Abstract
The potential interaction of fluctuating temperature and the virulence of entomopathogens has implications for biological control. The objective of this study was to investigate the effect of entomopathogenic fungi (Metarhizium brunneum, Beauveria bassiana) on noctuid pest caterpillars (Spodoptera littoralis, Heliothis virescens) under constant and fluctuating temperature regimes. The results revealed similar virulence of entomopathogenic fungi under fluctuating temperature (20–30 °C or 15–35 °C) compared with constant temperature (25 °C). Experiments with cotton leaves as food confirmed that S. littoralis was less susceptible to entomopathogenic fungi (M. brunneum) than H. virescens. Results of additional experiments with H. virescens larvae, B. bassiana, and artificial diet were comparable to experiments with M. brunneum and cotton leaves, despite that susceptibility to B. bassiana was three orders of magnitude lower than to M. brunneum. The fact that both fungus species showed reduced growth on medium under fluctuating temperatures when compared to constant temperatures did not translate to an interaction of temperature regime and the virulence against noctuid larvae. Our study implies that virulence studies with noctuid larvae under constant temperatures using plant material or artificial diet might be fair models also for environments with fluctuating temperatures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Key messages
-
Virulence of entomopathogenic fungi for noctuid larvae was tested at different temperature regimes.
-
Effects on mortality and development did not differ between fluctuating and constant temperatures.
-
Virulence was unaffected despite reduced fungal growth on medium under fluctuating temperature.
-
Results were comparable for noctuid larvae fed on cotton and larvae fed on artificial diet.
-
Assays at constant temperature are a fair model for environments with fluctuating temperature.
Introduction
In agroecosystems, a major threat to crop plants is herbivores feeding on vegetative and fruiting structures and reducing the quality and quantity of the yield. Herbivores themselves are attacked by natural enemies, such as pathogens, parasitoids and predators, which are considered beneficial from a human perspective. In integrated farming systems, measures are taken to preserve natural enemies and some natural enemies have been released deliberately for biological control purposes (Shennan 2008; Wijnands et al. 2012).
Every organism survives and grows within certain (physiological) temperature limits with best performance at a certain optimum temperature (Rezende and Bozinovic 2019; Wieser 1973). Insects are ectothermal, i.e., their body temperature follows the surrounding temperature and cannot be regulated metabolically. Therefore, temperature plays a major role for the physiology of insects and has a direct influence on movement ability, development rate, and reproduction. In the field, temperatures vary over the year (seasonality) and fluctuate over days and nights. Temperatures lower or higher than the physiological limits can often be tolerated if they occur for a short time only. In addition, special adaptations to heat stress (González-Tokman et al. 2020) or cold periods, such as overwintering (Danks 2006), have evolved in many insect species. However, permanent changes in temperature caused by global warming, i.e., higher or lower average temperatures and increasing frequencies of extreme weather events, can influence long-term population development (González-Tokman et al. 2020). Varying and fluctuating temperature may also affect the interaction among organisms including herbivores and natural enemies (Bahar et al. 2012; Inglis et al. 1999; Tougeron et al. 2021). Unfavorable temperatures, either short or long term, may thus represent one important stressor for insects in the field. Insects inhabiting agroecosystems, however, may experience additional stressors, such as unsuitable and highly disturbed habitats, toxicity caused by pesticides, or pressure by pathogens, predators and parasitoids. The study of multiple stressors is important to better understand ecological processes in the agroecosystem, in particular with regard to climate change (Kaunisto et al. 2016).
The potential interaction of fluctuating temperature and the virulence of entomopathogens has implications for biological control, because in many cases laboratory studies to identify and test new biocontrol agents are done under constant temperatures. Depending on the interaction, however, virulence observed under constant temperatures might not occur in the field under fluctuating temperature, which might influence the success of biological control programs.
Entomopathogenic fungi are applied widely as biological control agents against a range of different pests, including beetles, bugs, and caterpillars. Fluctuating temperature can reduce growth and infectivity of entomopathogenic fungi (Inglis et al. 1999). Other factors, such as the intrinsic thermal performance of different species and strains, acclimatization history, status of the immune system, nutritional status, or humidity, also may influence infectivity (Fargues and Luz 2000; Ferguson and Sinclair 2020; Gava et al. 2022; Kryukov et al. 2018; Rangel et al. 2008; Reinbacher et al. 2023; Wojda and Taszlow 2013).
Studies on the impact of fluctuating temperature on host–pathogen interactions are scarce, in particular for Lepidoptera. In a previous study, the interaction of fluctuating temperature, entomopathogenic fungi, and noctuid larvae was investigated (Ghazanfar et al. 2020), Metarhizium and Beauveria species were selected, because they are used frequently as an environmentally friendly way of pest control (Shah and Pell 2003). As model pest species, two noctuid caterpillars (Lepidoptera: Noctuidae), the old-world species Spodoptera littoralis Boisduval and the new world species Heliothis virescens (Fabricius), were used. Both species are serious pests of cotton, maize, and a range of other crops (CABI Compendium 2019, 2022). When second instar caterpillars were dipped in Beauveria bassiana (Bals.- Criv) Vuill. (Hypocreales: Cordycipitaceae) spore suspension and then reared on artificial diet until pupation, all H. virescens exposed to the fungus died at constant temperature (25 °C), while no effects compared with the unexposed control were observed at fluctuating temperature regimes (20–30 °C or 15–35 °C) with an average of 25 °C. S. littoralis was not affected by B. bassiana at any temperature regime. The aim of the current study was to confirm this surprisingly strong influence of fluctuating temperature on the infectivity of entomopathogenic fungi on H. virescens. Because the previous study has several methodological shortcomings, an improved and more robust experimental design was used in the current study. First, a strain of Metarhizium brunneum (Petch) (Hypocreales: Clavicipitaceae) with higher virulence against noctuid caterpillars including S. littoralis was selected. Second, caterpillars were reared exclusively on plant food (cotton leaves) rather than artificial diet to avoid artifacts caused by potential antimicrobial reagents in the diet. Third, each experiment was repeated at least three times and the temperature program was rotated among climate cabinets for each repetition to ensure consistency of the results. Fourth, the actual temperature in each climate cabinet was recorded during the experimental period. The objective of the study was to investigate the interaction between H. virescens and S. littoralis larvae and M. brunneum under constant and fluctuating temperature regimes. More specifically, we tested how (1) the development of both noctuid species and (2) the virulence of the fungus are affected by fluctuating compared with constant temperatures. Encouraged by the results of these experiments, we tried to verify the results by Ghazanfar et al. (2020) for H. virescens with an adjusted experimental setup using the same B. bassiana strain and artificial diet that were used in the original study.
Materials and methods
Cotton plants
Cotton, line DP393, was provided by Bayer Crop Science (St. Louis, USA). Seeds were planted in 1.3-L pots using heat-treated soil (60 °C for at least 24 h) to minimize soilborne pests and diseases. Plants were grown in a climate chamber at 25 °C, 70% relative humidity (RH), and a 16:8 h light:dark photoperiod. After 3–4 weeks, individual plants were transferred to 7-L pots using soil without heat treatment and 15 g slow release fertilizer mixed in the soil (Manna 3 M, Wilhelm Haug GmbH, Ammerbuch, Germany). Liquid NPK fertilizer was applied weekly (0.2%, Manna, Wilhelm Haug GmbH). Plants at the flowering stage (3–4 months old) were used for the experiments.
Insects
Heliothis virescens was provided by Bayer AG (Frankfurt, Germany), and S. littoralis was provided by Syngenta Crop Protection AG (Stein, Switzerland). For the production of eggs, groups of approximately 10 adults (equal sex ratio) were confined in transparent plastic cylinders (11 cm diameter, 15 cm high). The cylinders had a hole at the bottom, which was fitted with a cotton dental wick (1.5 cm diameter). The bottom of the wick was submerged in a glass dish with approximately 40% honey water (w/w) that served as food for the moths. The lid of the cylinders was cut wide for ventilation, and a paper tissue was placed between the lid and the cylinder as a substrate for egg laying. Honey water was replaced every second day and the paper tissue with eggs was replaced every 1 or 2 days. Eggs were incubated in ventilated plastic cylinders for 3 days until hatching. Hatched larvae were transferred to ventilated plastic boxes (14 × 20 cm) with a layer of solidified 2% water–agar (Agar–Agar, Kobe I, Carl Roth, Karlsruhe, Germany) at the bottom and two fresh cotton leaves. The agar limited drying out of the leaves. A new cotton leaf was added after 1 or 2 days, and the larvae were used for experiments when they just reached 2nd instar (day 3 after hatching). Adults and first instars were kept at constant 23 °C, while eggs were incubated at 25 °C. RH was set to 70%, and photoperiod to 16:8 h light:dark for all stages. After the experiments described below ended with the emergence of adults, some of the adults were further used to produce eggs for the next experimental repetition.
Interaction between S. littoralis and H. virescens reared on cotton and M. brunneum at fluctuating temperatures
Metarhizium brunneum, strain EAMb 09/01-Su, Spanish Type Culture Collection CECT 20784, was provided by the University of Cordoba, Department of Agronomy. The strain was isolated in 2009 and is known to be virulent against Spodoptera spp. and other noctuids (Resquín-Romero et al. 2016). To ensure infectivity, the fungus was passed through S. littoralis and H. virescens larvae before being used for the respective experiments. Spores collected from the hosts were transferred to selective medium, modified after Strasser et al. (1996); Sabouraud 2% glucose agar (SDA) supplemented with cycloheximide (0.05 g/L), streptomycin sulfate (0.6 g/L), tetracycline (0.05 g/L), and dodine (50 mg/L) and incubated at 22 °C and 70% RH without light. Well-grown plates with spores were stored at 4 °C. Two to 3 weeks before treatment, spores were transferred to selective medium and incubated once again to ensure that fresh spores were available for each experiment. On the setup-day of each experiment, spores were washed from one fully grown plate with 15 ml of 0.1% Tween-80 using a spatula, homogenized with a magnetic stirrer, and passed through a fine gauze to remove mycelium and agar residues (stock suspension). The spores in a 1:100 dilution of the stock were counted in a Thoma chamber. Appropriate dilutions of the stock (see below) were made with 0.1% Tween-80. After experiments were set up, diluted spore suspension (1:100 dilution of the stock) was pipetted onto a thin layer of 10% Sabouraud 4% glucose agar on a microscopic glass slide and incubated in a plastic container lined with wet tissue paper for 24 h at 25 °C and 70% RH without light. Germination (presence of germ tube growing from the spore) was confirmed for more than 95% of spores in all experiments.
To study the interaction between noctuid larvae and M. brunneum at fluctuating temperatures, young but fully grown cotton leaves were harvested and disks of 2.5 cm diameter were cut. Leaf disks were placed upside down in transparent plastic dishes (3 cm diameter) with a thin layer of 2% water–agar on the bottom. Second instar noctuids (< 24 h after molting) were dipped in spore suspension (5 × 107 spores/ml for S. littoralis and 1 × 105 spores/ml for H. virescens) or in 0.1% Tween-80 as a control treatment (Table 1). The selected spore concentrations were found to achieve approximately 20% mortality in preliminary dose–response assays (data not shown). Larvae were dipped typically in batches of 12 individuals, which were transferred with a fine brush from the rearing container to a custom-made plastic cylinder (1 cm diameter, 2 cm high) with gauze at the two ends. After submerging the cylinder for 5–10 s, larvae were placed individually on cotton leaf disks and the dishes were closed with tightly fitting, ventilated lids. Larvae were incubated in three climate cabinets (Panasonic MLR 352 H-PE). One-third of the larvae was incubated at constant 25 °C, one-third at temperature fluctuating daily symmetrically from 20 to 30 °C in 2.5 °C steps every 3 h (“small fluctuating temperature”), and the last third at temperature fluctuating from 15 to 35 °C in 5 °C steps every 3 h (“large fluctuating temperature”) (Table 1). The programmed daily mean temperature in all treatments was 25 °C. Realized temperature was recorded in each climate cabinet using two data loggers (Libero THi1, Elpro, Buchs, Switzerland). We ensured that disks from the same leaf and larvae from the same rearing container were distributed evenly among all fungus treatments and temperature regimes.
Two days after the start of the experiment (day 0 = dipping of larvae), survival was recorded and all larvae were transferred to new leaf disks. Further two days later, larvae were transferred to larger leaf disks (3 cm diameter) placed in plastic cylinders (4.5 cm diameter, 4 cm high) with a layer of agar at the bottom and a punctured lid for ventilation. From now on, survival was recorded and new leaf pieces were added daily. On day 8, all larvae were weighed and transferred to new cylinders (4.5 cm diameter, 6 cm high) with a layer of agar. Once larvae were expected to pupate soon (around day 10), they were transferred to new cylinders filled with ca. 2 cm of slightly moist vermiculite. Leaf pieces were provided until feeding stopped and larvae built a pupation chamber. The pupae were formed 2–3 days later, and the date of pupation was recorded. One or two days after pupation, pupae were weighed and transferred to a new cylinder with vermiculite. Cylinders with pupae were stored in gray ventilated plastic boxes with limited light access. When all larvae either died or pupated, the sex of the pupae was determined with a stereomicroscope (Armes et al. 1992). Pupae were checked daily, and the date of adult emergence was recorded. Throughout the experiment, handling of larvae was preferably done between 9 and 12 am when all cabinets had the same temperature (25 °C). Larvae dying during the experiment were placed on wet filter paper and incubated at constant 25 °C. Four days later, the dead larvae were examined for signs of fungus infection (presence of mycelium or spores).
We defined the following assay validity criteria: (1) mortality after 8 days at 25 °C constant temperature in the treatment without fungal exposure (control) below 20%; (2) mortality after 8 days at 25 °C constant temperature in the treatment with fungal exposure at least 10% higher than in the treatment without fungal exposure; (3) difference of mean temperature over the experiment period among climate cabinets below 0.5 °C. The experiment was repeated 5 times with S. littoralis and 3 times with H. virescens. One repetition with S. littoralis had to be discarded because of high mortality in the control treatment and one because of too high mean temperature in one climate cabinet. For the three valid repetitions, the temperature programs were rotated among the climate cabinets so that each cabinet ran each program for one repetition. Each repetition of the S. littoralis experiment had 17–21 replicates (i.e., individual larvae) per temperature regime in the treatments without fungal exposure and 29–31 replicates in the treatments with fungal exposure. For the H. virescens experiment, 20–22 replicates were set up per temperature regime in the non-fungus and 34–36 replicates in the fungus treatments.
In parallel with each experiment with noctuid larvae, we also assessed M. brunneum growth on selective medium. In 9–12 Petri dishes with medium, a hole was punched in the middle of the dish using a sterilized cork borer (0.5 cm diameter). From the spore suspension prepared for dipping larvae, 50 µl of a 1 × 107 spores/ml dilution was pipetted into the hole. The dishes were covered with a tray, distributed evenly to the three temperature programs, and incubated in the same cabinets as the larvae. After 16 days, the dishes were photographed and the area of each fungal colony was measured using ImageJ software (version 1.52a, National Institutes of Health, USA). Over the whole experimental period, 20 replicates per temperature regime became available.
Interaction between H. virescens reared on artificial diet and B. bassiana at fluctuating temperatures
To be able to directly compare the results of our experiments with the study by Ghazanfar et al. (2020), we repeated the experiment of this study using the experimental procedure described above with some modifications (Table 1). Beauveria bassiana (strain ATCC 74040, commercialized as Naturalis®) was used as fungal biocontrol agent. Host passage and fungus cultivation was as described for M. brunneum, but the final multiplication was done with complete medium (modified after Riba and Ravelojoana 1984), because B. bassiana growth on selective medium was not satisfactory. Because B. bassiana showed lower virulence than M. brunneum, a higher concentration of 1 × 108 spores/ml was used for dipping. Experiments were only conducted with H. virescens, because S. littoralis had not been susceptible for B. bassiana even at high concentrations (Ghazanfar et al. 2020). Instead of agar and cotton leaves, Heliothis stonefly diet (Ward Bioscience, EducaTec, Döttingen, Switzerland) was provided ad libitum in the rearing containers. Shortly before pupation, larvae were transferred to cylinders with vermiculite and a ball of artificial diet was added until the larvae stopped feeding and built a pupation chamber.
The same validity criteria were applied as for the experiment with M. brunneum. The experiment was repeated 5 times to obtain 3 valid repetitions. One repetition had to be discarded because of low fungal infections in the constant 25 °C treatment and another one because of a technical malfunction of one climate cabinet. Each repetition had 23–24 replicates per temperature regime in the control treatments and 34–36 replicates in the fungus treatments. Growth of B. bassiana was measured as described above in parallel with the experiments with H. virescens. Each temperature regime was tested with 12 replicates (4 per repetition).
Development of H. virescens on artificial diet at different constant temperatures
To get a better idea of the influence of constant temperature on H. virescens development, an additional experiment was performed. Five constant temperatures, i.e., 20, 22.5, 25, 27.5, and 30 °C, were set up in individual climate cabinets (Table 1). The setup was as described above for the experiment with artificial diet, while the dipping step was omitted. The experiment was conducted once with 50 replicates per temperature. Temperature was recorded with two data loggers per cabinet.
Data analyses
Life table data of the noctuids from the three feeding experiments with fluctuating temperature (Table 1) were analyzed with linear mixed effects models (LMER) or generalized linear mixed effects models (GLMER) using R statistical software (R version 3.6.3, The R Foundation for Statistical Computing, Vienna, Austria), package lme4. For all categorical factors, contrasts were set to orthogonal. Weight data were analyzed by LMER, time data by GLMER with Poisson distribution, and sex ratio and survival by GLMER with binomial distribution (logit-link function), including the fixed factors Fungus treatment (F), Temperature regime (T), and the interaction F × T. Effects of factors and interactions were determined from ANOVA tables with type III sum of squares (car package) with α = 0.05. Sex (S) was initially included full factorial in the models for pupal weight, pupation time, and emergence time to check for significant interactions with the other factors. Weak interactions (F × S) were present for pupal weight in the experiments with S. littoralis on cotton (p = 0.03) and with H. virescens on artificial diet (p = 0.04, Online Resource 1, Table S2). We therefore analyzed pupal weight for both sexes separately for all three experiments. Because Sex was significant for emergence time in the experiments with H. virescens, the factor remained in the models. For pupation time, however, it was removed from the model because it was never significant. Repetition was included in all models as a random factor initially and removed in case of singularity (not explaining any variation), resulting in a generalized linear model (GLM) instead of a GLMER. Significant differences between individual temperature treatments were determined with Tukey's tests (emmeans package). Some data for weight on day 8 were accidentally not saved in the experiment with H. virescens on artificial diet and B. bassiana (25 °C, no fungus: N = 13; with fungus: N = 9; 15–30 °C no fungus: N = 1).
Temperature and humidity data from both data loggers per cabinet were averaged over 24-h intervals and those daily values were averaged over the experimental period of each experiment (start of experiment day 0 to end of experiment when all adults emerged or pupae died).
Data for the experiment with H. virescens at different constant temperatures were analyzed in a similar way: weight data with linear models (LM), time data with GLMs with Poisson distribution, and survival and sex ratio with GLMs with binomial distribution. Sex was included in the initial models and removed if nonsignificant.
Results
Interaction between S. littoralis reared on cotton and M. brunneum at fluctuating temperatures
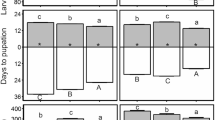
When second instar S. littoralis were dipped in 5 × 107 M. brunneum spores/ml suspension (day 0), survival in the following 8 days (on cotton) was significantly reduced by approximately 20% (Χ2 = 20.5, p < 0.0001, Fig. 1a, Table S1). There was no effect of the different temperature regimes (Χ2 = 0.76, p = 0.7) and no interaction of fungus treatment and temperature regime (Χ2 = 0.53, p = 0.8). No effect of fungus treatment and temperature regime on survival was observed for the subsequent development until adult emergence (Table 2). The effect of fungus treatment also was significant when the whole developmental period from day 0 to adult emergence was analyzed (Table 2). While weight on day 8 was not affected by fungus treatment (Χ2 = 0.17, p = 0.7) and temperature regime (Χ2 = 1.2, p = 0.6), the interaction also was nonsignificant (Χ2 = 3.0, p = 0.2, Fig. 1b, Table S1). Pupal weight of males was slightly (ca. 4%), but significantly, lower when L2 were dipped into spore suspension compared with the control treatment (Table 2, Fig. S1a). No such effect was observed for pupae of females (Table 2, Fig. S1b). Pupation time, emergence time, and sex ratio were not affected by fungus treatment and temperature regime (Table 2).
Survival from day 0 to day 8 (a, c, e) and weight on day 8 (b, d, f) of noctuid larvae when reared on cotton leaves or artificial diet under three temperature regimes: a, b Spodoptera littoralis fed cotton leaves, infested with 5 × 107 Metarhizium brunneum spores/ml; c, d Heliothis virescens fed cotton leaves, infested with 1 × 105 M. brunneum spores/ml; e, f H. virescens fed artificial diet, infested with 1 × 108 Beauveria bassiana spores/ml. In the no fungus treatments, larvae were dipped in 0.1% Tween-80 medium without spores. Larvae were incubated at 25 °C constant temperature, temperature fluctuating daily from 20 to 30 °C, or from 15 to 35 °C. In b, d, f, dots represent individual values, black rhombuses means, black horizontal lines medians, hinges 25th and 75th percentiles, and whiskers the smallest or largest values no further than 1.5 × interquartile range from the hinges. The number of replicates (n) is given. Results of GLMER and LMER with fixed factors Fungus (F) and Temperature (T) are presented in boxes (***P < 0.001). Letters display significant differences between fungus treatments
Of the larvae dying within the first 8 days of the experiment that were incubated on wet filter paper, fungal spores were observed on 6 out of 28 larvae (21%) at constant 25 °C, on 5 of 29 larvae (17%) at small fluctuating temperature, and on 2 of 33 larvae (6%) at large fluctuating temperature (Online Resource 1, Table S3).
Interaction between H. virescens reared on cotton and M. brunneum at fluctuating temperatures
Survival of H. virescens reared on cotton on day 8 after dipping into 1 × 105 M. brunneum spores/ml suspension was significantly reduced by approximately 20% (Χ2 = 20.8, p < 0.0001) without any measurable effects of temperature regime (Χ2 = 0.26, p = 0.9) and no significant interaction between fungus treatment and temperature regime (Χ2 = 1.48, p = 0.5, Fig. 1c, Table S1). This effect also was detectable over the whole developmental period (Table 3). Weight on day 8 was reduced significantly by almost 20% after fungus treatment (Χ2 = 15.5, p < 0.0001) and weight under small fluctuating temperature was significantly higher than under constant or large fluctuating temperature (Χ2 = 15.0, p = 0.0005), but there was no interaction between fungus treatment and temperature regime (Χ2 = 5.42, p = 0.1, Fig. 1d, Table S1). No effect of fungus treatment and temperature regime and no interaction was observed for survival from day 8 to adult emergence, male and female pupal weight, pupation time, emergence time, and sex ratio (Table 3, Fig. S1c, d).
Sporulation on larvae dying until day 8 was confirmed in 7 of 23 cases (30%) for 25 °C constant, 10 of 27 cases (37%) for small fluctuating, and 7 of 15 cases (47%) for large fluctuating temperature (Table S3).
Interaction between H. virescens reared on artificial diet and B. bassiana at fluctuating temperatures
In addition to the experiments with M. brunneum and cotton, H. virescens also was treated with 1 × 108 B. bassiana spores/ml and reared on artificial diet. Survival on day 8 was significantly reduced by almost 30% (Χ2 = 82.2, p < 0.0001, Fig. 1e, Table S1), while no effect of temperature regime (Χ2 = 0.48, p = 0.8) and no F × T interaction (Χ2 = 0.4, p = 0.8) was present (Fig. 1e). Similar to the other experiments, an overall effect on survival (day 0 to adult emergence) was significant (Table 4). On day 8 after treatment, larval weight was approximately 20% less in the fungus treatments than in the controls (Χ2 = 25.0, p < 0.0001, Fig. 1f, Table S1). No effect of the temperature regime (Χ2 = 0.08, p = 1.0) and no interaction was observed (Χ2 = 1.03, p = 0.6). Survival from day 8 to adult emergence, male and female pupal weight, pupation time, emergence time, and sex ratio were not affected by fungus treatment and temperature regime (Table 4, Fig. S1E, F).
Sporulation was observed on 30 of 33 dead larvae (91%) for 25 °C constant, 27 of 34 (79%) for small fluctuating, and 23 of 27 (85%) for large fluctuating temperature (Table S3).
Fungal growth on medium
Spore suspension was applied to the center of Petri dishes with selective medium and incubated at the different temperature regimes. After 16 days, the largest area was covered with mycelium at constant 25 °C for both fungal species (Table 5), followed by small fluctuating temperature. Smallest areas were covered at large fluctuating temperature. For M. brunneum, fungal growth at this temperature regime was only 30% compared to constant 25 °C. For B. bassiana, the effect was smaller (43%). Differences between temperature regimes were all significant (Table 5).
Development of H. virescens on artificial diet at different constant temperatures
When H. virescens development was investigated at constant temperatures ranging from 20 to 30 °C, 98–100% of the larvae survived until adult emergence (Table 6). Weight after 8 days increased from 20 to 27.5 °C and decreased again at 30 °C. In contrast, the highest pupal weight was obtained at 22.5 °C (males and females pooled). Pupation and emergence time decreased continuously with increasing temperature (Table 6). Fungal growth on selective medium was highest at 22.5 °C and lowest at 30 °C (Table 6). Details on statistical models for this experiment can be found in Online Resource 1, Table S4.
Discussion
Inoculation with fungal spores reduced larval survival
When exposed to fungal spores, survival of noctuid larvae 8 days after treatment was reduced in all conducted experiments, i.e., when S. littoralis and H. virescens were dipped into M. brunneum suspension and reared on cotton and when H. virescens were dipped into B. bassiana suspension and reared on artificial diet (Fig. 1). Furthermore, in both experiments with H. virescens, larval weight was reduced 8 days after treatment. Fungus treatment, however, did not affect H. virescens during their later development. Survival from day 8 to adult, pupal weight, pupation time, or emergence time were not affected in any of the experiments. In S. littoralis, no effect on weight after 8 days, but a small effect on male (not female) pupal weight was observed.
Entomopathogenic fungi usually lead to death within 3–5 days from the time of application (Inglis et al. 2001), which also was evident in the current study. Furthermore, surviving exposure may infer costs for the immune response (de Souza et al. 2020). In accordance with our results, effects on biological parameters including larval weight after exposure to sublethal B. bassiana concentrations also were reported for Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae) (de Souza et al. 2020).
To obtain an effect of approximately 20–30% reduction of survival after 8 days, the number of spores that needed to be applied was very different for the two noctuids and the two fungus species. Susceptibility toward M. brunneum was higher for H. virescens (1 × 105 spores/ml) than for S. littoralis (5 × 107), and susceptibility of H. virescens toward M. brunneum was higher than toward B. bassiana (1 × 108). Because of the low expected susceptibility of S. littoralis toward B. bassiana (Ghazanfar et al. 2020), no experiment with this noctuid / fungus combination was conducted. However, there might be other B. bassiana strains with higher virulence toward S. littoralis than the Naturalis® strain that we used (Resquín-Romero et al. 2016).
Fluctuating temperature had no effect on larval development
Development of insects is temperature dependent (Colinet et al. 2015; Rezende and Bozinovic 2019). Previous experiments showed that constant 15 °C is close to the lower limit for H. virescens and S. littoralis leading to a standstill of development (Ghazanfar et al. 2020; El-Malki 2000). With raising temperature, noctuid larvae developed faster (Table 6; Butler et al. 1979; El-Malki 2000). Weight is also increasing, but after reaching an optimum temperature, it is decreasing again. In our experiment with constant temperatures and H. virescens, the highest weight after 8 days was obtained at 27.5 °C, while the highest pupal weight was obtained at 22.5 °C (Table 6). Constant temperatures of 35 °C or higher were reported to cause high mortality in H. virescens and S. littoralis (Ghazanfar et al. 2020; Butler et al. 1979; El-Malki 2000). Similar results were obtained for other noctuid species, such as Spodoptera exigua (Hübner) (Lepidoptera: Noctuidae) (Karimi-Malati et al. 2014), Busseola fusca (Fuller) (Lepidoptera: Noctuidae) (Khadioli et al. 2014), Sesamia calamistis Hampson (Lepidoptera: Noctuidae) (Khadioli et al. 2014), and H. armigera (Mironidis and Savopoulou-Soultani 2014).
In any of the three experiments with either constant 25 °C, temperature fluctuating from 20 to 30 °C, or from 15 to 35 °C, no influence of the temperature regime on larval development was evident. This indicates that S. littoralis and H. virescens are well adapted to daily fluctuations from 15 to 35 °C, although the conditions in the highly fluctuating regime were close to the minimum and maximum physiological limits.
When reviewing the literature, Colinet et al. (2015) and Wu et al. (2015) concluded that effects of fluctuating temperature on insect development often differ from effects of constant temperature, because metabolic rate changes often are asymmetrical. Depending on the shape of the temperature–response curve, fluctuating temperatures may either increase (concave curve) or decrease (convex curve) the performance compared to a linear curve (Wu et al. 2015). For H. virescens developing on cotton, temperature fluctuations around a mean of 29 °C with a maximum of 36 or 38 °C led to decreased developmental rates compared with more moderate fluctuations with a maximum of 30, 32 or 34 °C (Moghal and Schneider 1998). The immature development of the noctuid H. armigera was similar under an alternating thermal regime (15–30 °C, mean 25 °C) compared with constant 25 °C, which aligns with the results of the present study (Mironidis and Savopoulou-Soultani 2014). For other Lepidoptera species, similar or better performance was reported under fluctuating temperature regimes compared with constant temperature, but high fluctuations or high mean temperatures can lead to adverse effects, dependent on the physiological characteristics of the species (Fischer et al. 2011; Brakefield and Kesbeke 1997; Xing et al. 2015; Bahar et al. 2012). In general, insects can develop at fluctuating temperatures that peak beyond their high or low physiological limits, but repairing direct cold or heat injuries is physiologically costly, which often results in delayed development (Colinet et al. 2015; Wu et al. 2015).
Fluctuating temperature reduced fungal growth
Fungal growth on medium was clearly influenced by temperature. In both fungal species, fluctuating temperature resulted in reduced growth when compared to the constant temperature with the same daily mean (25 °C), while higher fluctuation resulted in stronger growth reduction (Table 5). Furthermore, B. bassiana showed maximal growth at 22.5 °C constant temperature. The optimum temperature for most entomopathogenic Hyphomycetes is reported to be between 20 and 25 °C, while growth is possible within a range between 5 and 37 °C, depending on the local climate where the fungal isolates were collected from (Inglis et al. 2001; Fargues et al. 1992; Kryukov et al. 2012; Mwamburi et al. 2015; Hallsworth & Magan 1999; Gava et al. 2022). Similar to insects, also entomopathogenic fungi can survive short periods of heat, as shown for Metarhizium isolates that resumed normal growth after 3 days of cycled heat peaks (4 or 8 h at 40 °C), while some isolates showed a delay in recommencing growth after the stress event (Keyser et al. 2014). In contrast, growth recommenced immediately after exposure to 8 h cold treatments (Keyser et al. 2014). Conidia of various Metarhizium anisopliae (Metschn.) Sorokín (Hypocreales: Clavicipitaceae) isolates tolerated 12 h exposure to 40 °C, while germination rates decreased after 2 h at 45 °C (Rangel et al. 2005). Similarly, germination rates after 2 h at 45 °C varied among strains and species of Beauveria (Fernandes et al. 2008). At low temperatures (5 °C), most B. bassiana, but not Metarhizium isolates germinated well (Fernandes et al. 2008).When we incubated dead larvae from our experiments, fungal growth was confirmed in 6–47% of the cases. That hyphal growth and formation of spores was not always visible after the death of a larva could have had several reasons. First, it was often not possible to transfer the fragile dead caterpillars without damaging their body. Second, contamination by other fungi or bacteria might have occurred. Third, other reasons might have hindered the fungus to proliferate in the dead larva. Generally, B. bassiana seemed to be easier to cultivate from dead caterpillars than M. brunneum. Although fluctuating temperatures reduced fungal growth on medium, there was no clear pattern of temperature regime affecting growth in dead caterpillars.
Temperature regimes did not interact with fungus infection
The main purpose of this study was to investigate how fungal infections of noctuid larvae are influenced by fluctuating (20–30 °C and 15–35 °C) compared with constant temperature (25 °C). Our experiments, however, did not reveal any significant fungus treatment by temperature regime interaction. The adverse effect of temperature fluctuations on fungal growth on medium did not seem to limit the virulence for the noctuids. This is in contrast to Ghazanfar et al. (2020), who reported no effects of B. bassiana on H. virescens at fluctuating temperature, but 100% mortality at constant temperature. Because the study of Ghazanfar et al. (2020) was not replicated, we might speculate that the observed interaction in the previous paper was an artifact. One concern of using artificial diet to rear the infected larvae as done by Ghazanfar et al. (2020) was the possibility that antimicrobial reagents contained in the diet might influence virulence of the entomopathogenic fungi. While our experiments with cotton leaves and artificial diet did not allow to test for such influence, there was a similar lack of fungus/temperature interactions with both types of food.
There are only few studies looking at effects of fluctuating temperature compared with constant temperature on virulence of entomopathogenic fungi. The impact of fluctuating temperature on the infection and colonization of the grasshopper Melanoplus sanguinipes (Fabricius) (Orthoptera: Acrididae) by entomopathogenic fungi was studied by Inglis et al. (1999). For nymphs inoculated with B. bassiana and to a lesser extent Metarhizium flavoviride Gams & Roszypal (Hypocreales: Clavicipitaceae), the number of colony-forming units in the hemocoel and mortality decreased as the amplitude of temperature increased from 25 °C constant to 20–30 °C, 15–35 °C, and 10–40 °C. Lecuona et al. (2005) reported that the infection of the blood-sucking bug Triatoma infestans Klug (Hemiptera: Reduviidae) by B. bassiana was reduced when temperature cycles included high temperatures (34 °C) over long time periods (12 h) or when initial high temperatures were followed by an abrupt temperature fall (34–22 °C). The authors recommend to apply B. bassiana in the field late afternoon to avoid high temperatures and abrupt changes in temperature during the first stages of the infection cycle. Ferguson and Sinclair (2020) concluded that thermal history and acclimatization of both the host Gryllus veletis Alexander & Bigelow (Orthoptera: Gryllidae) and the fungus M. brunneum influenced the outcome of infection under constant and fluctuating temperature regimes. Furthermore, infections under fluctuating temperatures were a product of the relative host–pathogen performance under constant temperatures, which allowed to predict the outcome of these interactions under variable environments. Gava et al. (2022) advised to select strains with high virulence at fluctuating temperatures for the selection of suitable biological control agents, because thermotolerance and thus virulence against Ceratitis capitata (Wiedemann) (Diptera: Tephritidae) differed considerably among various strains of B. bassiana and M. anisopliae. Temperature changes can also impact immunity in insect hosts with effects on the susceptibility to infection by entomopathogens. For example, larvae of Galleria mellonella (L.) (Lepidoptera: Pyralidae) with induced diapause (15 °C) were susceptible to fungal infection by Cordyceps militaris (L.) Link (Hypocreales: Cordycipitaceae), while larvae at 25 °C could counteract infection (Kryukov et al 2018). In contrast, heat shocks (40 °C for 30 min) increased resistance of G. mellonella to infection with Bacillus thuringiensis Berliner (Bacillales: Bacillaceae) (Wojda & Taszlow 2013). In addition to temperature fluctuations, also fluctuations in humidity had a strong influence on the virulence of B. bassiana for Rhodnius prolixus Ståhl (Hemiptera: Reduviidae) (Fargues and Luz 2000). Finally, the nutritional state of the host insect (Reinbacher et al. 2023) and the pathogen (Rangel et al. 2008) can influence infectivity. In summary, although no interaction between temperature regime and fungal treatment was evident in the present experiments, infectivity of entomopathogenic fungi for insects can be influenced by fluctuating temperatures in combination with other factors, such as the intrinsic thermal performance of the different species and strains, acclimatization (thermal history), induction of immunity, other climatic factors (e.g., humidity), and nutritional state of host and pathogen.
Comparable conditions were crucial for this type of experiments
Key for this series of experiments was to maintain comparable temperature conditions (daily mean of 25 °C) in the different climate cabinets. To avoid artifacts due to temperature differences among cabinets, we conducted all experiments with three valid repetitions, while rotating climate cabinets, so that each climate cabinet was used for each temperature regime once. Second, we measured temperature (and humidity) in each cabinet with two data loggers and we also rotated data loggers randomly among cabinets. Third, we conducted preliminary experiments with data loggers and adjusted temperature programs to level out temperature differences among the cabinets. This required shifts in programmed temperature of up to 0.4 °C for one of our cabinets. It is important to notice that the temperatures programmed for the climate cabinets come with a certain temperature accuracy, which is specified as ± 0.3 °C for the used MLR-352H cabinets. Temperature-sensitive experiments thus need verification with data loggers. Because data loggers also come with a certain level of variation (± 0.5 °C for the used ELPRO libero THi1), the use of multiple data loggers is recommended.
From the experiment with different constant temperatures and H. virescens, we can estimate changes in developmental parameters based on a certain temperature difference. From 25 °C constant temperature, a difference of 0.1 °C resulted in a change of 5 mg weight after 8 days (2%), 0.5 mg pupal weight (0.2%), 0.1 days pupation time (1%), and 0.1 days emergence time (1%) (for detailed calculations see Table S5). The highest temperature sensitivity is thus for the weight after 8 days. For the same dataset (H. virescens fed artificial diet, 25 °C constant, N = 50), estimated effect sizes based on the obtained means and SDs that could be detected with t-tests were 20% for weight after 8 days, 5% for pupal weight, 4% for pupation time, and 4% for emergence time (Table S5). Thus, detectable effect sizes were at least 4 times higher than effects due to a 0.1 °C variation in the temperature settings. In our cabinets, the measured average temperatures were highly constant and did not differ at a level exceeding 0.1 °C over all repetitions, which renders artifacts due to differences in average temperatures unlikely (Table S6).
Conclusions
Our highly controlled laboratory study revealed similar effects of entomopathogenic fungi on the development of noctuid larvae under fluctuating temperature compared with constant temperature. When fed with cotton leaves, S. littoralis was less susceptible to M. brunneum than H. virescens. Experiments with H. virescens larvae, B. bassiana and artificial diet were comparable to the experiments with M. brunneum and cotton leaves, despite the fact that susceptibility to B. bassiana was three orders of magnitude lower than to M. brunneum. Although the entomopathogenic fungi showed reduced growth at fluctuating temperatures, no interaction of temperature regime on infectivity of noctuid larvae was observed. To avoid artifacts in temperature-sensitive experiments, it is crucial to ensure that temperature settings are accurate. This work implies that virulence studies under constant temperatures using plant material or artificial diet for noctuid larvae are suitable models also for environments with temperatures fluctuating within the physiological limits of the tested species.
Author contribution statement
All authors contributed to conceptualization, methodology, and reviewing and editing of the manuscript. Resources (fungal strains) were contributed by GG. MM performed data validation, formal analysis, investigation, data curation, writing of the original draft, and data visualization. All authors read and approved the final manuscript.
Data availability
The datasets generated and/or analyzed during the current study are available in the Supplementary Information to this publication (file “Online Resource 2_Data”).
References
Armes NJ, Bond GS, Cooter RJ (1992) The laboratory culture and development of Helicoverpa armigera. Natural Resources Institute, Bulletin 57, Chatham, UK.
Bahar MH, Soroka JJ, Dosdall LM (2012) Constant versus fluctuating temperatures in the interactions between Plutella xylostella (Lepidoptera: Plutellidae) and its larval parasitoid Diadegma insulare (Hymenoptera: Ichneumonidae). Environ Entomol 41:1653–1661. https://doi.org/10.1603/en12156
Brakefield PM, Kesbeke F (1997) Genotype–environment interactions for insect growth in constant and fluctuating temperature regimes. Proc R Soc Lond B Biol Sci 264:717–723. https://doi.org/10.1098/rspb.1997.0102
Butler GD Jr, Hamilton AG, Proshold FI (1979) Developmental times of Heliothis virescens and H. subflexa in relation to constant temperature. Ann Entomol Soc Am 72:263–266. https://doi.org/10.1093/aesa/72.2.263
Compendium CABI (2019) Heliothis virescens (tobacco budworm). https://doi.org/10.1079/cabicompendium.26774
Compendium CABI (2022) Spodoptera littoralis (cotton Leafworm). https://doi.org/10.1079/cabicompendium.51070
Colinet H, Sinclair BJ, Vernon P, Renault D (2015) Insects in fluctuating thermal environments. Annu Rev Entomol 60:123–140. https://doi.org/10.1146/annurev-ento-010814-021017
Danks HV (2006) Insect adaptations to cold and changing environments. Can Entomol 138:1–23. https://doi.org/10.4039/n05-802
de Souza TD, Fernandes FO, Sanches AC, Polanczyk RA (2020) Sublethal effects of different fungal isolates on Helicoverpa armigera (Lepidoptera: Noctuidae). Egypt J Biol Pest Control 30:141. https://doi.org/10.1186/s41938-020-00327-9
El-Malki KG (2000) Thermal requirements and prediction models of cotton leafworm Spodoptera littoralis (Boisd). In: Dugger P, Richter D (eds) Proceedings of the Beltwide Cotton Conferences, Vol. 2, 4–8 January 2000, San Antonio, Texas, United States, pp 1019–1021
Fargues J, Luz C (2000) Effects of fluctuating moisture and temperature regimes on the infection potential of Beauveria bassiana for Rhodnius prolixus. J Invertebr Pathol 75:202–211. https://doi.org/10.1006/jipa.1999.4923
Fargues J, Maniania N, Delmas J, Smits N (1992) Influence de la température sur la croissance in vitro d’hyphomycètes entomopathogènes. Agronomie 12:557–564. https://doi.org/10.1051/agro:19920708
Ferguson LV, Sinclair BJ (2020) Thermal variability and plasticity drive the outcome of a host-pathogen interaction. Am Nat 195:603–615. https://doi.org/10.1086/707545
Fernandes ÉKK, Rangel DEN, Moraes ÁML, Bittencourt VREP, Roberts DW (2008) Cold activity of Beauveria and Metarhizium, and thermotolerance of Beauveria. J Invertebr Pathol 98:69–78. https://doi.org/10.1016/j.jip.2007.10.011
Fischer K, Kölzow N, Höltje H, Karl I (2011) Assay conditions in laboratory experiments: is the use of constant rather than fluctuating temperatures justified when investigating temperature-induced plasticity? Oecologia 166:23–33. https://doi.org/10.1007/s00442-011-1917-0
Gava CAT, Leal CM, Vieira de Sá A, Paranhos BAJ (2022) Selecting thermal tolerant strains of entomopathogenic fungi to control Ceratitis capitata (Wiedeman) in tropical semi-arid conditions. Biol Control 176:105062. https://doi.org/10.1016/j.biocontrol.2022.105062
Ghazanfar MU, Hagenbucher S, Romeis J, Grabenweger G, Meissle M (2020) Fluctuating temperatures influence the susceptibility of pest insects to biological control agents. J Pest Sci 93:1007–1018. https://doi.org/10.1007/s10340-020-01215-9
González-Tokman D, Córdoba-Aguilar A, Dáttilo W, Lira-Noriega A, Sánchez-Guillén RA, Villalobos F (2020) Insect responses to heat: physiological mechanisms, evolution and ecological implications in a warming world. Biol Rev 95:802–821. https://doi.org/10.1111/brv.12588
Hallsworth JE, Magan N (1999) Water and temperature relations of growth of the entomogenous fungi Beauveria bassiana, Metarhizium anisopliae, and Paecilomyces farinosus. J Invertebr Pathol 74:261–266. https://doi.org/10.1006/jipa.1999.4883
Inglis GD, Duke GM, Kawchuk LM, Goettel MS (1999) Influence of oscillating temperatures on the competitive infection and colonization of the migratory grasshopper by Beauveria bassiana and Metarhizium flavoviride. Biol Control 14:111–120. https://doi.org/10.1006/bcon.1998.0666
Inglis GD, Goettel MS, Butt TM, Strasser H (2001) Use of hyphomycetous fungi for managing insect pests. In: Butt TM, Jackson C, Magan N (eds) Fungi as biocontrol agents. CABI Publishing, Wallingford, UK, pp 23–69
Karimi-Malati A, Fathipour Y, Talebi AA, Bazoubandi M (2014) Life table parameters and survivorship of Spodoptera exigua (Lepidoptera: Noctuidae) at constant temperatures. Environ Entomol 43:795–803. https://doi.org/10.1603/en11272
Kaunisto S, Ferguson LV, Sinclair BJ (2016) Can we predict the effects of multiple stressors on insects in a changing climate? Curr Opin Insect Sci 17:55–61. https://doi.org/10.1016/j.cois.2016.07.001
Keyser CA, Fernandes ÉKK, Rangel DEN, Roberts DW (2014) Heat-induced post-stress growth delay: a biological trait of many Metarhizium isolates reducing biocontrol efficacy? J Invertebr Pathol 120:67–73. https://doi.org/10.1016/j.jip.2014.05.008
Khadioli N, Tonnang ZEH, Ong’amo G, Achia T, Kipchirchir I, Kroschel J, Le Ru B (2014) Effect of temperature on the life history parameters of noctuid lepidopteran stem borers, Busseola fusca and Sesamia calamistis. Ann Appl Biol 165:373–386. https://doi.org/10.1111/aab.12157
Kryukov VY et al (2012) Change in the temperature preferences of Beauveria bassiana sensu lato isolates in the latitude gradient of Siberia and Kazakhstan. Microbiol 81:453–459. https://doi.org/10.1134/S002626171204011X
Kryukov VY, Tomilova OG, Yaroslavtseva ON, Wen T-C, Kryukova NA, Polenogova OV, Tokarev YS, Glupov VV (2018) Temperature adaptations of Cordyceps militaris, impact of host thermal biology and immunity on mycosis development. Fungal Ecol 35:98–107. https://doi.org/10.1016/j.funeco.2018.07.003
Lecuona RE, Rodriguez J, La Rossa FR (2005) Effect of constant and cyclical temperatures on the mortality of Triatoma infestans (Klug) (Hemiptera: Reduviidae) treated with Beauvaria bassiana (Bals.) Vuill. (Hyphomycetes). Neotrop Entomol 34:675–679. https://doi.org/10.1590/S1519-566X2005000400019
Mironidis GK, Savopoulou-Soultani M (2014) Development, survivorship, and reproduction of Helicoverpa armigera (Lepidoptera: Noctuidae) under constant and alternating temperatures. Environ Entomol 37:16–28. https://doi.org/10.1093/ee/37.1.16
Moghal MS, Schneider JC (1998) Effects of high temperature on development of Heliothis virescens (F.) on cotton fruiting structures in the laboratory. Southwest Entomol 23:123–126. Available online: https://www.sswento.org/_files/ugd/0646ec_3e4b0994a4f9494f855ae6e41243718e.pdf. Accessed 24 Oct 2023
Mwamburi LA, Laing MD, Miller RM (2015) Effect of surfactants and temperature on germination and vegetative growth of Beauveria bassiana. Environ Microbiol 46:67–74. https://doi.org/10.1590/S1517-838246120131077
Rangel DEN, Braga GUL, Anderson AJ, Roberts DW (2005) Variability in conidial thermotolerance of Metarhizium anisopliae isolates from different geographic origins. J Invertebr Pathol 88:116–125. https://doi.org/10.1016/j.jip.2004.11.007
Rangel DEN, Alston DG, Roberts DW (2008) Effects of physical and nutritional stress conditions during mycelial growth on conidial germination speed, adhesion to host cuticle, and virulence of Metarhizium anisopliae, an entomopathogenic fungus. Mycol Res 112:1355–1361. https://doi.org/10.1016/j.mycres.2008.04.011
Reinbacher L, Praprotnik E, Razinger J, Bacher S, Grabenweger G (2023) Influence of wireworm diet on its susceptibility to and control with the entomopathogenic fungus Metarhizium brunneum (Hypocreales: Clavicipitaceae) in laboratory and field settings. J Econ Entomol 116:108–118. https://doi.org/10.1093/jee/toac198
Resquín-Romero G, Garrido-Jurado I, Delso C, Ríos-Moreno A, Quesada-Moraga E (2016) Transient endophytic colonizations of plants improve the outcome of foliar applications of mycoinsecticides against chewing insects. J Invertebr Pathol 136:23–31. https://doi.org/10.1016/j.jip.2016.03.003
Rezende EL, Bozinovic F (2019) Thermal performance across levels of biological organization. Philos Trans R Soc Lond B Biol Sci 374:20180549. https://doi.org/10.1098/rstb.2018.0549
Riba G, Ravelojoana AM (1984) The parasexual cycle in the entomopathogenic fungus Paecilomyces fumoso-roseus (Wize) Brown and Smith. Can J Microbiol 30:922–926. https://doi.org/10.1139/m84-144
Shah PA, Pell JK (2003) Entomopathogenic fungi as biological control agents. Appl Microbiol Biotechnol 61:413–423. https://doi.org/10.1007/s00253-003-1240-8
Shennan C (2008) Biotic interactions, ecological knowledge and agriculture. Philos Trans R Soc Lond B Biol Sci 363:717–739. https://doi.org/10.1098/rstb.2007.2180
Strasser H, Forer A, Schinner F (1996). Development of media for the selective isolation and maintenance of virulence of Beauveria brongniartii. In: Jackson TA, Glare TR (eds) 3rd international workshop on microbial control of soil dwelling pests. AgResearch Lincoln, New Zealand, pp 125–130
Tougeron K, Ferrais L, Renard M-E, Hance T (2021) Effects of constant versus fluctuating temperatures on fitness indicators of the aphid Dysaphis plantaginea and the parasitoid Aphidius matricariae. Insects 12:855. https://doi.org/10.3390/insects12100855
Wieser W (1973) Effects of temperature on ectothermic organisms - ecological implications and mechanisms of compensation. Springer-Verlag, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-65703-0
Wijnands FG, Baur R, Malavolta C, Gerowitt B (2012) Integrated pest management - design and application of feasible and effective strategies. IOBC-WPRS, Lelystad, The Netherlands.
Wojda I, Taszłow P (2013) Heat shock affects host–pathogen interaction in Galleria mellonella infected with Bacillus thuringiensis. J Insect Physiol 59:894–905. https://doi.org/10.1016/j.jinsphys.2013.06.011
Wu T-H, Shiao S-F, Okuyama T (2015) Development of insects under fluctuating temperature: a review and case study. J Appl Entomol 139:592–599. https://doi.org/10.1111/jen.12196
Xing K, Ma C-S, Zhao F, Han J-C (2015) Effects of large temperature fluctuations on hatching and subsequent development of the diamondback moth (Lepidoptera: Plutellidae). Fla Entomol 98(651–659):659. https://doi.org/10.1653/024.098.0240
Acknowledgements
We are grateful to Syngenta, Stein, Switzerland (Oliver Kindler), for providing S. littoralis eggs, to Bayer, Frankfurt, Germany (Birgit Bollenbach-Wahl), for providing H. virescens pupae, to Bayer, St. Louis, USA, for providing cotton seeds, and to the University of Cordoba, Cordoba, Spain (Enrique Quesada-Moraga), for providing M. brunneum strain EAMb 09/01-Su. We further acknowledge Christian Schweizer, Fionna Knecht, and Tanja Sostizzo for help in producing plates with fungal spores and Mario Waldburger and Nina Häner for help in rearing the noctuids and performing experiments.
Funding
Open access funding provided by Agroscope. The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethics Approval
Not relevant to this study.
Consent to Participate
Not relevant to this study.
Consent to Publish
Not relevant to this study.
Additional information
Communicated by Salvatore Arpaia.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Meissle, M., Grabenweger, G. & Romeis, J. No interaction of fluctuating or constant temperature and virulence of entomopathogenic fungi in two noctuid species. J Pest Sci 97, 809–823 (2024). https://doi.org/10.1007/s10340-023-01673-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10340-023-01673-x