Abstract
The objective is to compare patients who underwent retrograde intrarenal surgery with and without a ureteral access sheath (UAS) using kidney injury molecule-1 (KIM-1) levels. We also examined the difference in kidney damage between standard and dual lumen UAS. Sixty patients diagnosed with kidney stones and scheduled for RIRS were randomized into three groups: RIRS without UAS (Group 1), 11Fr/13Fr Boston scientific Navigator™ UAS (Group 2), and 11Fr/13Fr dual lumen ClearPetra™ UAS (Group 3). Data were prospectively collected in consecutive patients. Urine KIM-1/Cr levels were measured preoperatively, at postoperative 4 h, and on a postoperative day 14. Stone size, location, number, pre- and postoperative stent use, operation time, stone-free rate (SFR), post-ureteroscopic lesion scale (PULS) grade, hospitalization duration, and complications were recorded. There was no significant difference in demographical parameters and preoperative KIM-1/Cr levels among the groups. Postoperative 4th-hour urine KIM-1/Cr levels were higher in patients without UAS than patients with UAS (1.86, 0.67, 0.63 Groups 1, 2, 3, respectively). In comparing group 1 with groups 2 and 3 separately, Group 1 had a statistically significantly higher value than both groups (p = 0.002, p = 0.001, respectively). According to UAS type, there was no significant difference between groups 2 and 3. The use of UAS during RIRS has been shown to reduce kidney injury in the evaluation with KIM-1. Different UAS types on kidney injury and which one can protect the kidneys more during the procedure; will be elucidated by prospective randomized studies involving larger patient groups and UAS types.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Urinary system stone disease always preserves its importance in urology practice. Its global prevalence ranges between 1 and 15% [1]. The main intention of minimally invasive kidney stone treatment is to make the patient stone-free with minimum damage. Following a new generation of flexible ureterorenoscopes and laser lithotriptors, retrograde intrarenal surgery (RIRS) has become an essential alternative in stone treatment. And today, RIRS and Extracorporeal shockwave lithotripsy (SWL) are suggested as the first option in treating kidney stones smaller than 2 cm in EAU Guidelines. Beyond, RIRS is also a most preferred modality in the primary treatment of patients with kidney stones, musculoskeletal deformations, bleeding diathesis, or obesity in which SWL treatment failed [2, 3].
Ureteral access sheath (UAS) is an auxiliary tool providing direct kidney access during RIRS surgery. Although UAS use was stated to be ineffective on stone-free rates in different studies [4, 5] the advantages are to facilitate easy access to a ureterorenoscope, reduce intrarenal pressure, facilitate the removal of stone fragments, and increase the visual quality of endoscope during the operation [3, 4]. It has been shown that the use of UAS may be associated with lower rates of systemic inflammatory response (SIRS) after ureteroscopy and it reduces the mean intrarenal pressure by more than half (94 vs. 41 mm Hg). In line with the sheath's effect on SIRS, intrarenal pressure, and effective irrigation; it has been shown that the increased irrigation pressure by ureteroscopy results in deeper tissue penetration of the ink in pig kidneys, with no significant increase in tissue penetration of the ink when using a UAS [6]. Today, parallel to the technological advancements, custom-designed dual-lumen UAS (DLUAS) is also available. In addition to the canal providing renal access, these catheters also have a second canal in which the irrigation fluid in the kidney is drained, which can reduce intrapelvic pressure during the operation owing to the drainage of renal irrigation fluid [7, 8].
RIRS, increased intrarenal pressure, obstruction, perfusion pressure, and irrigation fluid volume are independent predictors of SIRS development during RIRS [9] and inherently kidney injury develops. Knowledge about kidney damage during the intervention is almost negligible.
Biomarkers can help us understand the damage and underlying pathophysiological events during ureteroscopy. KIM-1, which is shed into urine after acute kidney damage, is a specific marker of renal tubular injury is secreted earliest in tubular injury. Although many aspects of UAS use during RIRS have been investigated to date, it has not been compared at the level of kidney injury biomarkers.
This study compared kidney injury in patients who underwent RIRS with standard UAS, DLUAS, and without a UAS by measuring KIM-1 values. Based on our knowledge, this is the first study in this field.
Materials and methods
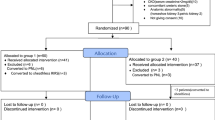
Ethical approval was received before the study (2019/2169). Verbal and written consent was also obtained from all participants. A total of 60 patients diagnosed with kidney stones through Abdominopelvic CT and who had RIRS operations were included in our study. Patients were divided into three groups through randomized prospectively. Each group consisted of 20 patients who were selected through randomization. The first group consisted of patients in whom a UAS wasn't used; the second group consisted of patients using STUAS, while the third group consisted of patients using DLUAS.
The use of UAS in the patients was randomly applied, in sequence, without UAS in the first patient, standard UAS in the second patient, and DLUAS in the third patient. Power analysis was conducted with the acquired definitive measurements to determine the size of the ideal sampling for the study. The effect size in the power analysis conducted according to postoperative 4th-hour KIM-1/Cr ratio, definitive measurements was calculated as d = 0.80. The sample size was calculated as 16 for groups, where the error level was 5%, and the power value was 95%. The study was completed when 60 patients were reached. The number of patient populations has reached a minimum of twenty for each subgroup.
Inclusion criteria
Healthy adult patients between 18 and 75 years of age, recognized to have CT-detected kidney stones with a dimension of 1 to 2 cm and a eGFR of 60 or higher, were included in the study.
Exclusion criteria
Patients under 18 and over 75 years of age, patients with pyuria, with ureter stone, positive urine culture and hydronephrosis, renal parenchyma thinning on the side of the stone, recent surgery history, preoperative DJ catheter presence, kidney malformation, chronic kidney diseases, solitary kidney, nephrotoxic agent use, patients who had stone surgery in the last 3 months and patients who cannot have ureterorenoscopy due to ureteral stricture, patients whose ureteral access sheath could not insert into the ureter were excluded from the study.
Surgical technique and follow-up
All surgical procedures were performed in a lithotomy position under general anesthesia by the same surgeon with 20 years of endourology experience. All patients had routine cystoscopy before flexible ureterorenoscopy. A guidewire was inserted in the kidney for safety under the guidance of fluoroscopy. Ureterorenoscopy with a rigid ureterorenoscope (Karl Storz 8/fr, Germany) was performed for all patients following cystoscopy. A second guidewire was inserted during ureterorenoscopy. A flexible ureteroscope (Flex-X2; Karl Storz, Germany) was used on all patients. A flexible ureterorenoscope was advanced directly to the kidney over the second guidewire with fluoroscopy in the first group. The second group placed the STUAS (Boston scientific Navigator UAS 11 fr/13 fr 46 cm/USA) by sliding it over the second guidewire and checking by fluoroscopy. A flexible ureterorenoscope was slid through UAS to get inside the kidney. In the third group, after sending DLUAS (ClearPetra 11 fr/13 fr 46 cm/China) to the kidney under fluoroscopy control, the kidney was reached by advancing a flexible ureterorenoscope through a UAS. The UASs are shown in Fig. 1. The stone was fragmented using a Holmium–YAG laser (Dornier Solvo 30 W/Germany,) of 200-micron diameter and with a 10 Hz/2.5 J laser setting. In patients where a DLUAS was used, the drainage channel was opened during surgery to reduce renal pressure. Irrigation fluid was kept at the height of 60 cm in all patients. Direct urinary tract radiography and/or Ultrasound was/were taken in postoperative 14th-day check. The stone size accepted for the complete stone-free condition was below 3 mm. DJ catheters of the patients who did not have significant residual stones in the postoperative 14th day control were removed.
Blood sampling and measurement of study parameters
Preoperative routine blood and urine tests were carried out for all patients. IV antibiotic prophylaxis was applied an hour before the operation. Abdominopelvic CT was used as the preoperative routine imaging method.
Considering the previous studies of Sabbisetti et al. and the follow-up planning of the patients, urine samples were collected preoperatively, at the 4th hour and 14th day after the operation, and centrifuged at 3000 rpm for 10 min (R). Acquired serum samples were separated into Eppendorf tubes and were kept at − 80 °C until the analysis. The samples were thawed on the study day, and urine KIM-1 (Elabscience Biotechnology Inc/USA) levels were measured. Then urine KIM-1/urine Cr rates were calculated and compared among the groups.
Statistical analysis
Statistical analysis was performed with SPSS 25.0 (Statistical Package, Chicago/USA). Categorical variables are described by frequencies and percentages. Continuous variables are presented as mean and standard deviations. Wilcoxon signed-rank and Friedman tests were used to analyzing the relationship between continuous variables before and after surgery. Independent T, Kruskal–Wallis and chi-square (χ2) tests were used to compare the relationship between categorical and continuous variables subgroups. Pearson correlation coefficients of continuous variables are calculated. A P value below 0.05 was considered statistically significant.
Results
A total of 60 patients who underwent RIRS due to kidney stones were included in the study. While the mean age of the patients was 48.2 ± 13.8, in Group 1, it was 50.5 ± 12.4; 47.95 ± 15.2 in Group 2 and 46.35 ± 14.1 in Group 3 (p = 0.68). Of all patients, 42 (70%) were male, and 18 (30%) were female. When the groups were evaluated separately, 13 (65%) men and 7 (35%) women in Group 1; In Group 2, 16 (80%) men and 4 (20%) women; and there were 13 (65%) men and 7 (35%) women in Group 3 (p = 0.49). According to the BMI measurements calculated in the preoperative measurements of the patients, the overall BMI average was 29.06 ± 4.3, while in Group 1, it was 29.8 ± 3.5; It was calculated as 28.6 ± 5.3 in Group 2 and 28.6 ± 4.1 in Group 3 (p = 0.46). When we examined the mean stone sizes, it was 14.08 ± 5.4 mm in all groups, while it was 14.95 ± 6.5 mm in Group 1; It was measured as 14.65 ± 5.6 mm in Group 2 and 12.65 ± 3.6 mm in Group 3. There was no statistically significant difference between stone sizes (p = 0.48). When stone densities were measured, the mean was 950 ± 289 HU, and it was measured as 1002 ± 331, 919 ± 263, 929 ± 278 HU in the groups, respectively (p = 0.57). While 39 patients had their stones on the right side, 21 patients had their stones on the left side, and no statistically significant difference was observed between the groups (p = 0.41). No statistically significant difference was observed among demographical, radiological, and surgical parameters and Preoperative urine KIM-1/Cr levels of the patients (p > 0.05) (Tables 1, 2).
There were 5 patients with postoperative fever in our study group of 60 patients. 2 of them are included in Group 1 and are grade 1 according to modified Clavien (treated only with antipyretic). 2 of them were included in group 2 and required postoperative ab treatment and were classified as modified Clavien 2. One of them is included in group 3 and classified as modified Clavien grade 1 (regressed only after antipyretic treatment).
Five (8.3%) patients had stones in the upper, 12 (20%) in the middle, 14 (23.3%) in the lower pole, 12 (20%) in the pelvis, and 17 (28.3%) was multiple and found in different calyces (Table 1). Regardless of the stone location of the patients, the infundibulopelvic angle was 49.05° in Group 1, 52.05° in Group 2, and 53.95° in Group 3 (p = 0.44). When we analyzed the surgery-related data, it was found that the mean operation time was 62.8 min, and there was no statistically significant difference between the groups (65 min for Group1, 63 min for Group 2, 60.5 min for Group 3) (p = 0.55). The mean length of stay in all groups was 1.62 days (1.75 days; 1.55 days; 1.55 days for the groups, respectively) (p = 0.99). The complete stone-free rate was 90% in all groups (p = 0.57).
43 (71.7%) patients had PULS grade 0, 12 (20%) grade 1, 4 (6.7%) grade 2, and 1 (1.7%) had grade 3 injuries. According to the Modified Clavien classification, 54 (90%) patients had grade 0, 4 (6.7%) grade 1, 2 (3.3%) grade 2 complications. There was no difference in SFR, ureteral damage, bleeding risk, and postoperative infectious complications between using or not using UAS (Table 1).
Preoperative urine KIM-1/Cr levels were 0.38 in Group 1, 0.28 in Group 2, and 0.32 in Group 3 (p = 0.57). The urine KIM-1/Cr levels taken in the postoperative 4th hour were 1.86 in Group-1, 0.67 in Group-2, and 0.63 in Group-3 (p = 0.021). Group-1 urine KIM-1/Cr level was significantly higher than Group 2 and 3 (p = 0.002 and p = 0.001, respectively). The urine KIM-1/Cr on the postoperative 14th day was not different among groups (p = 0.30) (Table 2, Fig. 2). Postoperative 4th hour KIM-1/Cr was observed to increase first and then decrease on the postoperative 14th day in all three groups (All parameters p < 0.001).
When we analyzed serum creatinine levels, the mean preoperative serum creatinine level was 0.87 ± 0.22, and there was no significant difference between the groups (0.84 ± 0.20; 0.89 ± 0.22; 0.82 ± 0.20 p:0.77, respectively).
The mean postoperative 4th hour serum creatinine level was 0.91 ± 0.23, and unlike KIM-1 levels, no significant difference was observed between the groups (0.93 ± 0.22; 0.98 ± 0.25; 0.87 ± 0.17 p:0.79, respectively).
On the postoperative 14th day, mean serum creatinine level was 0.85 ± 0.18 and no difference was observed between the groups (0.89 ± 0.27, 0.90 ± 0.24, 0.85 ± 0.19 p:0.65, respectively).
In the correlation analysis between KIM-1/Cr and the parameters; age, stone size, operation time, stone density, fluoroscopy time, hospitalization time, a positive correlation was detected between postoperative 4th hour KIM-1/Cr and hospitalization time (p = 0.036).
Discussion
UAS has many advantages, such as reducing intrapelvic pressure, providing easy and quick access to the collecting system, and lengthening the durability of flexible ureterorenoscopes. It has single and dual lumen types [3, 4]. To our knowledge, there is no study evaluating renal damage or comparing patients using standard and DLUAS yet. Although stone-free rates with and without UAS differ in different studies, the rates are similar between groups [5, 10]. The stone-free rates in our study were 85%, 95%, and 90% in the first group not using UAS, the second group using STUAS, and the third group using DLUAS, respectively. Although we achieved higher stone-free rates in the STUAS group, a statistically significant difference wasn't detected (p = 0.57). Stone-free data we acquired were similar to the literature.
Our primary endpoint is whether the use of DLUAS is beneficial compared to standard UAS and non-UAS patients in terms of kidney damage measured by KIM-1.
Our secondary endpoint is between the groups whether there is a difference in surgical outcomes, such as operation time, length of hospital stay, stone-free rate, perioperative and postoperative complications.
Our study's postoperative complications were detected at rates similar to the literature for groups using and not using UAS [5, 11,12,13]. The effect of UAS on postoperative complications wasn't observed. Although different studies were made on operation times, some provided higher results for UAS groups and some for non-UAS groups [14, 15]. No difference in operation time among the groups was detected. In addition, parallel to the literature, no difference among the groups in hospitalization times was seen [10, 16]. Berquet et al. In their study, in which they evaluated the duration of hospitalization, reported that there was no difference between the patients who used UAS and those who did not [10]. In their study, Traxer et al. reported that hospitalization was longer in patients who did not use UAS [15]. In a meta-analysis conducted in 2018, the difference between hospitalization times was not statistically significant [16]. In our study, the hospitalization was 1.67 days for all patients, 1.75 for Group 1, 1.55 for Group 2, and 1.55 for Group 3 (p = 0.99). Hospitalization times were found to be lower when compared to the literature. We think that the lower length of stay in the 2nd and 3rd groups using UAS is due to the decreased infective complications due to the decrease in intrapelvic pressure. A positive correlation was found between the KIM-1/Cr rate in postoperative 4th hour and hospitalization time based on the correlation analysis performed. Using a UAS during RIRS shortened the hospitalization duration and decreased renal damage.
KIM-1 excretion in the urine is highly specific for kidney injury, because no other organs have shown to express KIM-1 that could change its urinary concentration. [17] In a study, it was determined that KIM-1 was the best predictor of postoperative acute kidney injury.[18] One of the other markers are greatly affected by conditions, such as trauma and obesity, where there is an increased inflammatory state.[19] Moreover, some authors state that elevated KIM-1 levels may precede histological changes in AKI patients [20] Our study evaluated the increased pressure related to kidney damage by measuring KIM-1 levels, a phagocytic phosphatidylserine receptor present in kidney epithelial cells. It can specifically recognize phosphatidylserine epitopes on apoptotic tubule epithelial cells [21]. Urine KIM-1 levels were shown to increase after renal ischemia significantly [22]. Brian K et al. measured the pressures during the ureterorenoscopy procedure with a preoperatively inserted nephrostomy catheter and reported that the pressure was significantly lower for stones in all localizations when UAS was used [23]. Balasar et al. measured preoperative and postoperative urine KIM-1/Cr levels in patients with micro PNL, RIRS, and PNL. A significant decrease was detected in postoperative KIM-1/Cr levels in RIRS and PNL group spared to micro PNL at the end of the study (p = 0.010, p = 0.001, respectively)[24]. In another study, KIM-1/Cr and NGAL/Cr levels were detected 2 h after the operation at a statistically significantly increased level compared to preoperative levels (p = 0.04, p = 0.02, respectively). KIM-1 levels increasing in the postoperative 2nd hour were observed to decrease again to preoperative levels in the postoperative 24th hour (Preoperative:2.24 ± 1.14; Postoperative 2nd hour:5.16 ± 2.18; Postoperative 24th hour:2.42 ± 1.60) [21]. It was observed that KIM-1 levels, which increased at the postoperative 2nd hour, decreased to preoperative levels in the measurements at the postoperative 24th hour (Preop: 2.24 ± 1.14; Postop 2. Hour: 5.16 ± 2.18; Postop 24. Hour:2, 42 ± 1.60) [25]. Dağgülli et al. included 76 patients in their prospective controlled study to examine the use of biomarkers KIM-1, NAG, NGAL, and LFABP, which are indicators of AKI after PNL. Urine samples were collected 2 h before, 2 h after, and 24 h after surgery. The investigators concluded that the KIM-1/Cr, NAG/Cr, and NGAL/Cr ratios increased significantly at 24 h postoperatively (P < 0.05, compared with preoperative rates). [26].
In the studies conducted, there is an increase in renal flow due to a decrease in intrarenal resistance in the first phase in cases of unilateral ureteral obstruction. In the second stage, renal blood flow decreases after approximately 2–5 h.[27].
Also in the literature; There are studies showing that acute kidney injury reaches the postoperative 2nd, 3th, 4th, 6th hour maximum level after ischemia. In the light of this information, we preferred to measure the level of KIM at the postoperative 4th hour, when acute kidney injury can be detected [28, 29].
KIM-1 levels were checked preoperatively, postoperative 4th hour, and postoperative 14th days in our study. Preoperative KIM-1/Cr levels show the homogeneous distribution of the groups. However, KIM-1/Cr levels in the postoperative 4th hour were significantly different increases for the three groups (Table 2). In addition, KIM-1/Cr levels on the postoperative 14th day were decreased in all three groups, and there was no difference among the groups again. This increase in the fourth hour is an expected finding because of the damage formation occurring due to increased intrapelvic pressure during the surgery. As in other studies we mentioned above, the initial postoperative KIM-1/Cr increase is striking in our study. When postoperative 4th hour KIM-1/Cr levels were compared separately for Group-1 (non-UAS) with 2 (STUAS) and Group-1 with 3 (DLUAS), Group-1 had a significantly higher result again, and there was no significant difference among Groups-2 and 3. Although kidney damage in DLUAS use was less than in STUAS, this difference wasn't significant (p = 0.7). Although postoperative 14th day KIM-1/Cr levels decreased below the preoperative level in Groups-2 and 3, this decrease was sharper in Group-3 (p2vs3 = 0.35), showing that UAS use is more used efficient in postoperative kidney regeneration.
Contrary to the KIM-1 values, no difference was observed between the serum creatinine values at the postoperative 4th hour and 14th day. We think that KIM-1 being more specific for kidney damage and increasing earlier and serum creatinine measurement being less specific and increasing in the late period play a role.
Limitations of our study were the small sample size, lack of both pathologic findings, and intrapelvic pressure measurement. As the coronavirus disease 2019 (COVID-19) pandemic burgeoned, the medical community encountered many challenges. Our study also coincided with the pandemic times; we could number of increase the patients' even though we reached a sufficient number of patients in the groups [30].
Despite its small sample size, significantly lower KIM-1/Cr levels were shown in UAS using groups (STUAS and DLUAS) compared to those not using UAS. In the presence of this sample size limitation, the damage was less in the DLUAS group, although not statistically significant.
Conclusions
UAS is an essential tool that may benefit the patients and shorten hospitalization time by reducing kidney damage during ureterorenoscopy. As a result of our study, we detected significantly lower KIM-1/Cr levels in UAS using groups (STUAS and DLUAS) compared to the group not using UAS. According to our hypothesis between kidney damage and KIM-1, There was no statistically significant evidence that dual lumen UAS reduces kidney damage compared to standard UAS. Making new studies on this subject with more patients and new markers would clarify the issue more.
Data availability
All authors guarantee that all data and materials support their claims and comply with the standards.
References
Romero V, Akpinar H, Assimos DG (2010) Kidney stones: a global picture of prevalence, incidence, and associated risk factors. Rev Urol 12(2–3):e86
Türk C, Knoll T, Petrik A. Guidelines on Urolithiasis. Arnhem, the Netherlands: European Association of Urology, update March 2013. 2014.
Sönmez MG, Kara C, A new approach in ureteral access sheath locating in retrograde intrarenal surgery (RIRS) by endovisional technique. Archivio Italiano di Urologia e Andrologia, 2015. p. 286–290.
Stern JM, Yiee J, Park S (2007) Safety and efficacy of ureteral access sheaths. J Endourol 21(2):119–123
James O, Ekeruo WO, Scales CD Jr, Marguet CG, Springhart WP, Maloney ME et al (2005) Effect of ureteral access sheath on stone-free rates in patients undergoing ureteroscopic management of renal calculi. Urology 66(2):252–255
Loftus C, Byrne M, Monga M (2021) High pressure endoscopic irrigation: impact on renal histology. Int Braz J Urol. 47: 350–356.
Ng YH, Somani BK, Dennison A, Kata SG, Nabi G, Brown S (2010) Irrigant flow and intrarenal pressure during flexible ureteroscopy: the effect of different access sheaths, working channel instruments, and hydrostatic pressure. J Endourol 24(12):1915–1920
Zeng G, Wang D, Zhang T, Wan SP (2016) Modified access sheath for continuous flow ureteroscopic lithotripsy: a preliminary report of a novel concept and technique. J Endourol 30(9):992–996
Tokas T, Herrmann TR, Skolarikos A, Nagele U (2019) Pressure matters: intrarenal pressures during normal and pathological conditions, and impact of increased values to renal physiology. World J Urol 37(1):125–131
Berquet G, Prunel P, Verhoest G, Mathieu R, Bensalah K (2014) The use of a ureteral access sheath does not improve stone-free rate after ureteroscopy for upper urinary tract stones. World J Urol 32(1):229–232
Schoenthaler M, Buchholz N, Farin E, Ather H, Bach C, Bach T et al (2014) The Post-Ureteroscopic Lesion Scale (PULS): a multicenter video-based evaluation of inter-rater reliability. World J Urol 32(4):1033–1040
Wang HH, Huang L, Routh JC, Kokorowski P, Cilento BG, Nelson CP (2011) Use of the ureteral access sheath during ureteroscopy in children. J Urol 186(4S):1728–1733
Geraghty RM, Ishii H, Somani BK (2016) Outcomes of flexible ureteroscopy and laser fragmentation for treatment of large renal stones with and without the use of ureteral access sheaths: results from a university hospital with a review of literature. Scandinavian J Urol 50(3):216–219
Pardalidis NP, Papatsoris AG, Kapotis CG, Kosmaoglou EV (2006) Treatment of impacted lower third ureteral stones with the use of the ureteral access sheath. Urol Res 34(3):211–214
Traxer O, Wendt-Nordahl G, Sodha H, Rassweiler J, Meretyk S, Tefekli A et al (2015) Differences in renal stone treatment and outcomes for patients treated either with or without the support of a ureteral access sheath: the clinical research office of the endourological society ureteroscopy global study. World J Urol 33(12):2137–2144
Huang J, Zhao Z, AlSmadi JK, Liang X, Zhong F, Zeng T et al (2018) Use of the ureteral access sheath during ureteroscopy: a systematic review and meta-analysis. PLoS ONE 13(2):e0193600
Wasung ME, Chawla LS, Madero M (2015) Biomarkers of renal function, which and when? Clin Chim Acta 438:350–357
Liangos O, Tighiouart H, Perianayagam MC, Kolyada A, Han WK, Wald R, Jaber BL (2009) Comparative analysis of urinary biomarkers for early detection of acute kidney injury following cardiopulmonary bypass. Biomarkers 14(6):423–431
Taglieri N, Koenig W, Kaski JC (2009) Cystatin C and cardiovascular risk. Clin Chem 55(11):1932–1943
Perco P, Oberbauer R (2008) Kidney Injury Molecule-1 as a biomarker of acute kidney injury in renal transplant recipients. Nat Clin Pract Nephrol 4:362–363
Ichimura T, Asseldonk EJ, Humphreys BD, Gunaratnam L, Duffield JS, Bonventre JV (2008) Kidney injury molecule–1 is a phosphatidylserine receptor that confers a phagocytic phenotype on epithelial cells. J Clin Investig 118(5):1657–1668
Sabbisetti VS, Ito K, Wang C, Yang L, Mefferd SC, Bonventre JV (2013) Novel assays for detection of urinary KIM-1 in mouse models of kidney injury. Toxicol Sci 131(1):13–25
Auge BK, Pietrow PK, Lallas CD, Raj GV, Santa-Cruz RW, Preminger GM (2004) Ureteral access sheath provides protection against elevated renal pressures during routine flexible ureteroscopic stone manipulation. J Endourol 18(1):33–36
Balasar M, Pişkin MM, Topcu C, Demir LS, Gürbilek M, Kandemir A, Öztürk A (2016) Urinary Kidney Injury Molecule-1 levels in renal stone patients. World J Urol 34(9):1311–1316
Dede O, Dağguli M, Utanğaç M, Yuksel H, Bodakcı MN, Hatipoğlu NK et al (2015) Urinary expression of acute kidney injury biomarkers in patients after RIRS: it is a prospective, controlled study. Int J Clin Exp Med 8(5):8147
Daggülli M, Utangaç MM, Dede O, Bodakci MN, Hatipoglu NK, Penbegül N et al (2016) Potential biomarkers for the early detection of acute kidney injury after percutaneous nephrolithotripsy. Ren Fail 38(1):151–156
Cohen JJ, Harrington JT, Kassirer JP (1983) Pathophysiology of obstructive nephropathy. Kidney Int 23:414–426
Sabbisetti VS, Waikar SS, Antoine DJ, Smiles A, Wang C, Ravisankar A, Bonventre JV (2014) Blood Kidney Injury Molecule-1 is a biomarker of acute and chronic kidney injury and predicts progression to ESRD in type I diabetes. J Am Soc Nephrol 25(10):2177–2186
Dede O, Dağguli M, Utanğaç M, Yuksel H, Bodakcı MN, Hatipoğlu NK, Penbegül N (2015) Urinary expression of acute kidney injury biomarkers in patients after RIRS: it is a prospective, controlled study. Int J Clin Exp Med 8(5):8147
Corsello DP, Gotur DB, Carroll CL, Masud FN, Simpson SQ (2020) Impact of Small-N Studies During a Pandemic. Chest 158(4):1338–1340
Funding
The authors did not receive support from any organization for the submitted work. The authors state that they have no proprietary interest in the products named in this article. None of the authors involved in this study received financial support.
Author information
Authors and Affiliations
Contributions
GE: project development, manuscript writing, data managing, laboratory analysis, collection and storage of samples. MGS: data analysis, manuscript editing, editing of pictures and tables. AA: data analysis, manuscript Editing. CT: laboratory analysis, sample collection and storage. HA: data analysis, collection and storage of samples. SG: manuscript editing. MB: project development, manuscript editing, surgical application.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
None.
Ethics approval
Authors declared that the research was conducted according to the principles of the World Medical Association Declaration of Helsinki “Ethical Principles for Medical Research Involving Human Subjects”. The study was performed in the Clinical Ethic Committee of Necmettin Erbakan University Meram Medicine Faculty (No: 2020/2835).
Consent to participate
All participants give their consent to participate in the study.
Consent for publication
All participants give permission to publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ecer, G., Sönmez, M.G., Aydın, A. et al. Comparison of retrograde intrarenal stone surgery with and without a ureteral access sheath using kidney injury molecule-1 (KIM-1) levels: a prospective randomized study. Urolithiasis 50, 625–633 (2022). https://doi.org/10.1007/s00240-022-01345-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00240-022-01345-y