Abstract
We reviewed 33 original research studies assessing brain perfusion, using consensus guidelines from a “white paper” issued by the International Society for Magnetic Resonance in Medicine Perfusion Study Group and the European Cooperation in Science and Technology Action BM1103 (“Arterial Spin Labelling Initiative in Dementia”; https://www.cost.eu/actions/BM1103/). The studies were published between 2011 and 2023 and included participants with subjective cognitive decline plus; neurocognitive disorders, including mild cognitive impairment (MCI), Alzheimer’s disease (AD), frontotemporal lobar degeneration (FTLD), dementia with Lewy bodies (DLB) and vascular cognitive impairment (VCI); as well as schizophrenia spectrum disorders, bipolar and major depressive disorders, autism spectrum disorder, attention-deficit/hyperactivity disorder, panic disorder and alcohol use disorder. Hypoperfusion associated with cognitive impairment was the major finding across the spectrum of cognitive decline. Regional hyperperfusion also was reported in MCI, AD, frontotemporal dementia phenocopy syndrome and VCI. Hypoperfused structures found to aid in diagnosing AD included the precunei and adjacent posterior cingulate cortices. Hypoperfused structures found to better diagnose patients with FTLD were the anterior cingulate cortices and frontal regions. Hypoperfusion in patients with DLB was found to relatively spare the temporal lobes, even after correction for partial volume effects. Hyperperfusion in the temporal cortices and hypoperfusion in the prefrontal and anterior cingulate cortices were found in patients with schizophrenia, most of whom were on medication and at the chronic stage of illness. Infratentorial structures were found to be abnormally perfused in patients with bipolar or major depressive disorders. Brain perfusion abnormalities were helpful in diagnosing most neurocognitive disorders. Abnormalities reported in VCI and the remaining mental disorders were heterogeneous and not generalisable.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Arterial spin labelling (ASL) magnetic resonance imaging (MRI) can obtain absolute quantification of cerebral blood flow (CBF) and assess the corresponding metabolism of the brain. By inverting the longitudinal magnetisation of proton spins as they flow into the brain, ASL uses labelled arterial blood water as a diffusible tracer [1, 2]. Other imaging techniques that assess brain perfusion and metabolism, such as single-photon emission computed tomography (SPECT) and positron emission tomography (PET), rely on exogenous radioactive tracers delivered to (and cleared out of) the brain tissue. Computed tomography and dynamic contrast enhanced (DCE) or dynamic susceptibility contrast (DSC) MRI also can assess perfusion by using a bolus injection of an exogenous contrast agent whose dynamic passage through the brain tissue provides specific perfusion parameters. However, neither SPECT, PET, CT, DCE nor DSC MRI enable absolute quantification of CBF. ASL not only allows for absolute quantification (usually in mL/100 g/min) but also is completely non-invasive because it does not rely on the injection of any exogenous tracer [3,4,5]. Crucially, ASL is also being used in other organs, apart from the brain [6].
Since its inception in the early 1990s, ASL has greatly improved. Modifications in both MRI sequences and hardware have increased the image quality and reduced the corresponding scanning time [7]. The relatively short acquisition time and simplicity of repeated scanning has been advantageous for follow-up studies, making ASL a useful complement to conventional MRI protocols [3, 4]. To standardise ASL and expand its usage, a consensus statement in the form of a “white paper” issued by the International Society for Magnetic Resonance in Medicine Perfusion Study Group and the European Cooperation in Science and Technology Action BM1103 (“Arterial Spin Labelling Initiative in Dementia”; https://www.cost.eu/actions/BM1103/) was published in 2015 [8]. This consensus recommended the implementation of pseudo-continuous arterial spin labelling (pCASL) with a single post-labelling delay (PLD) time, background suppression and a three-dimensional (3D) readout [8]. This approach helped to standardise both the labelling strategy and MRI sequence acquisition, improve agreement in CBF measurements between studies and facilitate the use of ASL in clinical practice. An updated and expanded consensus on the “current state and guidance on ASL in clinical neuroimaging” was published in 2023 [5].
As the prevalence of neurocognitive disorders (NCD) increases and effective treatments become available, the need for early and accurate clinical diagnoses becomes urgent [9, 10]. Along with NCD like Alzheimer’s disease (AD) and dementia with Lewy bodies (DLB), a high incidence of dementia also occurs in psychosis [11], and psychiatric diagnoses often overlap specific NCD, such as the behavioural variant of frontotemporal dementia (bvFTD) and the corresponding phenocopy syndrome [12]. Many mental disorders are heterogeneous at the pathophysiological and clinical levels [13]. Various symptoms associated with several mental disorders may vary at the level of severity or proportion [14], a concept known as “dimension” (cf. domain) [15]. In the clinical setting, such heterogeneity across symptoms can delay diagnosis.
A growing body of evidence shows that functional brain changes precede structural ones and, hence, physiological parameters may aid in early diagnosis [4, 5, 16]. To the best of our knowledge, no previous study has reported on the clinical application of pCASL in assessing a wide range of mental conditions. For this reason, the purpose of the current review was to compare CBF measurements or detect regions of abnormal brain perfusion, as assessed by pCASL, in patients with neurocognitive or other mental disorders, relative to healthy control (HC) subjects or those with subjective cognitive decline (SCD) [17], as well as to detect brain perfusion abnormalities useful to distinguish patients with different clinical conditions.
Methods
Literature search and selection criteria
This review was conducted according to recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines [18]. PubMed, WebOfScience and PsycINFO databases were searched for articles published until June 2023 that included terms related to NCD and other mental disorders (i.e. “cognitive disorder”, “cognitive dysfunction”, “cognitive impairment”, “mild neurocognitive disorder”, “mild cognitive disorder”, “mild cognitive impairment”, “dementia”, “major neurocognitive disorder”, “major cognitive disorder”, “mental disorders”, “mental illness”, “psychiatric disorders” or “psychiatric illness”), as well as terms related to imaging and ASL (i.e. “arterial spin labeling”, “arterial spin labelling”, “ASL”, “brain”, “brain imaging” or “neuroimaging”).
Published, peer-reviewed, original research studies on the clinical application of pCASL in patients with mental conditions were eligible for inclusion. Whenever possible, we applied the nomenclature of the Diagnostic and Statistical Manual of Mental Disorders, fifth edition (DSM-5) [14]. From an MRI perspective, only pCASL studies using a single PLD time, background suppression and a 3D readout on scanners operating up to 3 Tesla were included, according to the 2015 consensus paper providing technical guidelines for ASL application [8]. Studies also were required to have a comparison group (HC subjects or those with SCD) or to focus on differential diagnosis. Subjects with SCD were defined as individuals with subjective (self-perceived) cognitive complaints worsening over time but without any confirmation on cognitive testing (i.e. a normal objective performance level) [17].
Excluded studies were those not reporting comparisons of CBF measurements in regions of interest (ROI) or not using a voxel-wise approach, studies addressing psychopharmacological or other therapeutic effects only, articles written in languages other than English, review articles (e.g. meta-analyses) and commentaries or editorials on the theoretical background of ASL.
Studies focused only on neurovascular coupling or functional brain connectivity using ASL also were excluded.
Analysis
All included studies were analysed to determine whether they reported decreased (hypoperfusion), similar or increased brain perfusion (hyperperfusion), relative to HC or SCD subjects or between distinct groups of patients, according to statistical thresholds used in the corresponding publications. When disclosed, we report measures of diagnostic value, such as sensitivity, specificity, positive predictive value and negative predictive value. Likewise, when applied to measure the ability of brain perfusion to distinguish patients from controls or patients with different clinical conditions, we report the area under the receiver operating characteristic (ROC) curve (i.e. the area under the curve [AUC] or summary of the ROC curve)—the integral of a graphical plot illustrating the diagnostic ability of a binary classifier as its discrimination threshold (i.e. cut-off value) varies. An AUC closer to 1.0 indicates better performance of the classifier at distinguishing between two groups. To determine the risk of bias of each study, we also applied a set of 12 predefined questions as a quality assessment tool [19].
Results
Figure 1 presents the PRISMA flow diagram for the search and selection of studies. After excluding duplicates, screening titles and abstracts and assessing articles for eligibility, 33 studies published between 2011 and 2023 were included in this review [20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52]. According to the aforementioned quality assessment tool [19], all included studies were rated fair to good. Nineteen (58%) comprised samples of subjects with SCD plus and patients with NCD [21,22,23,24, 26, 30,31,32, 34, 35, 37, 38, 43, 46,47,48,49,50,51,52], including mild cognitive impairment (MCI), AD, frontotemporal lobar degeneration (FTLD), DLB and vascular cognitive impairment (VCI), according to the corresponding diagnostic criteria [17, 53,54,55,56,57,58,59,60,61,62,63]. Given that subjects with SCD plus have “features that increase the likelihood of preclinical AD” [17], we report them as a distinct subgroup belonging to the spectrum of cognitive decline. Four (12%) of the 33 studies encompassed patients with schizophrenia spectrum disorders (SSD) [27, 29, 36, 39], four (12%) comprised patients with major depressive disorder (MDD) [20, 28, 33, 45] and three (9%) comprised patients with bipolar disorders (BD) [33, 40, 45]. Finally, the remaining four (12%) studies included patients with autism spectrum disorder (ASD) [25], attention-deficit/hyperactivity disorder (ADHD) [42], panic disorder [41] and alcohol use disorder [44].
Table 1 summarises findings of the six (18%) studies reporting measures of diagnostic value for brain perfusion, all in the setting of SCD plus and NCD [21, 26, 30, 37, 43, 50]. Supplementary Table 1 summarises the main characteristics of all studies included in this review (i.e. demographic data and the PLD time used in each study) [20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52].
Arterial spin labelling in subjective cognitive decline plus and neurocognitive disorders
Subjective cognitive decline plus
The only study comprising subjects with SCD plus found them to have lower values of blood flow in the left hippocampal head and both posterior cingulate cortices (PCC), relative to HC subjects [43]. A positive correlation was found between the left hippocampal head blood flow and auditory verbal learning test delayed recall scores (r = 0.386; p-value = 0.046). The differentiation between SCD plus and HC subjects was best achieved by a combined ROC analysis of CBF values from the left hippocampal head, left precuneus and both PCC, which yielded an AUC of 0.84, but no cut-off value was reported [43].
Mild cognitive impairment and Alzheimer’s disease
Table 2 summarises findings of the nine (27%) studies comparing patients with MCI to HC or SCD subjects [21, 24, 34, 35, 43, 47, 48, 50, 52]. Table 3 summarises findings of the 14 (42%) studies comparing patients with AD to HC or SCD subjects [21,22,23,24, 26, 30, 32, 35, 38, 47,48,49,50, 52]. One study comprised patients under medication (i.e. patients with AD taking acetylcholinesterase inhibitors) [38]. It is plausible that patients in the remaining studies on AD were not taking antidementia drugs.
Global (supratentorial) cortical hypoperfusion was found in patients with MCI and those with AD [21,22,23, 30, 35, 50]. One study compared patients with AD and HC subjects using two single PLD times (1500 and 2500 ms). In both circumstances, the same clusters of hypoperfusion were found in patients with AD, but the number of hypoperfused voxels was lower when using the longer PLD time. This particular difference was attributed to compensatory phenomena caused by the surrounding structures [26].
Some studies reported hyperperfusion in the frontal lobes, left precuneus, right thalamus and right temporal white matter of patients with MCI [24, 48]. Regional hyperperfusion also was reported in the thalami [24, 52], right paracentral lobule, white matter of the right temporal lobe, right striatum [24], supplementary motor areas, putamina, left somatosensory cortex [47] and left middle cingulate cortex of patients with AD [52], relative to control subjects. Hyperperfusion was attributed to compensatory phenomena counteracting pathophysiology.
Studies comprising patients with MCI reported global cortical [21] and regional hypoperfusion in the parietal and occipital lobes [21, 24, 35, 43, 50], both PCC [21, 34, 43] and the adjacent precunei (which partially overlap and border the PCC [64]) [34, 43], as well as in the frontal lobes [21, 35, 43, 48], temporal lobes [24, 35] and hippocampi [34, 43]. Although two studies comprising patients with MCI did not find a significant difference in brain perfusion between these patients and HC subjects [47, 52], a statistical interaction revealed that altered perfusion in the right hippocampus was influenced by the number of years of education (p-value corrected for multiple comparisons < 0.05) [52].
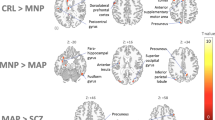
In patients with AD, 13 studies found hypoperfusion in the temporal lobes [21, 23, 24, 26, 30, 32, 35, 38, 47,48,49,50, 52], nine in the precunei and cingulate cortices [21, 23, 26, 30, 32, 38, 49, 50, 52] and nine in the parietal lobes [21, 23, 24, 26, 35, 38, 49, 50, 52]. The occipital [21, 23, 24, 30, 32, 35, 50] and frontal [21, 22, 26, 30, 35, 38, 48, 50] lobes also were found to be hypoperfused in AD. The most prominent changes were observed in the precunei, PCC and parietal regions [21, 23]. The hypoperfused structures found to aid in diagnosing patients with AD were the precunei and adjacent PCC (Fig. 2). ROC curves generated for CBF measurements in these regions revealed optimal cut-off values ranging from 31.6 to 37.8 ml/100 g/min to differentiate patients with AD from HC subjects or those with SCD (sensitivity: 77–84%; specificity: 69–81%; AUC: 0.83–0.89) [21, 26, 30].
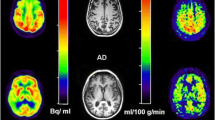
Cerebral blood flow maps of one patient with Alzheimer’s disease (AD, left column) and one patient with frontotemporal dementia (FTD, right column). The bottom row shows colour-coded cerebral blood flow maps coregistered onto high-resolution T1-weighted magnetic resonance images. Please note the occurrence of prominent hypoperfusion in the precunei and adjacent posterior cingulate cortices (thick arrows) of the patient with AD, as well as focal hypoperfusion in the anterior cingulate cortices of the patient with FTD (thin arrows). Please also note that the grey scale, not the colour scale, is different between patients to optimise the display of relative hypoperfusion. Reproduced under the Creative Commons Attribution 4.0 International License with permission of the authors: Steketee RME, Bron EE, Meijboom R, Houston GC, Klein S, Mutsaerts HJMM, Mendez Orellana CP, de Jong FJ, van Swieten JC, van der Lugt A, Smits M (2016) Early-stage differentiation between presenile Alzheimer's disease and frontotemporal dementia using arterial spin labeling MRI. Eur Radiol 26(1):244–253 [30]
One study by Benedictus et al. investigated the associations of normalised brain volumes and white matter hyperintensities volumes with CBF. It found that both smaller normalised brain volumes and larger white matter hyperintensities volumes were independently associated with lower cortical CBF in patients with AD, especially after statistical adjustment for hippocampal volume and concomitance of microbleeds and lacunar infarcts [22].
Six studies reported significant associations between brain perfusion and cognitive impairment [21, 26, 35, 38, 48, 49]. Three [21, 26, 49] used the Mini Mental State Examination (MMSE) [65]. One [38] used the Cognitive Ability Screening Instrument [66]. One [35] used separate measures to assess various cognitive domains (i.e. memory, attention, executive and visuospatial functioning, as well as language). Another [48] used the Wechsler memory scale—revised immediate-recall subtest [67]. All but one [48] of these studies demonstrated lower values of brain perfusion to be associated with worse cognitive performance in MCI and AD, mainly driven by strong correlations between lower CBF values and cognitive impairment in AD [21]. Patients with AD were found to have lower MMSE scores and decreased CBF, especially in the parietal lobes, precunei and middle and posterior cingulate cortices [21, 26]. Specifically, Binnewijzend et al. found a strong association between MMSE scores and CBF values [21]. A standardised β value of 0.48 (p-value ≤ 0.001) was found for the association between MMSE scores and CBF, corrected for partial volume effects, in the parietal lobes, precunei and adjacent PCC. The corresponding value of 0.42 (p-value ≤ 0.001) was found for the association between MMSE scores and global brain CBF [21]. A study by Liu et al. confirmed a similar association involving the left posterior cingulate cortex at a PLD time of 1500 ms (r = 0.628; p-value ≤ 0.001), but there was no correction for partial volume effects [26]. Finally, a study by Dong et al. found the left middle temporal gyrus perfusion to be positively correlated with MMSE scores (p-value corrected for multiple comparisons < 0.05) [49].
Frontotemporal lobar degeneration
Table 4 summarises findings of the six (18%) studies comparing patients with FTLD to HC or SCD subjects [23, 30,31,32, 37, 46]. Most patients had bvFTD, and some had other FTLD subtypes [30, 37, 46]. One study included a subgroup of seven patients with the following diagnostic criteria for phenocopy frontotemporal dementia (phFTD): presence of behavioural symptoms, imaging findings not consistent with bvFTD, no progression within (at least) one year after diagnosis, no C9ORF72 mutations and no other concurrent identifiable mental disorder. This study found hyperperfusion in the subcallosal areas of these seven patients, relative to HC subjects [31].
Patients with FTLD also were found to have lower CBF in the anterior cingulate cortices (ACC) and the frontal lobes [30, 37, 46]. One study found patients with semantic dementia and primary progressive nonfluent aphasia to have hypoperfusion predominantly in left brain hemispheric structures [46], but the sample size was small. Finally, Steketee et al. did not find a significant brain perfusion difference between patients with bvFTD and HC subjects [32].
The hypoperfused structures found to better help diagnose patients with FTLD were the ACC and frontal regions (Fig. 2). In fact, a ROC analysis yielded an optimal cut-off value of 46 ml/100 g/min for CBF in the ACC to differentiate patients with FTLD from HC subjects (sensitivity = 79%; specificity = 76%; AUC = 0.78) [30]. Slightly lower diagnostic values were found in another study (Table 1) that did not report any cut-off value [37].
Dementia with Lewy bodies
One study compared patients with DLB to subjects with SCD [23]. The study found a pattern of global cortical hypoperfusion in patients with DLB involving the frontal, parietal and occipital cortices, the precunei and adjacent PCC, as well as the cerebellum. Despite correction for partial volume effects, a relative sparing of perfusion in the temporal lobes was demonstrated [23], a finding mirroring the well-known structural sparing of these regions in DLB.
Vascular cognitive impairment
One study compared brain perfusion between patients with VCI and HC subjects [51]. Compared to the latter, patients with small vessel VCI were found to have hypoperfusion in the right middle frontal and superior temporal gyri. Patients with large vessel VCI were found to have hypoperfusion in the left inferior parietal, left inferior frontal and left middle frontal gyri. Conversely, patients with small vessel VCI were found to have hyperperfusion in the right hippocampus, cerebellar vermis and left middle and posterior cingulate cortices, whereas hyperperfusion was found in the right thalamus of patients with large vessel VCI. In addition, this study found decreased neurovascular coupling at high levels of the cortical hierarchy involved in cognitive control and emotion regulation [51].
Differential diagnosis of neurocognitive disorders
Mild cognitive impairment versus subjective cognitive decline plus
Patients with MCI were found to have lower CBF in the left precuneus and left thalamus, relative to subjects with SCD plus. Differentiating subjects with SCD plus from patients with MCI was best accomplished by combining CBF values from the left precuneus and thalamus whose ROC analysis yielded an AUC of 0.78, but no cut-off value was reported [43].
Alzheimer’s disease versus mild cognitive impairment
Table 5 summarises findings of the seven (21%) studies comparing patients with AD to those with MCI [21, 24, 35, 47, 48, 50, 52]. Even though patients with MCI or AD were found to have a similar hypoperfusion pattern, absolute CBF values were lower in those with AD [21, 35]. Specifically, hypoperfusion was found in the parietal [21, 35, 48, 50, 52], temporal [21, 47, 50, 52], frontal [35, 47, 48] and occipital [35, 50] lobes. Moreover, the precunei [21, 47] and adjacent PCC [21, 48], left posterior cingulate cortex [50, 52] and left anterior cingulate cortex [24] were further hypoperfused in patients with AD. Contrary to the abovementioned findings, hyperperfusion was reported in various regions of the right brain hemisphere [24], supplementary motor areas, striata and left precentral gyrus [47, 48], as well as in the orbitofrontal cortices, temporal poles, medial temporal lobes, both ACC, cerebellum [48] and in the left thalamus [52] of patients with AD, relative to patients with MCI.
Alzheimer’s disease versus frontotemporal lobar degeneration
Table 6 summarises findings of the four (12%) studies comparing patients with AD to those with FTLD [23, 30, 32, 50]. Despite some overlapping areas of hypoperfusion, all but one [50] of these studies found lower perfusion in the temporal cortices (i.e. right hippocampus, left superior and right inferior temporal gyri and right fusiform gyrus) [23, 32], both PCC [30] and orbitofrontal gyri [32] in patients with AD, relative to patients with FTLD. A ROC curve generated for CBF measurements in the PCC revealed an optimal cut-off value of 46.3 ml/100 g/min to differentiate patients with AD from those with FTLD (sensitivity = 69%; specificity = 68%; AUC = 0.74) [30].
Behavioural variant of frontotemporal dementia versus its phenocopy syndrome
Steketee et al. compared CBF values between patients with bvFTD and those with phFTD [31]. The subcallosal areas were found to be hyperperfused in patients with phFTD, relative to patients with bvFTD. This relative hyperperfusion may reflect compensatory phenomena counteracting incipient pathophysiology. Patients with bvFTD showed lower CBF in both straight gyri, the left superior and inferior frontal gyri and the left orbitofrontal gyrus, relative to patients with phFTD [31].
Alzheimer’s disease versus dementia with Lewy bodies
The study comprising patients with DLB also compared CBF values between these patients and those with AD. The ROI analysis showed more cortical hypoperfusion among patients with DLB, especially in the frontal, parietal and occipital cortices, as well as in the precunei and adjacent PCC [23].
Arterial spin labelling in psychiatric disorders
Schizophrenia spectrum disorders
Table 7 summarises findings of the four (12%) studies comparing brain perfusion between patients with SSD and HC subjects [27, 29, 36, 39]. Most patients had chronic symptoms (approximate mean duration of illness ≥ 10 years) and were on antipsychotic medication. In patients with SSD, hyperperfusion was found bilaterally in the temporal cortices [27, 29, 36], striata, thalami [27, 29, 36] and sensorimotor cortices [29, 36]. Furthermore, whereas the middle cingulate cortices were found to be hyperperfused in SSD [36], hypoperfusion was documented in the prefrontal cortices [29, 36, 39], ACC [27, 29, 36], occipital gyri [27, 29, 36], insular cortices [29, 36], parietal cortices [29, 39] and supplementary motor areas [36].
Two of the abovementioned studies [27, 29] reported associations between brain perfusion and Positive and Negative Syndrome Scale (PANSS) [68] scores. A positive correlation was found between perfusion in the left middle temporal gyrus and PANSS positive scores in women (partial rs = 0.344, p-value = 0.026) [29]. There was also a positive correlation between perfusion in the left inferior temporal gyrus and PANSS negative scores (r = 0.261, p-value = 0.009) [27]. Finally, a negative correlation was found between insular perfusion and PANSS negative scores (r = -0.238, p-value = 0.018) [27].
Bipolar disorders
Table 8 summarises findings of the three (9%) studies comparing brain perfusion between patients with BD and HC subjects [33, 40, 45]. Across the studies, participants had distinct characteristics. Specifically, one study [33] did not specify the type of bipolar disorder, one [40] included only patients with bipolar I disorder (i.e. with at least one manic episode) and one [45] comprised only patients with bipolar II disorder (i.e. with hypomanic and depressive episodes). Two of these three studies included only patients with BD experiencing a depressive episode [33, 45]. Participants in the remaining study [40] were under the effects of medication and clinically stable at the time of assessment. All participants of one study [45] were either drug naïve or unmedicated for at least six months.
The study by Zhao et al. found decreased CBF in the left dentate nucleus of the cerebellum in patients with BD, relative to HC subjects [33]. The study by Dai et al. used a dynamic pCASL approach and found increased perfusion fluctuations in the left fusiform gyrus and adjacent inferior temporal region of patients with bipolar I disorder, relative to HC subjects [40]. Additionally, a marginally significant increase in perfusion fluctuations was documented in the right temporal pole and adjacent inferior temporal region (p-value corrected for multiple comparisons = 0.063) [40]. The study by Chen et al. found hyperperfusion on the left side of the posterior lobe of the cerebellum (neocerebellum) and in the left middle temporal gyrus of patients with bipolar II disorder, relative to HC subjects [45].
Major depressive disorder
Table 9 summarises findings of the four (12%) studies comparing patients with MDD to HC subjects [20, 28, 33, 45]. All patients were experiencing a depressive episode at the time of assessment. Their average scores on the Hamilton Depression Rating Scale (HDRS) [69] indicated at least moderate severity of depression. Regarding medication, one study [20] comprised 65% of patients on antidepressant or antipsychotic medication, another [33] comprised 23% of patients on antidepressants, and two [28, 45] had no patients taking any relevant medication.
The study by Järnum et al. did not find significant CBF differences between patients with MDD and HC subjects [20]. However, this study conducted a subgroup analysis, dividing patients with MDD into remitting (n = 7) and non-remitting (n = 6) ones, depending on whether they did or did not have an HRDS-17 score ≤ 7 after a 6-month follow-up period on medication, respectively. At baseline, non-remitting patients with MDD showed significantly lower CBF in the frontal grey and white matter, both ACC, as well as in the temporal and parietal cortices, relative to HC subjects. Remitting patients with MDD showed no CBF differences, relative to HC subjects, but were found to have higher CBF in the parietal white matter (p-value = 0.04), relative to non-remitting patients [20]. The study by Kaichi et al. found hyperperfusion in various cortical regions of the left brain hemisphere. It also found hypoperfusion in the right lingual gyrus and the right superior temporal gyrus of patients [28]. The study by Zhao et al. found decreased CBF in patients with MDD at the superior cerebellar peduncles and the left dentate nucleus of the cerebellum [33]. The study by Chen et al. documented hyperperfusion in patients with MDD on the left side of the neocerebellum and in the left middle temporal gyrus, the latter significantly correlated with HDRS scores (r = 0.322, p-value = 0.002) [45].
Bipolar and major depressive disorders
Two (6%) of the included studies in this review assessed brain perfusion in samples of patients either with BD or MDD during a depressive episode [33, 45]. In the study by Zhao et al., decreased CBF in the left dentate nucleus of the cerebellum was found both in BD and MDD [33]. In the study by Chen et al., both patients with unmedicated bipolar II disorder and those with MDD had hyperperfusion on the left side of the neocerebellum and in the left middle temporal gyrus [45].
Other psychiatric disorders
One study on ASD compared brain perfusion between high-functioning autist children/adolescents and HC subjects matched for age, gender and intelligence quotient [25]. On average, patients with high-functioning autism scored 7.7 on the Autism Diagnostic Observation Schedule [70] severity scale, and had a Social Responsiveness Scale [71] total t score of 74.7. At the time of assessment, 41% of patients were receiving medication. A voxel-wise comparison demonstrated a pattern of widespread hyperperfusion at the frontotemporal regions, especially in the medial orbitofrontal cortices and both inferior frontal opercula, as well as in the left middle and inferior temporal gyri and the right precentral gyrus of patients with ASD, relative to HC subjects. By contrast, hypoperfusion was documented in the ACC of patients with ASD. Although this study found increased functional connectivity in the anterior module of the default mode network in patients, there was hypoperfusion in the same region [25].
One study on ADHD compared brain perfusion between patients with this condition and HC subjects [42]. Most (78%) patients had a predominantly inattentive presentation of ADHD. The remaining 22% had a combined presentation of ADHD symptoms. Widespread hypoperfusion was found in patients with ADHD in the left cerebral hemisphere, especially in the left insula, orbitofrontal cortex, putamen, globus pallidus, amygdala, supramarginal gyrus, Rolandic operculum, left temporal gyri, hippocampus, parahippocampal gyrus and left olfactory gyrus (p-value corrected for multiple comparisons < 0.05) [42].
One study on panic disorder compared brain perfusion between the afflicted patients and HC subjects [41]. Patients included in this study had at least one panic attack and were drug-free for at least two weeks prior to assessment. They had an average score of 13.2 on the Hamilton Rating Scale for Anxiety [72] and 9.7 on the Panic Disorder Severity Scale [73]. There was significant hypoperfusion and cortical thinning in the right fusiform gyrus in patients with panic disorder, relative to HC subjects. Furthermore, in patients with panic disorder, Z-scores calculated from perfusion values of the right fusiform gyrus were found to be negatively correlated both with the Hamilton Rating Scale for Anxiety (r = -0.512; p-value = 0.007) and Panic Disorder Severity Scale (r = -0.521; p-value = 0.006) scores [41].
Finally, one study on alcohol use disorder compared perfusion in the salience network of patients, relative to HC subjects (“social drinking controls”) [44]. Patients consumed an average of 30 drinks per week and had an average Alcohol Use Disorders Identification Test [74] score of 13.1. Patients with alcohol use disorder were found to have hypoperfusion in the insular cortices, relative to HC subjects. Furthermore, a negative correlation was found between perfusion in the ACC and Alcohol Use Disorders Identification Test scores (p-value corrected for multiple comparisons = 0.04), as well as between perfusion in the left frontal operculum and the number of drinks consumed per week (p-value corrected for multiple comparisons = 0.01) [44].
Discussion
Most studies included in this review comprised subjects with cognitive impairment, and most reported either global or regional brain hypoperfusion in subjects with SCD plus or patients with MCI, AD, FTLD, DLB or VCI, relative to HC or SCD subjects [21,22,23,24, 26, 30, 32, 34, 35, 38, 43, 47,48,49,50,51,52]. We also found reports of regional hyperperfusion in MCI [24], AD [47], phFTD [31] and VCI [51], mostly attributed to compensatory phenomena counteracting pathophysiology. Global cortical hypoperfusion was found either in patients with MCI or AD. Furthermore, hypoperfusion in the precunei and adjacent PCC was characteristic of patients with AD [21,22,23,24, 26, 30, 32, 35, 38, 47, 49, 52]. Crucially and as previously mentioned in the section of results, ROC curves generated for CBF measurements in these regions revealed optimal cut-off values, ranging from 31.6 to 37.8 ml/100 g/min, to diagnose patients with AD [21, 26, 30].
Apart from differentiating patients with AD from control subjects, a major finding of the current review was that brain hypoperfusion, as determined by pCASL, enables early detection of AD and thus serves as a biomarker of incipient cognitive impairment. Moreover, pCASL helps to differentiate subjects across the spectrum of cognitive decline, ranging from HC subjects to patients with AD [21, 35, 43]. In fact, a more pronounced hypoperfusion was documented in AD, relative to MCI [21, 35]; in MCI, relative to SCD plus [43]; and in SCD plus, relative HC subjects [43]. These findings support the notion that CBF could be incorporated into models of AD staging as an early biomarker [4, 35]. Furthermore, lower values of both global and regional CBF were found to be associated with the severity of cognitive impairment in SCD plus, MCI and AD [21, 26, 35, 38, 43]. Finally, one study found an association between larger white matter hyperintensities volumes and cortical hypoperfusion in AD [22]. Given that neurodegenerative and cerebrovascular pathology often coexist [75], this result speaks to the notion that ischaemic small vessel disease contributes to hypoperfusion in AD.
The only study on VCI in this review revealed both regional hypoperfusion and hyperperfusion in patients with small vessel and those with large vessel VCI [51]. Apart from brain perfusion, the study assessed neurovascular coupling. Brain regions with altered neurovascular coupling (i.e. altered perfusion or altered amplitude of low-frequency fluctuations in blood oxygen level-dependent functional MRI) were found to be localised at high levels of the cortical hierarchy, namely within the default mode network [51]. Nevertheless, given the heterogeneity of VCI, which can involve small or large vessels or the grey or white matter and can have a local or systemic cause [76], the aforementioned results should be complemented by those of future studies with larger samples and a longitudinal design.
Hypoperfused structures found to better help diagnosing patients with FTLD were the ACC and frontal regions [30]. Global brain hypoperfusion, with relative sparing of the temporal lobes, was found to be distinctive of DLB [23], even after correcting for partial volume effects, a pattern resembling the temporal lobe volume sparing typical of DLB. These findings represent a relevant clue for clinical diagnosis of both FTLD and DLB.
Although more studies on the differential diagnosis of NCD are warranted, pCASL was found to be helpful to discriminate patients with AD from those with FTLD (Fig. 2). As previously mentioned, a ROC curve generated for CBF measurements in the PCC revealed an optimal cut-off value of 46.3 ml/100 g/min to discriminate AD from FTLD in the early stage of disease progression [30]. In addition, compared to patients with AD, patients with DLB showed a more widespread pattern of cortical hypoperfusion, especially in the frontal, parietal and occipital cortices, as well as in the precunei and adjacent PCC [23].
Although brain perfusion could represent a biomarker of several psychiatric disorders [77], most studies included in this review did not find specific patterns of abnormal perfusion in SSD, BD or MDD [20, 27,28,29, 33, 36, 39, 40, 45]. The lack of findings suggests a predominance of other mechanisms underlying these conditions (e.g. abnormal neurotransmission, lack of synaptic plasticity and dysconnectivity) over microvascular or metabolic abnormalities. Such mechanisms are still poorly understood.
Perfusion abnormalities were reported in SSD with potential pathophysiological and clinical relevance [27, 29, 36, 39]. For instance, hyperperfusion in the temporal cortices and striata might mirror abnormally increased dopaminergic activity in these regions. Furthermore, hyperperfusion in the temporal cortices is consistent with the occurrence of auditory hallucinations in patients with SSD [78]. The association between increased perfusion in the left middle temporal gyrus and PANSS positive scores supports this idea [29]. Likewise, hypoperfusion in the prefrontal and anterior cingulate regions has been demonstrated, contributing to the well-established reduced neuropil hypothesis and to reduced effective connectivity found in the prefrontal regions and ACC of patients with schizophrenia [79]. It is also consistent with decreased dopaminergic activity in the prefrontal cortical pathway, possibly accounting for cognitive dysfunction and negative symptoms in these patients [80]. However, perfusion abnormalities in SSD were found to be more diffuse and topographically complex than the aforementioned, making them nearly impossible to detect by visual inspection in a clinical setting. In addition, most patients with SSD had chronic symptoms and were on antipsychotic medication. Given that cognitive impairment, grey matter loss and social isolation are expected to be more prominent in patients with chronic schizophrenia, these factors may confound interpretation and generalisation of the reported findings.
The studies on patients with BD or MDD were quite heterogeneous, particularly as patients with BD had distinct characteristics and medication schemes [33, 40, 45]. These differences could have prevented the identification of patterns of abnormal brain perfusion and hindered comparison of the reported results. Whereas three studies found discrepant brain perfusion abnormalities in patients with MDD, relative to HC subjects [28, 33, 45], another found no significant baseline differences [20]. However, a subgroup analysis of the latter study found remitting patients with MDD to have higher CBF in the parietal white matter, relative to non-remitting patients [20]. This result suggests that CBF, as assessed by pCASL, might be predictive of MDD remission, as has been demonstrated by the response of these patients to repetitive transcranial magnetic stimulation [81], a potential tool for individualised therapy.
Regarding studies assessing brain perfusion in samples of patients either with BD or MDD [33, 45], Chen et al. suggested that dysfunction of the neocerebellum might be involved in the pathophysiological processes of bipolar II disorder and MDD and that increased perfusion both in the neocerebellum and the left middle temporal gyrus could represent overlapping pathophysiology in BD and MDD [45]. The study by Zhao et al. contradicted these findings, showing hypoperfusion in the left dentate nucleus of the cerebellum in BD and MDD [33]. Curiously, a common theme in both studies is that each found abnormally perfused infratentorial structures in BD and MDD, indicating that microstructural and perfusion abnormalities in the prefrontal–thalamic–cerebellar circuit influence neurobiological processes of both disorders [33]. Nevertheless, it was not possible to determine whether the reported abnormalities represented a state or trait biomarker for these conditions, because all participants were experiencing a depressive episode [33, 45].
Although ASD and ADHD are both neurodevelopmental disorders according to the DSM-5, findings on ASD [25], ADHD [42], panic disorder [41] and alcohol use disorder [44] did not allow for any sort of generalisation due to only one study being identified for each condition. Additional drawbacks of the current review were the inability to include eligible studies on other prevalent psychiatric disorders [14] and the limited number of studies on SCD plus [43], DLB [23] and VCI [51]. Stringent application of the ASL technical guidelines (i.e. pCASL sequences with a single PLD time) [8] further reduced the sample of eligible studies. Given that sequences incorporating multi-PLD times are under development, studies reporting on their clinical contribution are warranted in the near future. Moreover, some studies on NCD included subjects with SCD as a control group [21,22,23, 35], instead of HC subjects. Although this might be regarded as a limitation, including such populations might better reflect a typical clinical scenario. Finally, in the current review, only one study reported results based on a longitudinal design [20].
Nineteen (58%) of the included studies [20,21,22,23, 25, 26, 30, 31, 33,34,35,36,37,38, 43, 44, 46, 48, 50] reported absolute CBF measurements (in mL/100 g/min). Six of these [22, 25, 34, 36, 37, 48] just reported global brain or grey matter CBF. The remaining 14 (42%) studies [24, 27,28,29, 32, 39,40,41,42, 45, 47, 49, 51, 52] reported regions of abnormal brain perfusion. The reported findings were demonstrated at the between-subject level in all studies [20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52]. Only one study [30] also presented an exemplary illustration on the utility of pCASL for clinical diagnosis at the within-subject level (Fig. 2).
Clinical heterogeneity (i.e. differences in patient characteristics) across samples may explain the diversity of results reported by the studies reviewed herein, particularly demographic factors (age, sex and education), concurrent medication and comorbidities. Likewise, the significance of abnormal global or regional brain perfusion depends on the understanding of physiological correlates at the neurovascular level. Nevertheless, our results support the use of biomarkers for the diagnosis of NCD and other mental disorders. Molecular, biological (e.g. liquid biopsy) and imaging-based (e.g. morphometric, perfusion-based, quantitative susceptibility mapping [82]) biomarkers are of particular interest. Prior reviews [4, 83, 84], as well as current guidance on ASL in the routine clinical setting [5], also support pCASL as being a reliable biomarker for the detection and diagnosis of NCD and its incipient forms. Implementation in clinical practice requires caution, however. For instance, the effect of perfusion modifiers (e.g. caffeine, age and blood gases) must be considered for diagnostic accuracy. Some of these factors alter CBF measurements in a similar way as pathological conditions and, therefore, can hinder accurate diagnoses [85].
In conclusion, brain perfusion abnormalities were found to help diagnose most NCD. Abnormalities reported in VCI and other mental disorders were heterogeneous and not generalisable, but some promising reports indicate an increasing role for the assessment of brain perfusion by pCASL, especially if this advanced MRI technique is combined with evaluation of neurovascular coupling or with functional and effective brain connectivity analyses.
References
Alsop DC, Detre JA (1998) Multisection cerebral blood flow MR imaging with continuous arterial spin labeling. Radiology 208(2):410–416. https://doi.org/10.1148/radiology.208.2.9680569
Williams DS, Detre JA, Leigh JS et al (1992) Magnetic resonance imaging of perfusion using spin inversion of arterial water. Proc Natl Acad Sci 89(1):212–216
Grade M, Hernandez Tamames JA, Pizzini FB et al (2015) A neuroradiologist’s guide to arterial spin labeling MRI in clinical practice. Neuroradiology 57(12):1181–1202. https://doi.org/10.1007/s00234-015-1571-z
Haller S, Zaharchuk G, Thomas DL et al (2016) Arterial spin labeling perfusion of the brain: emerging clinical applications. Radiology 281(2):337–356. https://doi.org/10.1148/radiol.2016150789
Lindner T, Bolar DS, Achten E et al (2023) Current state and guidance on arterial spin labeling perfusion MRI in clinical neuroimaging. Magn Reson Med 89(5):2024–2047. https://doi.org/10.1002/mrm.29572
Taso M, Aramendia-Vidaurreta V, Englund EK et al (2023) Update on state-of-the-art for arterial spin labeling (ASL) human perfusion imaging outside of the brain. Magn Reson Med 89(5):1754–1776. https://doi.org/10.1002/mrm.29609
Golay X, Guenther M (2012) Arterial spin labelling: final steps to make it a clinical reality. MAGMA 25(2):79–82. https://doi.org/10.1007/s10334-012-0308-9
Alsop DC, Detre JA, Golay X et al (2015) Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: a consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn Reson Med 73(1):102–116. https://doi.org/10.1002/mrm.25197
Alzheimer’s Association Report (2021) Alzheimer’s disease facts and figures. Alzheimers Dement 17(3):327–406. https://doi.org/10.1002/alz.12328
Sims JR, Zimmer JA, Evans CD et al (2023) Donanemab in early symptomatic Alzheimer disease: the TRAILBLAZER-ALZ 2 randomized clinical trial. JAMA 330(6):512–527. https://doi.org/10.1001/jama.2023.13239
Jonas K, Abi-Dargham A, Kotov R (2021) Two hypotheses on the high incidence of dementia in psychotic disorders. JAMA Psychiat 78(12):1305–1306. https://doi.org/10.1001/jamapsychiatry.2021.2584
Gossink FT, Dols A, Kerssens CJ et al (2016) Psychiatric diagnoses underlying the phenocopy syndrome of behavioural variant frontotemporal dementia. J Neurol Neurosurg Psychiatry 87(1):64–68. https://doi.org/10.1136/jnnp-2014-308284
Silveira C, Marques-Teixeira J, de Bastos-Leite AJ (2012) More than one century of schizophrenia: an evolving perspective. J Nerv Ment Dis 200(12):1054–1057. https://doi.org/10.1097/NMD.0b013e318275d249
American Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders: DSM-5. American Psychiatric Press, Washington DC
Insel T, Cuthbert B, Garvey M et al (2010) Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry 167(7):748–751. https://doi.org/10.1176/appi.ajp.2010.09091379
Fallmar D, Haller S, Lilja J et al (2017) Arterial spin labeling-based Z-maps have high specificity and positive predictive value for neurodegenerative dementia compared to FDG-PET. Eur Radiol 27(10):4237–4246. https://doi.org/10.1007/s00330-017-4784-1
Jessen F, Amariglio RE, van Boxtel M et al (2014) A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimers Dement 10(6):844–852. https://doi.org/10.1016/j.jalz.2014.01.001
Page MJ, McKenzie JE, Bossuyt PM et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71. https://doi.org/10.1136/bmj.n71
National Heart, Lung and Blood Institute (2013) Study quality assessment tools, https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed 18 January 2024)
Jarnum H, Eskildsen SF, Steffensen EG et al (2011) Longitudinal MRI study of cortical thickness, perfusion, and metabolite levels in major depressive disorder. Acta Psychiatr Scand 124(6):435–446. https://doi.org/10.1111/j.1600-0447.2011.01766.x
Binnewijzend MA, Kuijer JP, Benedictus MR et al (2013) Cerebral blood flow measured with 3D pseudocontinuous arterial spin-labeling MR imaging in Alzheimer disease and mild cognitive impairment: a marker for disease severity. Radiology 267(1):221–230. https://doi.org/10.1148/radiol.12120928
Benedictus MR, Binnewijzend MA, Kuijer JP et al (2014) Brain volume and white matter hyperintensities as determinants of cerebral blood flow in Alzheimer’s disease. Neurobiol Aging 35(12):2665–2670. https://doi.org/10.1016/j.neurobiolaging.2014.06.001
Binnewijzend MA, Kuijer JP, van der Flier WM et al (2014) Distinct perfusion patterns in Alzheimer’s disease, frontotemporal dementia and dementia with Lewy bodies. Eur Radiol 24(9):2326–2333. https://doi.org/10.1007/s00330-014-3172-3
Ding B, Ling HW, Zhang Y et al (2014) Pattern of cerebral hyperperfusion in Alzheimer’s disease and amnestic mild cognitive impairment using voxel-based analysis of 3D arterial spin-labeling imaging: initial experience. Clin Interv Aging 9:493–500. https://doi.org/10.2147/CIA.S58879
Jann K, Hernandez LM, Beck-Pancer D et al (2015) Altered resting perfusion and functional connectivity of default mode network in youth with autism spectrum disorder. Brain Behav 5(9):e00358. https://doi.org/10.1002/brb3.358
Liu Y, Zeng X, Wang Z et al (2015) Different post label delay cerebral blood flow measurements in patients with Alzheimer’s disease using 3D arterial spin labeling. Magn Reson Imaging 33(9):1019–1025. https://doi.org/10.1016/j.mri.2015.05.001
Zhu J, Zhuo C, Qin W et al (2015) Altered resting-state cerebral blood flow and its connectivity in schizophrenia. J Psychiatr Res 63:28–35. https://doi.org/10.1016/j.jpsychires.2015.03.002
Kaichi Y, Okada G, Takamura M et al (2016) Changes in the regional cerebral blood flow detected by arterial spin labeling after 6-week escitalopram treatment for major depressive disorder. J Affect Disord 194:135–143. https://doi.org/10.1016/j.jad.2015.12.062
Ma X, Wang D, Zhou Y et al (2016) Sex-dependent alterations in resting-state cerebral blood flow, amplitude of low-frequency fluctuations and their coupling relationship in schizophrenia. Aust N Z J Psychiatry 50(4):334–344. https://doi.org/10.1177/0004867415601728
Steketee RM, Bron EE, Meijboom R et al (2016) Early-stage differentiation between presenile Alzheimer’s disease and frontotemporal dementia using arterial spin labeling MRI. Eur Radiol 26(1):244–253. https://doi.org/10.1007/s00330-015-3789-x
Steketee RM, Meijboom R, Bron EE et al (2016) Structural and functional brain abnormalities place phenocopy frontotemporal dementia (FTD) in the FTD spectrum. NeuroImage Clin 11:595–605. https://doi.org/10.1016/j.nicl.2016.03.019
Steketee RM, Meijboom R, de Groot M et al (2016) Concurrent white and gray matter degeneration of disease-specific networks in early-stage Alzheimer’s disease and behavioral variant frontotemporal dementia. Neurobiol Aging 43:119–128. https://doi.org/10.1016/j.neurobiolaging.2016.03.031
Zhao L, Wang Y, Jia Y et al (2016) Cerebellar microstructural abnormalities in bipolar depression and unipolar depression: a diffusion kurtosis and perfusion imaging study. J Affect Disord 195:21–31. https://doi.org/10.1016/j.jad.2016.01.042
Dolui S, Vidorreta M, Wang Z et al (2017) Comparison of PASL, PCASL, and background-suppressed 3D PCASL in mild cognitive impairment. Hum Brain Mapp 38(10):5260–5273. https://doi.org/10.1002/hbm.23732
Leeuwis AE, Benedictus MR, Kuijer JPA et al (2017) Lower cerebral blood flow is associated with impairment in multiple cognitive domains in Alzheimer’s disease. Alzheimers Dement 13(5):531–540. https://doi.org/10.1016/j.jalz.2016.08.013
Zhu J, Zhuo C, Xu L et al (2017) Altered coupling between resting-state cerebral blood flow and functional connectivity in schizophrenia. Schizophr Bull 43(6):1363–1374. https://doi.org/10.1093/schbul/sbx051
Anazodo UC, Finger E, Kwan BYM et al (2018) Using simultaneous PET/MRI to compare the accuracy of diagnosing frontotemporal dementia by arterial spin labelling MRI and FDG-PET. NeuroImage Clinical 17:405–414. https://doi.org/10.1016/j.nicl.2017.10.033
Huang CW, Hsu SW, Chang YT et al (2018) Cerebral perfusion insufficiency and relationships with cognitive deficits in Alzheimer’s disease: a multiparametric neuroimaging study. Sci Rep 8(1):1541. https://doi.org/10.1038/s41598-018-19387-x
Kim J, Plitman E, Nakajima S et al (2019) Modulation of brain activity with transcranial direct current stimulation: targeting regions implicated in impaired illness awareness in schizophrenia. Eur Psychiatry 61:63–71. https://doi.org/10.1016/j.eurpsy.2019.06.007
Dai W, Chen M, Duan W et al (2020) Abnormal perfusion fluctuation and perfusion connectivity in bipolar disorder measured by dynamic arterial spin labeling. Bipolar Disord 22(4):401–410. https://doi.org/10.1111/bdi.12856
Ni MF, Wang XM, Wang HY et al (2020) Regional cortical thinning and cerebral hypoperfusion in patients with panic disorder. J Affect Disord 277:138–145. https://doi.org/10.1016/j.jad.2020.07.139
Tan YW, Liu L, Wang YF et al (2020) Alterations of cerebral perfusion and functional brain connectivity in medication-naive male adults with attention-deficit/hyperactivity disorder. CNS Neurosci Ther 26(2):197–206. https://doi.org/10.1111/cns.13185
Yang Z, Rong Y, Cao Z et al (2021) Microstructural and cerebral blood flow abnormalities in subjective cognitive decline plus: diffusional kurtosis imaging and three-dimensional arterial spin labeling study. Front Aging Neurosci 13:625843. https://doi.org/10.3389/fnagi.2021.625843
Butcher TJ, Chumin EJ, West JD et al (2022) Cerebral blood flow in the salience network of individuals with alcohol use disorder. Alcohol Alcohol 57(4):445–451. https://doi.org/10.1093/alcalc/agab062
Chen F, Gong J, Chen G et al (2022) Shared and specific characteristics of regional cerebral blood flow and functional connectivity in unmedicated bipolar and major depressive disorders. J Affect Disord 309:77–84. https://doi.org/10.1016/j.jad.2022.04.099
Ssali T, Narciso L, Hicks J et al (2022) Concordance of regional hypoperfusion by pCASL MRI and (15)O-water PET in frontotemporal dementia: is pCASL an efficacious alternative? NeuroImage Clin 33:102950. https://doi.org/10.1016/j.nicl.2022.102950
Gao Z, Zhou S, Zhu W et al (2023) Sex-dependent changes in emotional memory associated with cerebral blood flow alterations during Alzheimer’s disease progression. Neuroradiology 65(4):751–763. https://doi.org/10.1007/s00234-022-03099-1
Camargo A, Wang Z, Alzheimer’s Disease Neuroimaging Initiative (2023) Hypo- and hyper-perfusion in MCI and AD identified by different ASL MRI sequences. Brain Imaging Behav 17(3):306–319. https://doi.org/10.1007/s11682-023-00764-8
Dong H, Guo L, Yang H et al (2023) Association between gray matter atrophy, cerebral hypoperfusion, and cognitive impairment in Alzheimer’s disease. Front Aging Neurosci 15:1129051. https://doi.org/10.3389/fnagi.2023.1129051
Mao C, You H, Hou B et al (2023) Differentiation of Alzheimer’s disease from frontotemporal dementia and mild cognitive impairment based on arterial spin labeling magnetic resonance imaging: a pilot cross-sectional study from PUMCH dementia cohort. J Alzheimers Dis 93(2):509–519. https://doi.org/10.3233/JAD-221023
Ruan Z, Sun D, Zhou X et al (2023) Altered neurovascular coupling in patients with vascular cognitive impairment: a combined ASL-fMRI analysis. Front Aging Neurosci 15:1224525. https://doi.org/10.3389/fnagi.2023.1224525
Zhu W, Gao Z, Li H et al (2023) Education reduces cognitive dysfunction in Alzheimer’s disease by changing regional cerebral perfusion: an in-vivo arterial spin labeling study. Neurol Sci 44(7):2349–2361. https://doi.org/10.1007/s10072-023-06696-x
Petersen RC, Doody R, Kurz A et al (2001) Current concepts in mild cognitive impairment. Arch Neurol 58(12):1985–1992. https://doi.org/10.1001/archneur.58.12.1985
Petersen RC, Stevens JC, Ganguli M et al (2001) Practice parameter: early detection of dementia: mild cognitive impairment (an evidence-based review). Neurology 56(9):1133–1142. https://doi.org/10.1212/wnl.56.9.1133
Petersen RC (2004) Mild cognitive impairment as a diagnostic entity. J Intern Med 256(3):183–194. https://doi.org/10.1111/j.1365-2796.2004.01388.x
Albert MS, DeKosky ST, Dickson D et al (2011) The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7(3):270–279. https://doi.org/10.1016/j.jalz.2011.03.008
McKhann G, Drachman D, Folstein M et al (1984) Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 34(7):939–944. https://doi.org/10.1212/wnl.34.7.939
McKhann GM, Knopman DS, Chertkow H et al (2011) The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7(3):263–269. https://doi.org/10.1016/j.jalz.2011.03.005
Neary D, Snowden JS, Gustafson L et al (1998) Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology 51(6):1546–1554. https://doi.org/10.1212/wnl.51.6.1546
Gorno-Tempini ML, Hillis AE, Weintraub S et al (2011) Classification of primary progressive aphasia and its variants. Neurology 76(11):1006–1014. https://doi.org/10.1212/WNL.0b013e31821103e6
Rascovsky K, Hodges JR, Knopman D et al (2011) Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 134(Pt 9):2456–2477. https://doi.org/10.1093/brain/awr179
McKeith IG, Dickson DW, Lowe J et al (2005) Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology 65(12):1863–1872. https://doi.org/10.1212/01.wnl.0000187889.17253.b1
Skrobot OA, Black SE, Chen C et al (2018) Progress toward standardized diagnosis of vascular cognitive impairment: Guidelines from the Vascular Impairment of Cognition Classification Consensus Study. Alzheimers Dement 14(3):280–292. https://doi.org/10.1016/j.jalz.2017.09.007
Leech R, Sharp DJ (2014) The role of the posterior cingulate cortex in cognition and disease. Brain 137(Pt 1):12–32. https://doi.org/10.1093/brain/awt162
Folstein MF, Folstein SE, McHugh PR (1975) “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12(3):189–198. https://doi.org/10.1016/0022-3956(75)90026-6
Teng EL, Hasegawa K, Homma A et al (1994) The Cognitive Abilities Screening Instrument (CASI): a practical test for cross-cultural epidemiological studies of dementia. Int Psychogeriatr 6(1):45–58. https://doi.org/10.1017/s1041610294001602
Chelune GJ, Bornstein RA and Prifitera A (1990) The Wechsler memory scale—revised. In: Advances in Psychological Assessment Volume 7. Springer, Boston, pp 65–99. https://doi.org/10.1007/978-1-4613-0555-2_3
Kay SR, Fiszbein A, Opler LA (1987) The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 13(2):261–276. https://doi.org/10.1093/schbul/13.2.261
Hamilton M (1960) A rating scale for depression. J Neurol Neurosurg Psychiatry 23:56–62. https://doi.org/10.1136/jnnp.23.1.56
Lord C, Risi S, Lambrecht L et al (2000) The Autism Diagnostic Observation Schedule-Generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord 30(3):205–223
Constantino JN, Davis SA, Todd RD et al (2003) Validation of a brief quantitative measure of autistic traits: comparison of the social responsiveness scale with the autism diagnostic interview-revised. J Autism Dev Disord 33(4):427–433. https://doi.org/10.1023/a:1025014929212
Hamilton M (1959) The assessment of anxiety states by rating. Br J Med Psychol 32(1):50–55. https://doi.org/10.1111/j.2044-8341.1959.tb00467.x
Shear MK, Brown TA, Barlow DH et al (1997) Multicenter collaborative panic disorder severity scale. Am J Psychiatry 154(11):1571–1575. https://doi.org/10.1176/ajp.154.11.1571
Saunders JB, Aasland OG, Babor TF et al (1993) Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption—II. Addiction 88(6):791–804. https://doi.org/10.1111/j.1360-0443.1993.tb02093.x
Bastos-Leite AJ, van der Flier WM, van Straaten EC et al (2007) The contribution of medial temporal lobe atrophy and vascular pathology to cognitive impairment in vascular dementia. Stroke 38(12):3182–3185. https://doi.org/10.1161/STROKEAHA.107.490102
Barkhof F, Fox NC, Bastos-Leite AJ et al (2011) Vascular dementia. In: Neuroimaging in dementia. 1st ed, Springer, Berlin, pp 137–176. https://doi.org/10.1007/978-3-642-00818-4_6
Theberge J (2008) Perfusion magnetic resonance imaging in psychiatry. Top Magn Reson Imaging 19(2):111–130. https://doi.org/10.1097/RMR.0b013e3181808140
Marti-Bonmati L, Lull JJ, Garcia-Marti G et al (2007) Chronic auditory hallucinations in schizophrenic patients: MR analysis of the coincidence between functional and morphologic abnormalities. Radiology 244(2):549–556. https://doi.org/10.1148/radiol.2442060727
Bastos-Leite AJ, Ridgway GR, Silveira C et al (2015) Dysconnectivity within the default mode in first-episode schizophrenia: a stochastic dynamic causal modeling study with functional magnetic resonance imaging. Schizophr Bull 41(1):144–153. https://doi.org/10.1093/schbul/sbu080
Goldman-Rakic PS, Castner SA, Svensson TH et al (2004) Targeting the dopamine D1 receptor in schizophrenia: insights for cognitive dysfunction. Psychopharmacology 174(1):3–16. https://doi.org/10.1007/s00213-004-1793-y
Weiduschat N, Dubin MJ (2013) Prefrontal cortical blood flow predicts response of depression to rTMS. J Affect Disord 150(2):699–702. https://doi.org/10.1016/j.jad.2013.04.049
Liu C, Wei H, Gong NJ et al (2015) Quantitative susceptibility mapping: contrast mechanisms and clinical applications. Tomography 1(1):3–17. https://doi.org/10.18383/j.tom.2015.00136
Wierenga CE, Hays CC, Zlatar ZZ (2014) Cerebral blood flow measured by arterial spin labeling MRI as a preclinical marker of Alzheimer’s disease. J Alzheimers Dis 42(Suppl 4):S411-419. https://doi.org/10.3233/JAD-141467
Meeter LH, Kaat LD, Rohrer JD et al (2017) Imaging and fluid biomarkers in frontotemporal dementia. Nat Rev Neurol 13(7):406–419. https://doi.org/10.1038/nrneurol.2017.75
Clement P, Mutsaerts HJ, Vaclavu L et al (2018) Variability of physiological brain perfusion in healthy subjects: a systematic review of modifiers. J Cereb Blood Flow Metab 38(9):1418–1437. https://doi.org/10.1177/0271678X17702156
Funding
Open access funding provided by FCT|FCCN (b-on).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Ethical approval and informed consent
N/A.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ferreira, R., Bastos-Leite, A.J. Arterial spin labelling magnetic resonance imaging and perfusion patterns in neurocognitive and other mental disorders: a systematic review. Neuroradiology 66, 1065–1081 (2024). https://doi.org/10.1007/s00234-024-03323-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-024-03323-0