Abstract
Objectives
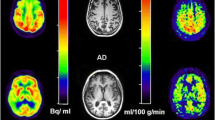
To compare pseudo-continuous arterial spin-labelled (PCASL) magnetic resonance imaging (MRI) measured quantitative cerebral blood flow (CBF) of patients with frontotemporal dementia (FTD), dementia with Lewy Bodies (DLB), Alzheimer’s disease (AD) and controls, in a region of interest (ROI) and voxel-wise fashion.
Methods
We analysed whole-brain 3D fast-spin-echo PCASL images of 20 FTD patients, 14 DLB patients, 48 AD patients and 50 controls from the Amsterdam Dementia Cohort. Regional CBF patterns were compared using analyses of variance for repeated measures. Permutation tests were used for voxel-wise comparisons. Analyses were performed using uncorrected and partial volume corrected (PVC) maps. All analyses were corrected for age and sex.
Results
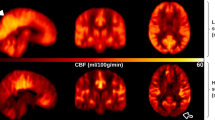
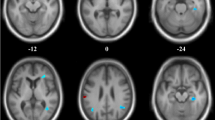
There was an interaction between diagnosis and region (p < 0.001), implying differences in regional CBF changes between diagnostic groups. In AD patients, CBF was decreased in all supratentorial regions, most prominently so in the posterior regions. DLB patients showed lowest CBF values throughout the brain, but temporal CBF was preserved. Supratentorial PVC cortical CBF values were lowest in the frontal lobes in FTD patients, and in the temporal lobes in AD patients.
Conclusions
Patients with AD, FTD and DLB display distinct patterns of quantitative regional CBF changes. 3D-PCASL may provide additional value in the workup of dementia patients.
Key points
• Patterns of regional CBF changes differ between AD, FTD and DLB patients
• CBF is lower throughout the brain in DLB than AD and FTD
• 3D-PCASL MRI is a potential non-invasive and easily accessible alternative to FDG-PET
• 3D-PCASL MRI may be of additional value in the workup of dementia



Similar content being viewed by others
Abbreviations
- Abeta:
-
Amyloid beta
- NGMV:
-
Normalized grey matter volume
- PCASL:
-
Pseudo-continuous arterial spin-labelling
- PPC:
-
Precuneus and posterior cingulate
- PVC:
-
Partial volume corrected
- P-tau:
-
Phosphorylated-tau
- T-tau:
-
Total-tau
- WMH:
-
Vascular white matter hyperintensities
References
Bohnen NI, Djang DS, Herholz K, Anzai Y, Minoshima S (2012) Effectiveness and safety of 18F-FDG PET in the evaluation of dementia: a review of the recent literature. J Nucl Med 53:59–71
McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM (1984) Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 34:939–944
McKeith IG, Dickson DW, Lowe J et al (2005) Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology 65:1863–1872
Neary D, Snowden JS, Gustafson L et al (1998) Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology 51:1546–1554
Sluimer JD, van der Flier WM, Karas GB et al (2009) Accelerating regional atrophy rates in the progression from normal aging to Alzheimer’s disease. EurRadiol 19:2826–2833
Whitwell JL, Josephs KA, Rossor MN et al (2005) Magnetic resonance imaging signatures of tissue pathology in frontotemporal dementia. Arch Neurol 62:1402–1408
Beyer MK, Larsen JP, Aarsland D (2007) Gray matter atrophy in Parkinson disease with dementia and dementia with Lewy bodies. Neurology 69:747–754
Jack CR Jr, Knopman DS, Jagust WJ et al (2010) Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol 9:119–128
Colloby SJ, Fenwick JD, Williams ED et al (2002) A comparison of (99m)Tc-HMPAO SPET changes in dementia with Lewy bodies and Alzheimer’s disease using statistical parametric mapping. Eur J Nucl Med Mol Imaging 29:615–622
Lobotesis K, Fenwick JD, Phipps A et al (2001) Occipital hypoperfusion on SPECT in dementia with Lewy bodies but not AD. Neurology 56:643–649
Mosconi L, Tsui WH, Herholz K et al (2008) Multicenter standardized 18F-FDG PET diagnosis of mild cognitive impairment, Alzheimer’s disease, and other dementias. J Nucl Med 49:390–398
Dai W, Garcia D, de Bazelaire C, Alsop DC (2008) Continuous flow-driven inversion for arterial spin labeling using pulsed radio frequency and gradient fields. Magn Reson Med 60:1488–1497
Binnewijzend MA, Kuijer JP, Benedictus MR et al (2013) Cerebral blood flow measured with 3D pseudocontinuous arterial spin-labeling MR imaging in Alzheimer disease and mild cognitive impairment: a marker for disease severity. Radiology 267:221–230
McKhann GM, Knopman DS, Chertkow H et al (2011) The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7:263–269
Rascovsky K, Hodges JR, Knopman D et al (2011) Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 134:2456–2477
Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA (1987) MR signal abnormalities at 1.5T in Alzheimer’s dementia and normal aging. AJR Am J Roentgenol 149:351–356
Buxton RB, Frank LR, Wong EC, Siewert B, Warach S, Edelman RR (1998) A general kinetic model for quantitative perfusion imaging with arterial spin labeling. Magn Reson Med 40:383–396
Smith SM (2002) Fast robust automated brain extraction. Hum Brain Mapp 17:143–155
Jenkinson M, Smith S (2001) A global optimisation method for robust affine registration of brain images. Med Image Anal 5:143–156
Zhang Y, Brady M, Smith S (2001) Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging 20:45–57
Asllani I, Borogovac A, Brown TR (2008) Regression algorithm correcting for partial volume effects in arterial spin labeling MRI. Magn Reson Med 60:1362–1371
Nichols TE, Holmes AP (2002) Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp 15:1–25
Smith SM, Nichols TE (2009) Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage 44:83–98
Alsop DC, Detre JA, Grossman M (2000) Assessment of cerebral blood flow in Alzheimer’s disease by spin-labeled magnetic resonance imaging. Ann Neurol 47:93–100
Dai W, Lopez OL, Carmichael OT, Becker JT, Kuller LH, Gach HM (2009) Mild cognitive impairment and alzheimer disease: patterns of altered cerebral blood flow at MR imaging. Radiology 250:856–866
Johnson NA, Jahng GH, Weiner MW et al (2005) Pattern of cerebral hypoperfusion in Alzheimer disease and mild cognitive impairment measured with arterial spin-labeling MR imaging: initial experience. Radiology 234:851–859
Kim SM, Kim MJ, Rhee HY et al (2013) Regional cerebral perfusion in patients with Alzheimer’s disease and mild cognitive impairment: effect of APOE Epsilon4 allele. Neuroradiology 55:25–34
Mak HK, Chan Q, Zhang Z et al (2012) Quantitative assessment of cerebral hemodynamic parameters by QUASAR arterial spin labeling in Alzheimer’s disease and cognitively normal elderly adults at 3-tesla. J Alzheimers Dis 31:33–44
McKeith IG (2006) Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the Consortium on DLB International Workshop. J Alzheimers Dis 9:417–423
Taylor JP, Firbank MJ, He J et al (2012) Visual cortex in dementia with Lewy bodies: magnetic resonance imaging study. Brit J Psychiatry 200:491–498
Fong TG, Inouye SK, Dai W, Press DZ, Alsop DC (2011) Association cortex hypoperfusion in mild dementia with Lewy bodies: a potential indicator of cholinergic dysfunction? Brain Imaging Behav 5:25–35
Ishii K, Soma T, Kono AK et al (2007) Comparison of regional brain volume and glucose metabolism between patients with mild dementia with lewy bodies and those with mild Alzheimer’s disease. J Nucl Medicine 48:704–711
Shimizu S, Hanyu H, Kanetaka H, Iwamoto T, Koizumi K, Abe K (2005) Differentiation of dementia with Lewy bodies from Alzheimer’s disease using brain SPECT. Dement Geriatr Cogn Disord 20:25–30
Albin RL, Minoshima S, D’Amato CJ, Frey KA, Kuhl DA, Sima AA (1996) Fluoro-deoxyglucose positron emission tomography in diffuse Lewy body disease. Neurology 47:462–466
Sato A, Sato Y, Uchida S (2002) Regulation of cerebral cortical blood flow by the basal forebrain cholinergic fibers and aging. Auton Neurosci 96:13–19
Du AT, Jahng GH, Hayasaka S et al (2006) Hypoperfusion in frontotemporal dementia and Alzheimer disease by arterial spin labeling MRI. Neurology 67:1215–1220
Hu WT, Wang Z, Lee VM, Trojanowski JQ, Detre JA, Grossman M (2010) Distinct cerebral perfusion patterns in FTLD and AD. Neurology 75:881–888
Chen Y, Wolk DA, Reddin JS et al (2011) Voxel-level comparison of arterial spin-labeled perfusion MRI and FDG-PET in Alzheimer disease. Neurology 77:1977–1985
Jueptner M, Weiller C (1995) Review: does measurement of regional cerebral blood flow reflect synaptic activity? Implications for PET and fMRI. NeuroImage 2:148–156
Campbell AM, Beaulieu C (2006) Pulsed arterial spin labeling parameter optimization for an elderly population. J Magn Reson Imaging 23:398–403
Acknowledgments
The authors thank Ajit Shankaranarayanan of GE Healthcare for providing the 3D pseudo-continuous ASL sequence that was used to obtain data for this paper. The study was supported by the Alzheimer Center of the VU University Medical Center Amsterdam, the Netherlands.
The scientific guarantor of this publication is Prof. Dr. Frederik Barkhof. The authors of this manuscript declare relationships with the following companies:
M.A.A. Binnewijzend, J.P.A. Kuijer, W.M. van der Flier, M.R. Benedictus, C.M. Möller, Y.A.L. Pijnenburg and A.W. Lemstra report no disclosures.
N.D. Prins serves on the advisory board of Boehringer Ingelheim and Envivo. He has been a speaker at symposia organised by Janssen and Novartis. NDP has a senior fellowship at the Alzheimer Center VUmc partly supported by Vereniging AEGON and receives research support from the Brain Foundation of the Netherlands and Alzheimer Nederland. NDP receives no personal compensation for the activities mentioned above.
M.P. Wattjes serves as a consultant for Biogen-Idec. Dr. Wattjes receives research support from Biogen Idec, Bayer Healthcare, Roche and Janssen Cilag.
B.N.M. van Berckel receives research support from the Alzheimer Assistance Foundation, the Center for Translational Molecular Imaging, the Alzheimer Association, De Hersenstichting Nederland and the Internationale Stichting Alzheimer Onderzoek. Dr. van Berckel receives no personal compensation from these organizations.
P. Scheltens receives grant support (for the institution; the VUmc Alzheimer Center, see also below) from GE Healthcare, Danone Researcrh and MERCK. In the past 2 years he has received speaker’s fees (paid to the institution) from Lilly, GE Healthcare, Lundbeck, Danone and Jansen AI-Pfizer.
Research of the VUmc Alzheimer Center is part of the neurodegeneration research program of the Neuroscience Campus Amsterdam. The VUmc Alzheimer Center is supported by Alzheimer Nederland and Stichting VUmc fonds. The clinical database structure was developed with funding from Stichting Dioraphte.
F. Barkhof serves on the editorial boards of Brain, European Radiology, Radiology, Multiple Sclerosis and Neuroradiology and serves as a consultant for Bayer-Shering Pharma, Sanofi-Aventis, Biogen-Idec, EVA, Synthon BV, Merck-Serono, Jansen Alzheimer Immunotherapy, Novartis and Roche. Dr. Barkhof receives research support from the Dutch MS Society (EU-FP7). Dr. Barkhof has received consulting fees or honoraria for the consultancy mentioned above. Dr. Barkhof has received speaker’s fees from the Serono Symposia Foundation.
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article. The authors state that this work has not received any funding. One of the authors (W.M. Van der Flier) has significant statistical expertise. Institutional review board approval was obtained. Written informed consent was obtained from all subjects (patients) in this study. Approval from the institutional animal care committee was not required because there were no animal subjects involved in this study. Some study subjects or cohorts have been previously reported elswhere by Binnewijzend et al. (Radiology 267:221–230, 2013). Methodology: retrospective, case-control study, performed at one institution.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Binnewijzend, M.A.A., Kuijer, J.P.A., van der Flier, W.M. et al. Distinct perfusion patterns in Alzheimer’s disease, frontotemporal dementia and dementia with Lewy bodies. Eur Radiol 24, 2326–2333 (2014). https://doi.org/10.1007/s00330-014-3172-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-014-3172-3