Abstract
To date, evidence supporting the efficacy of tricuspid valve (TV) repair in interrupting the progression of systemic right ventricular (RV) adverse remodeling in hypoplastic left heart syndrome (HLHS) is conflicting. We conducted a systematic review and meta-analysis of scientific literature to assess the impact of TV repair in effectively modifying the prognosis of patients with HLHS. We conducted a systematic review of PubMed, Web of Science, and Scopus databases. A random-effect meta-analysis was performed and transplant-free survival, freedom from TV regurgitation, and TV reoperation data were reconstructed using the published Kaplan–Meier curves. Nine studies were included, comprising 203 HLHS patients undergoing TV repair and 323 HLHS controls. The estimated transplant-free survival at 1, 5, and 10 years of follow-up was 75.5% [95% confidence interval (CI) = 67.6–84.3%], 63.6% [95% CI = 54.6–73.9%], and 61.9% [95% CI = 52.7–72.6%], respectively. Transplant-free survival was comparable to HLHS peers without TV regurgitation (p = 0.59). Five-year freedom from recurrence of TV regurgitation and freedom from TV reoperation was 57% [95% CI = 46.7–69.7%] and 63.6% [95% CI = 54.5–74.3%], respectively. Younger age and TV repair at the time of Norwood operation increased the risk of TV regurgitation recurrence and the need for TV reoperation. Our meta-analysis supports the efficacy of TV repair in favorably modifying the prognosis of patients with HLHS and TV regurgitation, reestablishing a medium-term transplant-free survival which is comparable to HLHS peers. However, durability of surgery and long-term fate of TV and RV performance are still unclear.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The inclusion of the morphologically tricuspid valve (TV) into the systemic circulation by means of the Fontan palliation pathway triggers a premature deterioration of its competence [1,2,3,4,5,6]. Right ventricular (RV) dominance exposes univentricular patients to an increased risk of clinically significant atrioventricular valve regurgitation, which translates into poor early and long-term transplant-free survival [7,8,9,10]. Hypoplastic left heart syndrome (HLHS) represents the prototype of this condition [11] and 15–25% of affected patients are expected to require surgical management of TV regurgitation (TVR) during their palliation course [12,13,14,15,16,17].
The underlying pathophysiology of systemic TVR entails a vicious cycle where all the constituents of the TV apparatus, as well as the ventricular myocardium, are involved (Fig. 1). The systemic afterload imposes a pressure stress on the RV, which undergoes a remodeling process eventually leading to ventricular dilatation [18]. Papillary muscles displacement, leaflet tethering, and TV annulus enlargement contribute to the loss of coaptation [19, 20]. Also, intrinsic abnormalities of the TV leaflets can be present [15]. The development of TVR generates additional RV volume overload, which further deteriorates RV performance and aggravates TVR itself, finally resulting in failing Fontan circulation [21].
To date, the role of surgical TV repair in successfully interrupting this vicious cycle is still unclear. In fact, evidence supporting the efficacy of TV repair in reverting the (un)natural progression of RV adverse remodeling and preventing TVR recurrence in HLHS is conflicting [14, 20, 22,23,24]. This ultimately turns into a poorly predictable long-term outcome even for HLHS patients in whom a successful TV repair has been achieved [12, 14,15,16]. Moreover, the very small sample size of surgical cohorts dramatically limits the generalization of findings. We conducted a systematic review and meta-analysis of scientific literature to assess the impact of TV repair in effectively modifying the prognosis of patients with HLHS, the risk of TVR recurrence, and the need for reintervention.
Materials and Methods
Data Collection
A systematic review was conducted according to the PRISMA [25] and MOOSE [26] guidelines. This study was prospectively registered on the PROSPERO database (CRD42023396529). The PubMed, Web of Science, and Scopus databases were systematically searched in January 2023, by two authors (M.P. and M.A.P.). Any eligibility disagreement was resolved by discussion among all the authors and then agreement by consensus. Ethics approval and patient consent were obtained by each research group. Our institutional Ethics Review Board waived the need for ethics approval for the meta-analysis. Data available on request to the corresponding author.
Inclusion Criteria
After duplicates removal, the manuscripts were firstly screened on the title and abstract and then underwent full-text revision, using the following inclusion criteria: (1) study population composed of patients affected by HLHS with TVR undergoing TV repair; (2) studies reporting survival, and/or TVR recurrence, and/or risk of reoperation for TVR displayed as Kaplan-Meier curves; (3) papers written in English after 1970.
Exclusion Criteria
Excluded studies were the ones: (1) enrolling patients undergoing TV replacement as a primary surgical attempt for TVR; (2) enrolling univentricular patients without a specific anatomical diagnosis of HLHS; (3) series without surgical treatment of TVR; (4) case reports and series with less than five patients; (5) reviews and meta-analyses; (6) not full-text manuscripts.
Data Extraction
Two authors (M.P. and M.A.P.) extracted data to a pre-set Excel abstraction form. Extracted data were: publication year, number of patients, number of controls, cohort period, age at study, follow-up period, gender, the timing of TV repair (classified as: during the Norwood procedure; interstage I; during the bidirectional Glenn; interstage II; during Fontan operation; after Fontan operation), the specific surgical technique for TV repair, transplant-free survival, patients at risk, freedom from TVR recurrence, freedom from reoperation for TVR, early (in-hospital) mortality, early (in-hospital) reoperation rate for TVR. When available, transplant free-survival and patients at risk were extracted also for control patients (i.e. those affected by HLHS without clinically significant TVR, not requiring TV repair) from the selected studies. Transplant-free survival curves were reconstructed by selecting only those studies in whom estimates were measured starting from the day of the Norwood operation, to allow for a comparison with controls.
Quality Assessment
The risk of bias at the study level was assessed by two reviewers (M.P. and L.V.) using the Appraisal tool for Cross-Sectional Studies (AXIS) [27]. The AXIS 20-item tool assesses the quality of cross-sectional studies based on the following criteria: clarity of aims/objectives and target population; appropriate study design and sampling framework; justification for the sample size; measures taken to address non-responders and the potential for response bias; risk factors/outcome variables measured in the study; clarity of methods and statistical approach; appropriate result presentation, including internal consistency; justified discussion points and conclusion; discussion of limitations; and identification of ethical approval and any conflicts of interest. The scoring system conforms to a “yes”, “no”, or “do not know/comment” design. We classified the studies into four quality categories based on the number of “yes” answers for each of the 20 questions included in the AXIS tool [28]: “high” (> 15 positive answers), “medium” (between 10 and 15), “low” (between 5 and 9), and “very low” (< 5).
Study Description
The study characteristics are presented descriptively as mean and standard deviation (SD) or median (interquartile range [IQR]) in the case of quantitative variables, depending on the data reported in the study, and as absolute and relative frequencies in the case of categorical variables.
Meta-analysis
Time to Event Endpoints. The transplant-free survival and time to event data were reconstructed using the algorithm indicated by Guyot et al [29]. The global Log-rank test was reported on the plot. The pooled hazard ratios (HR) were calculated via the Cox regression model on the reconstructed individual patient data with their related confidence interval (CI). A frailty term has been included in the model to account for correlation within the data reconstructed in the same study. Survival curves were obtained with the Kaplan-Meier method. Outcomes were presented as pooled proportions for data synthesis.
Other Endpoints
A random-effect meta-analysis has been carried out on the study outcomes. The heterogeneity is estimated from the studies’ intervention effects and standard errors included in the meta-analysis via Der Simonian and Laird Estimator [30]. The I² measure has been considered to quantify the heterogeneity. The measure expresses the percentage of between-study variability related to heterogeneity rather than chance [31]. The study-specific estimates with 95% CI have been reported representing the pooled meta-analytical estimate in a forest plot.
Effect Modifiers
Univariable meta-regression models have been computed to assess whether the study characteristics may act as effect modifiers on the final meta-analysis estimate. Considered variables for meta-regression were: publication year, age at surgery, percentage of patients at Norwood stage, and follow-up time. Given the unavailability of patients’ gender data in most of the studies, this variable was not included in the meta-regression model.
Publication Bias
The publication bias has been visually assessed by considering a Funnel plot representation. A funnel plot is a scatter plot of the study-specific effect sizes (log odds ratio or mean difference) against the standard error on the ordinate axis. When there is no publication bias, the data points in such a plot should form a roughly symmetrical, upside-down funnel. The symmetry has been also assessed by considering the linear regression test of the Egger Test for asymmetry in the funnel plot.
Computations were performed in R 4.0.1 [32] system with metaphor and IPDfromKM packages [33, 34].
Results
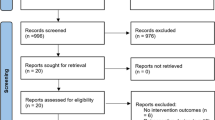
After the removal of duplicates, a total of 319 manuscripts were identified; full-text eligibility was assessed for 35 of them and, finally, 9 articles could be included (Fig. 2; Table 1) [12,13,14,15,16, 22, 35,36,37]. Figure 3 summarizes the quality assessment of selected reports using AXIS tool. Quality resulted in being high in 4 (44%) of studies and medium in 5 (56%). Quality assessment of each manuscript is provided in Supplemental Fig. 1.
Quality assessment of manuscripts using AXIS tool: selected studies (n=9) sorted by overall quality (panel A) and rate of fulfilment of each quality item of AXIS tool across papers (panel B). Blue color indicates AXIS criteria fully satisfied; red color indicates AXIS criteria not satisfied; green color indicates AXIS criteria not evaluable
Patient Characteristics
We identified a total of 203 patients who underwent surgical repair of TVR across series. The median/mean age at TV repair ranged from 0.02 to 1.9 years (Table 1). The majority of operations occurred concomitantly to the scheduled palliation procedures: 50 (24.6%) at the time of Norwood operation, 9 (4.4%) during interstage I period, 79 (38.9%) at bidirectional cavo-pulmonary connection (bidirectional Glenn) surgery, 15 (7.4%) during interstage II period, 61 (30%) at Fontan operation, and 9 (4.4%) after Fontan completion (Supplemental Fig. 2). The median/mean follow-up ranged from 0.4 to 7.9 years.
Surgical Strategy
The most common surgical technique for TV repair was commissuroplasty (139/191 patients, 72.8%), followed by annuloplasty (113/191, 59.2%), neo chordae implantation (24/191, 12.6%), leaflet adaptation (20/191, 10.5%), cleft closure (16/191, 8.4%), edge-to-edge stitch (12/191, 6.3%), and other less frequent procedures (13/191, 6.8%). The study of Nakata and colleagues[37] did not report a detailed description of TV repair techniques for HLHS patients, thus was excluded from this sub-analysis. Table 2 and Supplemental Fig. 3 summarize the adopted surgical techniques in each selected manuscript.
Early Outcomes and Transplant-free Survival
From pooled analysis of the included studies, in-hospital mortality after TV repair was 9% [95% CI = 1–21%; I2 = 76.9%, p < 0.001, Fig. 4). The rate of patients undergoing TV repair at the time of Norwood operation acted as a modifier effect on the meta-analysis (estimate 0.004 [95% CI: 0.0005–0.007] per 1% increase of Norwood rate, p = 0.024). Age at surgery (estimate − 0.16 [95% CI: − 0.33–0.01], p = 0.066), follow-up period (estimate − 0.011 [95% CI: − 0.097–0.076], p = 0.810), and cohort period (estimate − 0.024 [95% CI: − 0.065–0.017], p = 0.253) did not present a modifier effect on the analysis.
The pooled risk of early (in-hospital) TV reoperation resulted to be 1% [95% CI = 0–5%; I2 = 32.9%, p = 0.15, Fig. 4). None of the considered variables had a modifier effect on the meta-analysis.
Five manuscripts [13, 15, 16, 22, 35] (for a total of 104 patients) reported Kaplan-Meier curves with a follow-up starting from the time of Norwood operation, which allowed for the reconstruction of transplant-free survival data. The meta-analysis conducted on the identified studies estimated a transplant-free survival at 1, 2, 5, and 10 years of follow-up of 75.5% [95% CI = 67.6–84.3%], 69.4% [95% CI = 60.9–79%], 63.6% [95% CI = 54.6–73.9%], and 61.9% [95% CI = 52.7–72.6%], respectively (Fig. 5). Age at surgery (HR: 0.66, 95% CI = 0.20–2.19, p = 0.497), the rate of patients undergoing TV repair at the time of Norwood operation (HR: 1.00, 95% CI = 0.99–1.01, p = 0.631), follow-up time (HR: 0.93, 95% CI = 0.77–1.14, p = 0.491), and publication year (HR: 0.95, 95% CI = 0.84–1.08, p = 0.437) did not act as effect modifiers on the meta-analysis.
Pooled transplant-free survival of patients undergoing TV repair did not differ from the one of 323 patients with HLHS without TVR used as controls [13, 15, 16, 35] (p = 0.59, Fig. 5). When selecting those patients with TVR requiring surgery (n = 84) only from studies reporting controls [13, 15, 16, 35], transplant-free survival between the two groups was still comparable (p = 0.88, Supplemental Fig. 4), with a pooled HR of mortality of 1.12 (95% CI = 0.77–1.62, p = 0.568).
Freedom from TVR Recurrence and Freedom from Reoperation
Four studies [13, 22, 36, 37] (including a total of 91 patients) reported estimates of freedom from recurrence of clinically significant TVR after TV repair. Pooled analysis revealed freedom from TVR recurrence at 1, 2, 5, and 10 years of follow-up of 65.9% [95% CI = 56.7–76.7%], 63.2% [95% CI = 53.8–74.3%], 57% [95% CI = 46.7–69.7%], and 48.7% [95% CI = 37.3–63.7%], respectively (Fig. 6). Age at surgery had a modifier effect on the freedom from regurgitation (HR: 0.32, 95% CI = 0.19–0.54, p < 0.001), with younger patients experiencing an increased risk of recurrence of TVR. Similarly, the rate of patients undergoing TV repair at the time of Norwood operation acted as an effect modifier (HR: 1.02, 95% CI = 1.00–1.03, p = 0.021), increasing the risk of TVR recurrence. Follow-up time (HR: 1.27, 95% CI = 0.33–4.96, p = 0.730) and publication year (HR: 1.04, 95% CI = 0.85–1.27, p = 0.708) had not a modifier effect on the meta-analysis.
Five studies [13,14,15,16, 37] (for a total of 115 patients) estimated the freedom from TV reoperation after TV repair. Pooled analysis showed freedom from TV reoperation at 1, 2, 5, and 10 years of follow-up of 77% [95% CI = 69.4–85.4%], 71.4% [95% CI = 63.1–80.7%], 63.6% [95% CI = 54.5–74.3%], and 63.6% [95% CI = 54.5–74.3%], respectively (Fig. 6). Age at surgery acted as an effect modifier (HR: 0.05, 95% CI = 0.01–0.25, p < 0.001) and younger patients displayed an increased risk of TV reoperation. The rate of patients requiring TV repair at the time of Norwood operation had a modifier effect on the freedom from TV reoperation (HR: 1.02, 95% CI = 1.01–1.02, p < 0.001). Neither follow-up time (HR: 0.85, 95% CI = 0.67–1.07, p = 0.173) nor publication year (HR: 1.08, 95% CI = 0.92–1.26, p = 0.351) acted as effect modifiers on the meta-analysis.
Publication Bias
The funnel plots, in addition to the traditional bands to identify publication bias, contain three shaded regions; these regions identified statistically significant effects for a significance level between 0.1 and 0.05, 0.05 and 0.01, and < 0.01. Concerning the in-hospital mortality outcome only two studies fail outside the funnel plot bounds indicating a controlled publication bias; the only reporting a significant effect despite the high standard error is Nakata et al [37].
The studies reporting the early (in-hospital) TV reoperation rate outcome fall all inside the funnel plot bounds indicating a substantial absence of publication bias.
Discussion
Up to 1/4 of children affected by HLHS are projected to develop clinically significant TVR necessitating surgical repair during their single-ventricle palliation course [8, 9, 38]. Given the intrinsic pathophysiological relationship between TVR and RV myocardial remodeling triggered by its inclusion in the systemic circulation (Fig. 1), the role of surgical TV repair in interrupting this vicious cycle and positively modifying the long-term prognosis of HLHS patients is still undefined. We specifically addressed this topic by performing a meta-analysis of scientific literature which revealed that patients undergoing TV repair display comparable transplant-free survival to HLHS peers without clinically significant TVR. On the other hand, the durability of surgical repair seems to be limited and a significant quote of patients will necessitate more than one surgical procedure on the TV.
The loss of systemic TV competence represents a bad prognostic factor at every stage of univentricular palliation, impacting interstage I mortality [9], interstage II mortality [8], and Fontan completion outcomes [39]. In the multicenter prospective cohort of the Single Ventricle Reconstruction Trial, 11/549 patients required TV repair at the time of Norwood procedure [40], 44/393 at stage II operation [8], and 29/327 at Fontan [39]. However, the outcomes of TV surgery have not been specifically discussed by the investigators. More recently, in a sub-analysis of the Australia and New Zealand Fontan Registry, patients with HLHS exhibited poor long-term freedom from atrio-ventricular valve failure, which was demonstrated to be associated with RV contractile dysfunction and failure of Fontan circulation [17]. Interestingly, the observed inferior prognosis of Fontan patients requiring atrio-ventricular valve surgery resulted to be mainly driven by a disproportionate effect that atrio-ventricular valve regurgitation displayed in the RV-dominant population only [10]. These findings suggest that a successful TV surgery may redefine the natural history of TVR in patients with RV dominance. Unfortunately, HLHS patients represented only 35% (205/581) of the original right-dominant cohort, with only 42/205 cases requiring TV repair [10], thus limiting the applicability of findings.
By performing a systematic review of scientific literature, we sought to define if TV surgery can effectively modify the prognosis of HLHS patients with TVR. Keeping in mind the intrinsic limitations of a meta-analysis of retrospective observational studies, in this delicate cohort we observed a pooled transplant-free survival at 1, 5, and 10 years of follow-up of 75.5% [95% CI = 67.6–84.3%], 63.6% [95% CI = 54.6–73.9%], and 61.9% [95% CI = 52.7–72.6%], respectively, which parallels the prognosis of the general population of HLHS from large multicenter studies [38, 41]. In fact, both our overall cohort and the cases selected only from studies reporting controls (Supplemental Fig. 4) displayed a similar prognosis to HLHS peers without TVR (p = 0.59 and p = 0.88, respectively). Our results may imply that TV repair, together with the improved pre- and post-operative medical management [42, 43], can restore the original prognosis of HLHS patients with TVR, counteracting the deleterious effect that untreated TVR has on the patient’s survival.
Insights from 3-dimensional echocardiography have revealed that the mechanisms of TVR in HLHS entail flattening and dilatation of TV annulus, together with leaflet prolapse and tethering [19, 20]. Surgical repair is proven to effectively address annular enlargement, commissural regurgitant jets, and posterior leaflet prolapse [19]. However, septal leaflet tethering, which is directly related to RV cavity dilatation and contractility [20], is poorly modified by surgical efforts [19] and represents a risk factor for failure of TV repair [19]. The complex interdependence of TVR and RV myocardium might account for the high rates of TVR recurrence that we estimated through our meta-analysis. Almost half of the patients will experience a relapse of significant (≥ moderate) TVR at a medium follow-up (Fig. 6), translating into the need for a second surgical repair in most cases. Meta-regression analysis confirmed that a younger age at TV repair and TV repair occurring at the time of Norwood operation can augment the risk of TVR recurrence and the reoperation rate. We speculate that the higher technical complexity of TV surgery in smaller patients and, possibly, the presence of TV structural abnormalities or more compromised RV function may be the major drivers of the increased hazards in this subgroup of patients [36].
Our results should be carefully interpreted in light of the relatively short mean follow-up times of included studies (Table 1). Although a favorable RV remodeling process has been documented early after TV repair in HLHS [23, 36], which may sustain the positive effect of surgery on the patient’s survival, the very-long term fate of TV in HLHS is still to define. We may hypothesize that the observed high rates of TV recurrence and the need for TV reoperation indicate a strong interdependence of TVR and myocardial performance, which has been recognized to progressively decline when the RV is adopted as the systemic pumping chamber [6, 10, 44]. In this view, a later deflection of survival estimates from HLHS peers without TVR cannot be excluded, imposing strict and structured clinical surveillance even in patients in whom a successful TV repair has been achieved.
Limitations
Conducting a meta-analysis of observational studies possesses intrinsic limitations that our study has to account for. Unfortunately, the only large randomized clinical trial enrolling HLHS patients (the Single Ventricle Reconstruction Trial) has not specifically addressed the effects of TV repair on outcomes, thus it could not be included in our meta-analysis. We hope that our work could stimulate novel analysis of this precious source of clinical data on HLHS. Comparing outcomes of cases vs. controls that have been enrolled from different populations may generate a selection bias. However, when we compared cases vs. controls extracted from the same studies we did not observe a statistically significant modification of our results (see Supplemental Fig. 4). In order to define the source of heterogeneity among studies we investigated the modifier effect of a relatively small number of variables and we cannot exclude that other parameters may contribute to the studies heterogeneity. The individual patient data reconstruction of the Kaplan–Meier curves allows the characterization of long-term endpoints for large composed cohorts of patients. However, this pooled estimate does not account for the patient’s specific characteristics and possible confounding factors affecting the outcome. Finally, the relatively short mean follow-up times of included studies don’t allow reliable inferences on the very long-term fate of TV competence and patients’ survival after TV repair.
Conclusions
At a medium-term follow-up, TV repair can effectively modify the prognosis of patients with HLHS and loss of systemic TV competence, reestablishing a comparable transplant-free survival to HLHS peers without TVR. However, the durability of surgery seems to be time-dependent and a significant quota of patients will experience TVR recurrence, requiring more than one surgical procedure on the TV. The intrinsic relationship between TV competence and RV remodeling dictates careful and pro-active surveillance of this delicate population.
Data availability
Data available on request to the corresponding author.
Abbreviations
- CI:
-
confidence interval
- HLHS:
-
hypoplastic left heart syndrome
- HR:
-
hazard ratio
- IQR:
-
interquartile range
- LV:
-
left ventricle
- RV:
-
right ventricle
- SD:
-
standard deviation
- TV:
-
tricuspid valve
- TVR:
-
tricuspid valve regurgitation
References
West C, Maul T, Feingold B, Morell VO (2019) Right ventricular dominance is associated with inferior outcomes after the extracardiac fontan. World J Pediatr Congenit Hear Surg 10:416–423. https://doi.org/10.1177/2150135119843887
Pollak U, Abarbanel I, Salem Y et al (2022) Dominant ventricular morphology and early postoperative course after the fontan procedure. World J Pediatr Congenit Hear Surg 13:346–352. https://doi.org/10.1177/21501351221081246
Ghelani SJ, Colan SD, Azcue N et al (2018) Impact of ventricular morphology on fiber stress and strain in fontan patients. Circ Cardiovasc Imaging 11:e006738. https://doi.org/10.1161/CIRCIMAGING.117.006738
Bossers SSM, Kapusta L, Kuipers IM et al (2015) Ventricular function and cardiac reserve in contemporary fontan patients. Int J Cardiol 196:73–80. https://doi.org/10.1016/j.ijcard.2015.05.181
Moon J, Shen L, Likosky DS et al (2020) Relationship of ventricular morphology and atrioventricular valve function to long-term outcomes following fontan procedures. J Am Coll Cardiol 76:419–431. https://doi.org/10.1016/j.jacc.2020.05.059
Ponzoni M, Azzolina D, Vedovelli L et al (2022) Ventricular morphology of single-ventricle hearts has a significant impact on outcomes after fontan palliation: a meta-analysis. Eur J Cardio-Thoracic Surg 62:ezac535. https://doi.org/10.1093/ejcts/ezac535
King G, Buratto E, Cordina R et al (2023) Atrioventricular septal defect in fontan circulation: right ventricular dominance, not valve surgery, adversely affects survival. J Thorac Cardiovasc Surg 165:424–433. https://doi.org/10.1016/j.jtcvs.2022.04.011
Schwartz SM, Lu M, Ohye RG et al (2014) Risk factors for prolonged length of stay after the stage 2 procedure in the single-ventricle reconstruction trial. J Thorac Cardiovasc Surg 147:1791–1798e4. https://doi.org/10.1016/j.jtcvs.2013.07.063
Ghanayem NS, Allen KR, Tabbutt S et al (2012) Interstage mortality after the norwood procedure: results of the multicenter single ventricle reconstruction trial. J Thorac Cardiovasc Surg 144:896–906. https://doi.org/10.1016/j.jtcvs.2012.05.020
King G, Buratto E, Celermajer DS et al (2022) Natural and modified history of atrioventricular valve regurgitation in patients with fontan circulation. J Am Coll Cardiol 79:1832–1845. https://doi.org/10.1016/j.jacc.2022.02.022
Mahle WT, Hu C, Trachtenberg F et al (2018) Heart failure after the Norwood procedure: an analysis of the single ventricle reconstruction trial. J Hear Lung Transpl 37:879–885. https://doi.org/10.1016/j.healun.2018.02.009
Hoda M, Jaquiss RDB, James L, Thankavel PP (2022) Mechanical tricuspid valve replacement in hypoplastic left heart syndrome: an institutional experience. JTCVS Open 11:363–372. https://doi.org/10.1016/j.xjon.2022.06.015
Wamala I, Friedman KG, Saeed MY et al (2022) Tricuspid valve repair concomitant with the Norwood operation among babies with hypoplastic left heart syndrome. Eur J Cardio-Thoracic Surg 62:ezac033. https://doi.org/10.1093/ejcts/ezac033
Alsoufi B, Sinha R, McCracken C et al (2018) Outcomes and risk factors associated with tricuspid valve repair in children with hypoplastic left heart syndrome†. Eur J Cardio-Thoracic Surg 54:993–1000. https://doi.org/10.1093/ejcts/ezy198
Ono M, Mayr B, Burri M et al (2020) Tricuspid valve repair in children with hypoplastic left heart syndrome: impact of timing and mechanism on outcome. Eur J Cardio-Thoracic Surg 57:1083–1090. https://doi.org/10.1093/ejcts/ezaa004
Ruzmetov M, Welke KF, Geiss DM, Fortuna RS (2014) Outcomes of tricuspid valve repair in children with hypoplastic left heart syndrome. J Card Surg 29:698–704. https://doi.org/10.1111/jocs.12414
King G, Buratto E, Daley M et al (2022) Impact of aortic atresia after fontan operation in patients with hypoplastic left heart syndrome. Ann Thorac Surg 116:95–102. https://doi.org/10.1016/j.athoracsur.2022.09.018
Hormaza VM, Conaway M, Schneider DS, Vergales JE (2019) The effect of right ventricular function on survival and morbidity following stage 2 palliation: an analysis of the single ventricle reconstruction trial public data set. Congenit Heart Dis 14:274–279. https://doi.org/10.1111/chd.12722
Shigemitsu S, Mah K, Thompson RB et al (2021) Tricuspid valve tethering is associated with residual regurgitation after valve repair in hypoplastic left heart syndrome: a three-dimensional echocardiographic study. J Am Soc Echocardiogr 34:1199–1210. https://doi.org/10.1016/j.echo.2021.06.007
Kutty S, Colen T, Thompson RB et al (2014) Tricuspid regurgitation in hypoplastic left heart syndrome. Circ Cardiovasc Imaging 7:765–772. https://doi.org/10.1161/CIRCIMAGING.113.001161
Padalino MA, Ponzoni M, Castaldi B et al (2022) Surgical management of failing fontan circulation: results from 30 cases with 285 patient-years follow-up. Eur J cardio-thoracic Surg Off J Eur Assoc Cardio-thoracic Surg 61:338–345. https://doi.org/10.1093/ejcts/ezab450
Sugiura J, Nakano T, Oda S et al (2014) Effects of tricuspid valve surgery on tricuspid regurgitation in patients with hypoplastic left heart syndrome: a non-randomized series comparing surgical and non-surgical cases. Eur J Cardio-Thoracic Surg 46:8–13. https://doi.org/10.1093/ejcts/ezt613
Ugaki S, Khoo NS, Ross DB et al (2013) Tricuspid valve repair improves early right ventricular and tricuspid valve remodeling in patients with hypoplastic left heart syndrome. J Thorac Cardiovasc Surg 145:446–450. https://doi.org/10.1016/j.jtcvs.2012.10.040
Ohye RG, Gomez CA, Goldberg CS et al (2006) Repair of the tricuspid valve in hypoplastic left heart syndrome. Cardiol Young 16:21–26. https://doi.org/10.1017/S1047951106000722
Moher D, Shamseer L, Clarke M et al (2015) Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 4:1. https://doi.org/10.1186/2046-4053-4-1
Stroup DF (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA. https://doi.org/10.1001/jama.283.15.2008
Downes MJ, Brennan ML, Williams HC, Dean RS (2016) Development of a critical appraisal tool to assess the quality of cross-sectional studies (AXIS). BMJ Open 6:e011458. https://doi.org/10.1136/bmjopen-2016-011458
Bull C, Byrnes J, Hettiarachchi R, Downes M (2019) A systematic review of the validity and reliability of patient-reported experience measures. Health Serv Res 54:1023–1035. https://doi.org/10.1111/1475-6773.13187
Guyot P, Ades A, Ouwens MJ, Welton NJ (2012) Enhanced secondary analysis of survival data: reconstructing the data from published kaplan-meier survival curves. BMC Med Res Methodol 12:9. https://doi.org/10.1186/1471-2288-12-9
Higgins JPT, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21:1539–1558. https://doi.org/10.1002/sim.1186
Higgins JPT, Green S (2011) Cochrane handbook for systematic reviews of interventions
R Core Team (2022) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/
Viechtbauer W (2010) Conducting Meta-analyses in R with the meta for package. J Stat Softw 36:3. https://doi.org/10.18637/jss.v036.i03
Liu N, Zhou Y, Lee JJ (2021) IPDfromKM: reconstruct individual patient data from published kaplan-meier survival curves. BMC Med Res Methodol 21:111. https://doi.org/10.1186/s12874-021-01308-8
Huang S-C, Chen Y-S, Chang C-I, Chiu I-S (2016) Outcome of tricuspid valve plasty in norwood stage i operation. Circ J 80:1362–1370. https://doi.org/10.1253/circj.CJ-15-1371
Bautista-Hernandez V, Brown DW, Loyola H et al (2014) Mechanisms of tricuspid regurgitation in patients with hypoplastic left heart syndrome undergoing tricuspid valvuloplasty. J Thorac Cardiovasc Surg 148:832–840. https://doi.org/10.1016/j.jtcvs.2014.06.044
Nakata T, Fujimoto Y, Hirose K et al (2010) Atrioventricular valve repair in patients with functional single ventricle. J Thorac Cardiovasc Surg 140:514–521. https://doi.org/10.1016/j.jtcvs.2010.05.024
Newburger JW, Sleeper LA, Gaynor JW et al (2018) Transplant-free survival and interventions at 6 years in the SVR Trial. Circulation 137:2246–2253. https://doi.org/10.1161/CIRCULATIONAHA.117.029375
Ravishankar C, Gerstenberger E, Sleeper LA et al (2016) Factors affecting fontan length of stay: results from the single ventricle reconstruction trial. J Thorac Cardiovasc Surg 151:669-675e1. https://doi.org/10.1016/j.jtcvs.2015.09.061
Mah DY, Cheng H, Alexander ME et al (2016) Heart block following stage 1 palliation of hypoplastic left heart syndrome. J Thorac Cardiovasc Surg 152:189–194. https://doi.org/10.1016/j.jtcvs.2016.03.074
Ohye RG, Sleeper LA, Mahony L et al (2010) Comparison of Shunt types in the norwood procedure for single-ventricle lesions. N Engl J Med 362:1980–1992. https://doi.org/10.1056/NEJMoa0912461
Baba K, Ohtsuki S, Kamada M et al (2009) Preoperative management for tricuspid regurgitation in hypoplastic left heart syndrome. Pediatr Int 51:399–404. https://doi.org/10.1111/j.1442-200X.2008.02731.x
Pigula FA, Mettler B (2017) Management of tricuspid regurgitation in patients with hypoplastic left heart syndrome. Semin Thorac Cardiovasc Surg 29:64–69. https://doi.org/10.1053/j.semtcvs.2017.02.004
d’Udekem Y, Iyengar AJ, Galati JC et al (2014) Redefining expectations of long-term survival after the fontan procedure. Circulation 130:S32–S38. https://doi.org/10.1161/CIRCULATIONAHA.113.007764
Acknowledgements
Figures inspired by BioRender.com.
Funding
Open access funding provided by Università degli Studi di Padova within the CRUI-CARE Agreement. None.
Author information
Authors and Affiliations
Contributions
MP, DA, LV, and MAP performed data extraction and wrote the paper. LV, DA, and DG provided statistical expertise. DG, VV, and MAP supervised the project.
Corresponding author
Ethics declarations
Conflict of interest
The authors report no relationships that could be construed as a conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
PROSPERO registration number: CRD42023396529
Supplementary Information
Below is the link to the electronic supplementary material.
246_2023_3256_MOESM1_ESM.tif
Results of quality assessment using AXIS tool for each study included in the meta-analysis. Supplementary material 1 (TIF 215.0 kb)
246_2023_3256_MOESM2_ESM.tif
Timing of TV repair during single-ventricle palliation course in the included studies (n=9). TV: tricuspid valve. Supplementary material 2 (TIF 32.4 kb)
246_2023_3256_MOESM3_ESM.tif
Techniques for TV repair reported in the selected studies (n=8). TV: tricuspid valve. Supplementary material 3 (TIF 36.8 kb)
246_2023_3256_MOESM4_ESM.tif
Pooled Kaplan–Meier curves of transplant-free survival of patients requiring TV repair (n=84) selected only from studies reporting controls (n=323), with estimated HR across studies. CI: confidence interval; HR: hazard ratio; TV: tricuspid valve. Supplementary material 4 (TIF 149.4 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ponzoni, M., Azzolina, D., Vedovelli, L. et al. Tricuspid Valve Repair Can Restore the Prognosis of Patients with Hypoplastic Left Heart Syndrome and Tricuspid Valve Regurgitation: A Meta-analysis. Pediatr Cardiol (2023). https://doi.org/10.1007/s00246-023-03256-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00246-023-03256-0