Abstract
Background
Due to its partially superficial course, the saphenous nerve is vulnerable to injury by trauma or surgery potentially leading to painful neuroma formation. Different surgical techniques to treat neuroma have been described, but so far, no one has proven to be superior to the others. The aim of this study was therefore to identify factors influencing the outcome of revision surgery in saphenous nerve neuropathic pain in our department.
Methods
From 2010 to 2020, a total of 29 consecutive patients with neuropathic pain and suspected neuroma of the saphenous nerve underwent revision surgery. A medical chart review was performed to collect patient-, pain-, and treatment-specific factors. Outcomes were registered.
Results
Post revision surgery in neuropathic pain of the saphenous nerve, 16 (55.2%) patients suffered from persisting pain. In multivariable logistic regression models evaluating the risk of persisting pain post saphenous nerve revision surgery, both smoking and preoperative opiate consumption represented independent predictors of higher risk for persisting pain.
Conclusions
Patients with injury to the saphenous nerve should be coached toward smoking cessation. Whenever possible, forgoing opiate treatment might be beneficial.
Level of evidence: Level IV, Risk/Prognostic.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
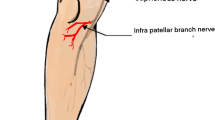
The purely sensory saphenous nerve is the largest cutaneous branch of the femoral nerve. It becomes subcutaneous around 10 cm above the medial epicondyle of the femur and then passes along the tibial side of the leg, accompanied by the great saphenous vein [1]. At its superficial location at knee level, the nerve is vulnerable to injury by trauma or surgery around the knee joint [2]. Injury to a nerve may lead to neuroma formation as the result of irregular or disorganized neuronal regeneration [3]. Additionally, per se uninjured nerves running through a surgical field can be affected by scar tethering, also leading to neuropathic pain. These affections of the nerve can cause saphenous nerve–originated neuropathic pain leading to severe disability. Patients will have neuropathic pain in a defined neural anatomical distribution along with a history of nerve injury [4]. Saphenous nerve neuroma is often caused by an injury during prior surgery like knee arthroscopy [5]. Different surgical techniques to treat neuroma have been described. Techniques involving neuroma excision and implantation/burying in muscle or bone have historically been the most commonly used [6,7,8]. Several studies have shown that a significant number of patients will still report severe persistent pain despite surgical treatment [4, 8].
The aim of this study was to evaluate the outcomes of surgical revision of the saphenous nerve in neuropathic pain in our department and to identify outcome influencing factors.
Patients and methods
This retrospective cohort study was performed with the approval of the local ethics committee. We identified all patients who underwent surgery on the saphenous nerve or its branches for neuropathic pain and suspected neuroma of the saphenous nerve at our department for plastic surgery and hand surgery between March 2010 and December 2020. The initial query yielded 29 consecutive patients who were all included in the analysis.
A medical chart review was performed to collect patient-, pain-, and treatment-specific factors. Pain relief following diagnostic injection, if performed, was noted based on the medical charts. Symptoms pre- and postoperatively were noted, and follow-up was defined as the date of surgery until the last visit to our department. The treatment or trauma leading to neuropathic pain of the sural nerve was registered as well as the time between first symptoms and surgical treatment.
Outcome of interest
The main outcome of interest was absence of pain postoperatively.
Statistical analysis
Descriptive statistics included frequencies and proportions for categorical variables. Means, medians, and ranges were reported for continuously coded variables. The chi-square was used to assess the statistical significance in proportions differences. The t-test and Kruskal–Wallis test were used to evaluate the statistical significance of means and median differences. Univariable logistic regression models were used to test the relationship between absence of pain postoperatively and several variables, namely age, sex, smoking status, type of trauma/surgery leading to neuroma formation, time duration between saphenous nerve injury and revision surgery, presence of a psychiatric history, positive diagnostic injection, presence of a Tinel sign, and opiate or Lyrica use before revision surgery.
R software environment for statistical computing and graphics (version 3.4.3) was used for all statistical analyses. All tests were two-sided with a level of significance set at P < 0.05.
Results
Between 2010 and 2020, 29 consecutive saphenous nerve revision surgeries for chronic saphenous nerve–associated neuropathic pain were performed in 10 male and 19 female patients with a median age of 45 years (IQR, 29 to 53 years) (Table 1). Post saphenous nerve revision surgery, 16 (55.2%) patients suffered from persisting pain. The median time between symptom causing event and surgery was 28.5 months (IQR 17–68). Neuropathic pain was secondary to traumatic injury in 8 cases, specifically deep skin lacerations around the knee, attributable to a prior surgery in 17 cases (orthopedic surgeries including knee joint arthroscopies, corrective osteotomies, fracture fixations, and ligament reconstructions) and due to other reasons in 4 cases (2 patients with prior sclerotherapy of varicose veins, one patient with a cystic lesion from the medial meniscus, and one idiopathic case). All patients relied on pain medication prior to saphenous nerve revision surgery (Table 2). Other treatments prior to surgery included physical therapy (n = 11, 37.9%), opiate pain medication (n = 10, 34.5%), neuropathic pain medication (pregabalin) (n = 14, 48.3%) or topical anesthetics (n = 17, 58.6%), or a combination of the aforementioned. A diagnostic injection prior to surgery was performed in 19 patients (65.5%). Of those, 18 patients (94.7%) reported significant relief after injection; one patient (5.3%) reported no relief (Table 2).
Prior to revision surgery, all patients complained of pain (n = 29). Numbness was reported in 13 cases, hyperesthesia in 8 cases, and a Tinel sign was documented in the medical records in 26 patients (Table 1). Table 3 illustrates/summarizes the surgical approach, as well as postoperative symptoms and treatments. Patients were treated with neurolysis only (10 patients) if the nerve was found to be in continuity but scar tethered. If the nerve showed a macroscopic injury resulting in neuroma, neurectomy was performed far enough proximal to the neuroma to allow for the distal nerve end to be surrounded by healthy fatty tissue (8 patients). In cases of combined neuroma(s) and scar tethering, neurectomy was performed and the nerve was stripped proximal to Hunter’s canal using a ring stripper (11 patients). Mean follow-up time was 205 days.
In univariable logistic regression models evaluating the risk of persisting pain post saphenous nerve revision surgery, smoking could be identified as a predictor of higher risk for persisting pain. As female gender and opiate consumption both presented a p value of 0.06 in the univariable models, they were also included in the multivariable logistic regression models. In multivariable logistic regression models evaluating the risk of persisting pain post revision surgery (see Table 4), smoking (odds ratio [OR]: 17.5, p = 0.02) and preoperative opiate consumption (OR: 20.8, p = 0.03) represented independent predictors of higher risk for persisting pain.
Discussion
Prior studies have demonstrated that surgical treatment of neuroma pain does not tend to be effective in all patients [9]. Different factors can lead to a symptomatic affection of the saphenous nerve around the knee, especially surgeries in this area. Mochida and Kikuchi showed that these type of surgery are associated with a 22.2% incidence rate of sensory disturbances in the area where the infrapatellar branch is distributed, and they suggested that the incidence can be minimized by clarifying the distribution of the infrapatellar nerve branch in relation to palpable landmarks [10]. Numerous surgical techniques have been described for neuroma treatment, but so far, no significant differences could be identified between these different surgical techniques [11]. We also found no difference between different surgical techniques, but instead, this study was focused on identifying predictors for persisting pain. Our study yielded several noteworthy findings.
First, smoking could be identified as a predictor of higher risk for persisting pain. Smoking has been identified as a risk factor for neuropathic pain even in the absence of physical trauma to a nerve [12]. The same correlation could be demonstrated after brachial plexus injury [13]. It has been suggested that quitting to smoke might have a direct effect on the severity of neuropathic pain [14]. Behavioral therapy including smoking cessation in combination with physical therapy can be a first-line intervention for patients with chronic pain [15]. It might therefore be worthwhile to suggest smoking cessation to patients after nerve injury.
Second, opiate consumption prior to neuroma surgery represented an independent predictor of higher risk for persisting pain. According to a Cochrane review published in 2013, the analgesic efficacy of opioids in chronic neuropathic pain is subject to considerable uncertainty [16]. Other pain-related studies have shown that patients using opioids preoperatively have less favorable outcomes [17]. A study on injury-related hyperemia of the rat sciatic vasa nervorum found that exogenous opioids dampen early inflammatory microvasodilation and could have important influences on the nerve regenerative milieu. With these factors in mind, the indication for opioids after nerve injury should always be evaluated especially critical. Also, studies have shown that pain scores will be higher in patients with prior opioid exposure as these patients might have less coping reserves [18].
Third, we did not find a correlation between persisting pain and the duration of pain like it has been suggested by Stokvis et al. [19]. In the treatment of neuropathic pain of the peripheral nerves, secondary hyperalgesia, due to sensitization in the central nervous system, has to be considered a risk factor for persisting pain after neuroma surgery. If central sensitization evolves such that nociceptive signaling becomes independent of inputs from primary afferents, then treating the peripheral nerve may no longer be therapeutically useful [20]. However, these mechanisms, their time frame, and contributing factors are not yet fully understood, suggesting that neuroma surgery might be effective even longer periods after injury to the nerve, like in our cohort.
Fourth for patient with chronic pain, not only a full relief of symptoms but also a reduction of overall pain might be considered a favorable outcome. We saw a reduction or full relief of pain in 55% (16/29) of our patients. Reduction of pain severity has shown a direct relationship with changes in function, anxiety, depression, and sleep in patient-reported outcomes [21]. Therefore, surgical intervention might still be considered a partial success as long as pain reduction is achieved and should be discussed with the patient as such prior to surgery.
There are several limitations to this study. It is a retrospective study and thus may be prone to observer bias. As peripheral nerve surgeons, we are aware that there might be other factors affecting outcomes of neuropathic pain management. Thus, our conclusions will need to be supported by prospective data for greater impact. Additionally, as we are presenting consecutive cases, patients were operated on by different surgeons. These adhere to a department standard concerning the postoperative regime, but minor deviations based on personal preference might have been made. Another critical point is that we do not have a quantification of pain or amount of pain medication consumed for all patients postoperative. Therefore, the question whether or not a pain reduction potentially significant to the patient could be achieved was not answered; this however could have a significant influence on patients’ quality of life and ability to return to work.
Lastly, we did not specifically assess and describe functional outcomes or document the return to work, which are key factors when evaluating the outcome of neuropathic pain treatment.
Conclusions
Surgical treatment of peripheral neuroma remains a clinical challenge. Patients should be coached toward smoking cessation in the presence of peripheral nerve injury. Whenever possible, forgoing of opiate treatment in the presence of peripheral nerve injury might be beneficial. Prospective studies are required to support the findings of this investigation.
References
Bromberg MB (2003) Saphenous nerve. Encycl Neurol Sci [Internet]. Elsevier. [cited 2022 May 9]. p. 198–200. Available from: https://linkinghub.elsevier.com/retrieve/pii/B0122268709009023
Ducic I, Levin M, Larson EE et al (2010) Management of chronic leg and knee pain following surgery or trauma related to saphenous nerve and knee neuromata. Ann Plast Surg 64:35–40
Watson J, Gonzalez M, Romero A et al (2010) Neuromas of the hand and upper extremity. J Hand Surg 35:499–510
Anantavorasakul N, Lans J, Macken AA et al (2020) Surgery for lower extremity symptomatic neuroma: long-term outcomes. J Plast Reconstr Aesthet Surg 73:1456–1464
Regev GJ, Ben Shabat D, Khashan M et al (2021) Management of chronic knee pain caused by postsurgical or posttraumatic neuroma of the infrapatellar branch of the saphenous nerve. J Orthop Surg 16:464
Dellon AL, Mackinnon SE (1986) Treatment of the painful neuroma by neuroma resection and muscle implantation. Plast Reconstr Surg 77:427–436
Koch H, Hubmer M, Welkerling H et al (2004) The treatment of painful neuroma on the lower extremity by resection and nerve stump transplantation into a vein. Foot Ankle Int 25:476–481
Lans J, Gamo L, DiGiovanni CW et al (2019) Etiology and treatment outcomes for sural neuroma. Foot Ankle Int 40:545–552
Poppler LH, Parikh RP, Bichanich MJ et al (2018) Surgical interventions for the treatment of painful neuroma: a comparative meta-analysis. Pain 159:214–223
Mochida H, Kikuchi S (1995) Injury to infrapatellar branch of saphenous nerve in arthroscopic knee surgery. Clin Orthop Relat Res 320:88–94
Schur MD, Sochol KM, Lefebvre R et al (2021) Treatment of iatrogenic saphenous neuroma after knee arthroscopy with excision and allograft reconstruction. Plast Reconstr Surg Glob Open 9:e3403
Çelik SB (2017) The evaluation of the neuropathic pain in the smokers. Ağrı - J Turk Soc Algol [Internet]. [cited 2022 May 29]; Available from: http://www.agridergisi.com/jvi.aspx?pdir=agri&plng=eng&un=AGRI-68815
Zhou Y, Liu P, Rui J et al (2017) The associated factors and clinical features of neuropathic pain after brachial plexus injuries: a cross-sectional study. Clin J Pain 33:1030–1036
Richards JS, Kogos SC, Ness TJ et al (2005) Effects of smoking on neuropathic pain in two people with spinal cord injury. J Spinal Cord Med 28:330–332
Iida H, Yamaguchi S, Goyagi T, et al. (2022) Consensus statement on smoking cessation in patients with pain. J Anesth [Internet]. [cited 2022 Oct 23]; Available from: https://link.springer.com/10.1007/s00540-022-03097-w
McNicol ED, Midbari A, Eisenberg E (2013) Opioids for neuropathic pain. Cochrane Pain, Palliative and supportive care group, editor. Cochrane Database Syst Rev [Internet]. [cited 2022 May 29]; Available from: https://doi.wiley.com/10.1002/14651858.CD006146.pub2
Zwaans WAR, Verhagen T, Roumen RMH et al (2015) Factors determining outcome after surgery for chronic groin pain following a lichtenstein hernia repair. World J Surg 39:2652–2662
Rapp SE, Ready BL, Nessly ML (1995) Acute pain management in patients with prior opioid consumption: a case-controlled retrospective review. Pain 61:195–201
Stokvis A, Henk Coert J, van Neck JW (2010) Insufficient pain relief after surgical neuroma treatment: prognostic factors and central sensitisation. J Plast Reconstr Aesthet Surg 63:1538–1543
Campbell JN, Meyer RA (2006) Mechanisms of neuropathic pain. Neuron 52:77–92
van Seventer R, Serpell M, Bach FW et al (2011) Relationships between changes in pain severity and other patient-reported outcomes: an analysis in patients with posttraumatic peripheral neuropathic pain. Health Qual Life Outcomes 9:17
Funding
Open access funding provided by University of Zurich
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design and manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Zurich, BASEC No. 2021–00643 20 April 2021.
Conflict of interest
Inga S. Besmens, Sophie Brackertz, Viviane Nietlispach, Andreas Schiller, Sophie Knipper, Pietro Giovanoli, and Maurizio Calcagni declare no conflict of interest.
Patient consent
Written informed consent was obtained from all participants included in this study in accordance with the local ethics committee requirements.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
I. S. Besmens and S. Brackertz contributed equally to this work and share first authorship.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Besmens, I.S., Brackertz, S., Nietlispach, V. et al. A cohort study on neuropathic pain of the saphenous nerve—factors influencing surgical outcome. Eur J Plast Surg 46, 397–402 (2023). https://doi.org/10.1007/s00238-022-02024-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00238-022-02024-2