molitor larvae that would affect the overall productivity of the mass-rearing system. For biocontrol of T. molitor, our results indicate that the efficacies of the two tested entomopathogens may be lowered at elevated CO2 concentrations, which has implications for understanding the reliability of biocontrol of storage pests. To ensure meaningful conclusions, we suggest it is crucial to consider CO2 effects (i.e., through monitoring and using pertinent CO2 concentrations) when studying any insect pathogen systems that are likely to be exposed to elevated CO2 in their natural or artificially maintained environments.
Data Availability
The datasets generated for the current study are available from the corresponding author on reasonable request.
References
Keeling RF, Keeling CD (2017) Atmospheric monthly in situ CO2 data - Mauna Loa observatory, Hawaii (Archive 2023-06-04). Scripps CO2 Program Data UC San Diego Library Digital Collections. https://doi.org/10.6075/J08W3BHW
Oonincx D, van Itterbeeck J, Heetkamp MJW, van den Brand H, van Loon JJA, van Huis A (2010) An exploration on greenhouse gas and ammonia production by insect species suitable for animal or human consumption. PLoS ONE 5:1–7. https://doi.org/10.1371/journal.pone.0014445
Gorres CM, Kammann C (2020) First field estimation of greenhouse gas release from European soil-dwelling Scarabaeidae larvae targeting the genus Melolontha. PLoS ONE 15:1–14. https://doi.org/10.1371/journal.pone.0238057
Howe JA, Smith AP (2021) The soil habitat. In: Gentry TJ, Fuhrmann JJ, Zuberer DA (eds) Principles and applications of soil microbiology, 3 edn. Elsevier, Amsterdam, pp 23–55
Bollazzi M, Forti LC, Roces F (2012) Ventilation of the giant nests of Atta leaf-cutting ants: does underground circulating air enter the fungus chambers? Insectes Sociaux 59:487–498. https://doi.org/10.1007/s00040-012-0243-9
Katariya L, Ramesh PB, Borges RM (2018) Dynamic environments of fungus-farming termite mounds exert growth-modulating effects on fungal crop parasites. Environ Microbiol 20:971–979. https://doi.org/10.1111/1462-2920.14026
Eilenberg J, Jensen AB (2018) Prevention and management of diseases in terrestrial invertebrates. In: Hajek AE, Shapiro-Ilan DI (eds) Ecology of invertebrate diseases 1ed. John Wiley & Sons Ltd, Hoboken NJ, pp 495–526
Kok R (2021) Preliminary project design for insect production: part 1-overall mass and energy/heat balances. J Insects Food Feed 7:499–509. https://doi.org/10.3920/jiff2020.0055
van Huis A (2017) Edible insects and research needs. J Insects Food Feed 3:3–5. https://doi.org/10.3920/JIFF2017.x002
Sogari G, Amato M, Biasato I, Chiesa S, Gasco L (2019) The potential role of insects as feed: a multi-perspective review. Animals 9:1–15. https://doi.org/10.3390/ani9040119
Cortes Ortiz JA, Ruiz AT, Morales-Ramos JA, Thomas M, Rojas MG, Tomberlin JK, Yi L, Han R, Giroud L, Jullien RL (2016) Insect mass production technologies. In: Dossey AT, Morales-Ramos JA, Rojas MG (eds) Insects as sustainable food ingredients: production, processing and food applications. Elsevier, pp 153–201
Li L, Xie B, Dong C, Hu D, Wang M, Liu G, Liu H (2015) Rearing Tenebrio molitor L. (Coleptera: Tenebrionidae) in the Lunar Palace 1 during a 105-day multi-crew closed integrative BLSS experiment. Life Sci Space Res 7:9–14. https://doi.org/10.1016/j.lssr.2015.08.002
Deveau M, Chen C-P, Johanson G, Krewski D, Maier A, Niven KJ, Ripple S, Schulte PA, Silk J, Urbanus JH, Zalk DM, Niemeier RW (2016) The global landscape of occupational exposure limits-implementation of harmonization principles to guide limit selection. J Occup Environ Hyg 12:127–144. https://doi.org/10.1080/15459624.2015.1060327
Health and Safety Executive (HSE) (2020) EH40/2005 workplace exposure limits, 4 edn. TSO (The Stationery Office), Norwich, pp 1–61
The national institute for occupational safety and health (2019) Carbon dioxide. https://www.cdc.gov/niosh/npg/npgd0103.html Accessed 27.01.2022
European Chemicals Agency. Substance regulatory obligations. https://echa.europa.eu/legislation-obligation/-/obligations/100.004.271 Accessed 27.01.2022
Ramos-Elorduy J, Gonzalez EA, Hernandez AR, Pino JM (2002) Use of Tenebrio molitor (Coleoptera: Tenebrionidae) to recycle organic wastes and as feed for broiler chickens. J Econ Entomol 95:214–220. https://doi.org/10.1603/0022-0493-95.1.214
Abalone R, Gaston A, Bartosik R, Cardoso L, Rodriguez J (2011) Gas concentration in the interstitial atmosphere of a wheat silo-bag. Part I: model development and validation. J Stored Prod Res 47:268–275. https://doi.org/10.1016/j.jspr.2011.05.004
Zhang SB, Zhai HC, Huang SX, Cai JP (2014) A site-directed CO2 detection method for monitoring the spoilage of stored grains by insects and fungi in Chinese horizontal warehouses. J Stored Prod Res 59:146–151. https://doi.org/10.1016/j.jspr.2014.07.002
Fleurat-Lessard F (2017) Integrated management of the risks of stored grain spoilage by seedborne fungi and contamination by storage mould mycotoxins - an update. J Stored Prod Res 71:22–40. https://doi.org/10.1016/j.jspr.2016.10.002
Eilenberg J, Vlak JM, Nielsen-LeRoux C, Cappellozza S, Jensen AB (2015) Diseases in insects produced for food and feed. J Insects Food Feed 1:87–102. https://doi.org/10.3920/jiff2014.0022
Slowik AR, Herren P, Bessette E, Lim FS, Hernández-Pelegrín L, Savio C (2023) Harmful and beneficial symbionts of Tenebrio molitor and their implications for disease management. J Insects Food Feed 9:1381–1395. https://doi.org/10.3920/JIFF2022.0171
Oppert B, Ellis RT, Babcock J (2010) Effects of Cry1F and Cry34Ab1/35Ab1 on storage pests. J Stored Prod Res 46:143–148. https://doi.org/10.1016/j.jspr.2010.01.003
Eski A, Murat Gezgin M (2022) Susceptibility of different life stages of Tenebrio molitor (Coleoptera: Tenebrionidae) to indigenous entomopathogenic fungi. J Stored Prod Res 98:1–8. https://doi.org/10.1016/j.jspr.2022.102008
Herren P, Hesketh H, Meyling NV, Dunn AM (2023) Environment-host-parasite interactions in mass-reared insects. Trends Parasitol 39:588–602. https://doi.org/10.1016/j.pt.2023.04.007
Borisade OA, Magan N (2015) Resilience and relative virulence of strains of entomopathogenic fungi under interactions of abiotic stress. Afr J Microbiol Res 9:988–1000. https://doi.org/10.5897/AJMR2015.7416
Cummins EP, Selfridge AC, Sporn PH, Sznajder JI, Taylor CT (2014) Carbon dioxide-sensing in organisms and its implications for human disease. Cell Mol Life Sci 71:831–845. https://doi.org/10.1007/s00018-013-1470-6
Passalacqua KD, Varadarajan A, Byrd B, Bergman NH (2009) Comparative transcriptional profiling of Bacillus cereus sensu lato strains during growth in CO2-bicarbonate and aerobic atmospheres. PLoS ONE 4:1–20. https://doi.org/10.1371/journal.pone.0004904
Argôlo-Filho R, Loguercio L (2013) Bacillus thuringiensis is an environmental pathogen and host-specificity has developed as an adaptation to human-generated ecological niches. Insects 5:62–91. https://doi.org/10.3390/insects5010062
Wakil W, Ghazanfar MU, Yasin M (2014) Naturally occurring entomopathogenic fungi infecting stored grain insect species in Punjab, Pakistan. J Insect Sci 14:1–7. https://doi.org/10.1093/jisesa/ieu044
Bernhard K, Jarrett P, Meadows M, Butt J, Ellis DJ, Roberts GM, Pauli S, Rodgers P, Burges HD (1997) Natural isolates of Bacillus thuringiensis: Worldwide distribution, characterization, and activity against insect pests. J Invertebr Pathol 70:59–68. https://doi.org/10.1006/jipa.1997.4669
Moonjely S, Bidochka MJ (2019) Generalist and specialist Metarhizium insect pathogens retain ancestral ability to colonize plant roots. Fungal Ecol 41:209–217. https://doi.org/10.1016/j.funeco.2019.06.004
St. Leger RJ, Wang JB (2020) Metarhizium: Jack of all trades, master of many. Open Biology 10:1–26. https://doi.org/10.1098/rsob.200307
Jurat-Fuentes JL, Jackson TA (2012) Bacterial entomopathogens. In: Vega FE, Kaya HK (eds) Insect Pathology, 2 edn. Academic Press, Eastbourne, pp 265–349
Wood JP, Meyer KM, Kelly TJ, Choi YW, Rogers JV, Riggs KB, Willenberg ZJ (2015) Environmental persistence of Bacillus anthracis and Bacillus subtilis spores. PLoS ONE 10:1–17. https://doi.org/10.1371/journal.pone.0138083
R Core Team (2021) R: A language and environment for statistical computing. https://www.R-project.org/ Accessed 01.02.2023
Ritz C, Baty F, Streibig JC, Gerhard D (2015) Dose-response analysis using R. PLoS ONE 10:1–13. https://doi.org/10.1371/journal.pone.0146021
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48. https://doi.org/10.18637/jss.v067.i01
Therneau TM (2020) coxme: Mixed effects cox models. R package version 2.2–16. https://CRAN.R-project.org/package=coxme Accessed 01.03.2023
Hothorn T, Bretz F, Westfall P (2008) Simultaneous inference in general parametric models. Biom J 50:346–363. https://doi.org/10.1002/bimj.200810425
Hajek AE, Davis CI, Eastburn CC, Vermeylen FM (2002) Deposition and germination of conidia of the entomopathogen Entomophaga maimaiga infecting larvae of gypsy moth, Lymantria dispar. J Invertebr Pathol 79:37–43. https://doi.org/10.1016/s0022-2011(02)00010-1
Lord JC (2009) Efficacy of Beauveria bassiana for control of Tribolium castaneum with reduced oxygen and increased carbon dioxide. J Appl Entomol 133:101–107. https://doi.org/10.1111/j.1439-0418.2008.01322.x
Schmidt S, Bos N, Murphy R, Koné NGA, Silué KS, Meyling NV, Poulsen M (2023) Make the environment protect you from disease: elevated CO2 inhibits antagonists of the fungus-farming termite symbiosis. Front Ecol Evol 11:1–9. https://doi.org/10.3389/fevo.2023.1134492
Daniels JA, Krishnamurthi R, Rizvi SSH (1985) A review of effects of carbon dioxide on microbial growth and food quality. J Food Prot 48:532–537. https://doi.org/10.4315/0362-028X-48.6.532
Trunet C, Mtimet N, Mathot AG, Postollec F, Leguerinel I, Couvert O, Broussolle V, Carlin F, Coroller L (2020) Suboptimal Bacillus licheniformis and Bacillus weihenstephanensis spore incubation conditions increase heterogeneity of spore outgrowth time. Appl Environ Microbiol 86:1–14. https://doi.org/10.1128/aem.02061-19
Nicolas G, Sillans D (1989) Immediate and latent effects of carbon dioxide on insects. Ann Rev Entomol 34:97–116. https://doi.org/10.1146/annurev.en.34.010189.000525
Helenius IT, Krupinski T, Turnbull DW, Gruenbaum Y, Silverman N, Johnson EA, Sporn PHS, Sznajder JI, Beitel GJ (2009) Elevated CO2 suppresses specific Drosophila innate immune responses and resistance to bacterial infection. PNAS 106:18710–18715. https://doi.org/10.1073/pnas.0905925106
Irwin DG, Smith LW, Pratt JJ (1972) Effects of carbon dioxide and nitrogen on the secretion of parabenzoquinones by Tribolium castaneum (Herbst). J Stored Prod Res 8:213–219. https://doi.org/10.1016/0022-474X(72)90042-2
Attygalle AB, Blankespoor CL, Meinwald J, Eisner T (1991) Defensive secretion of Tenebrio molitor (Coleoptera: Tenebrionidae). J Chem Ecol 17:805–809. https://doi.org/10.1007/BF00994202
Pedrini N, Ortiz-Urquiza A, Huarte-Bonnet C, Fan Y, Juárez MP, Keyhani NO (2015) Tenebrionid secretions and a fungal benzoquinone oxidoreductase form competing components of an arms race between a host and pathogen. PNAS 112:3651–3660. https://doi.org/10.1073/pnas.1504552112
Acknowledgements
We would like to thank Ÿnsect for providing the initial T. molitor population. Furthermore, we would like to thank Alan Bloomfield and Daisy Fairchild for their technical assistance.
Funding
This work was done within the project ‘Insect Doctors’ which has received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No. 859850.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. P.H. performed the experimental work, data collection, and analysis. P.H. wrote the first draft of the manuscript. N.V.M., A.M.D., C.S., and H.H. critiqued the manuscript for intellectual content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Herren, P., Dunn, A.M., Meyling, N.V. et al. Effect of CO2 Concentrations on Entomopathogen Fitness and Insect-Pathogen Interactions. Microb Ecol 87, 34 (2024). https://doi.org/10.1007/s00248-024-02347-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00248-024-02347-6