Abstract
Purpose
This retrospective cross-sectional cohort study investigated the influence of posture on lordosis (LL), length of the spinal canal (LSC), anteroposterior diameter (APD L1-L5), dural cross-sectional area (DCSA) of the lumbar spinal canal, and the prevalence of redundant nerve roots (RNR) using positional magnetic resonance imaging (MRI) (0.6 T).
Methods
Sixty-eight patients with single-level degenerative central lumbar spinal stenosis (cLSS) presenting with RNR in the standing position (STA) were also investigated in supine (SUP) or neutral seated (SIT) and flexed seated (FLEX) positions. Additionally, 45 patients complaining of back pain and without MRI evidence of LSS were evaluated. Statistical significance was set at p < 0.05.
Results
Controls (A) and patients with cLSS (B) were comparable in terms of mean age (p = 0.88) and sex (p = 0.22). The progressive transition from STA to FLEX led to a comparable decrease in LL (p = 0.97), an increase in LSC (p = 0.80), and an increase in APD L1-L5 (p = 0.78). The APD of the stenotic level increased disproportionally between the different postures, up to 67% in FLEX compared to 29% in adjacent non-stenotic levels (p < 0.001). Therefore, the prevalence of RNR decreased to 49, 26, and 4% in SUP, SIT, and FLEX, respectively.
Conclusion
The prevalence of RNR in standing position was underestimated by half in supine position. Body postures modified LL, LSC, and APD similarly in patients and controls. Stenotic levels compensated for insufficient intraspinal volume with a disproportionate enlargement when switching from the STA to FLEX.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Experimental cadaver studies [1, 2], myelographic and CT-myelographic investigations [3], and positional MRI studies [4–10] have shown that posture influences lumbar lordosis (LL), length of the spinal canal (LSC), segmental anteroposterior diameter (APD), and dural cross-sectional area (DCSA) of the lumbar spine. Transitioning from the extended spinal canal (standing) to the inflected (bent forward sitting) decreases the lordotic angle, increases the length of the lumbar spine, and increases the capacitance of the spinal canal.
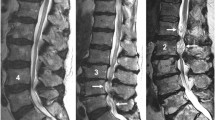
However, additional parameters are required to diagnose patients with degenerative central lumbar spinal stenosis (cLSS) presenting with redundant nerve roots (RNR) confirmed by MRI. First, we investigated the influence of four relevant postures on daily living. These are as follows: STA, which increases pain when lasts a long time; SUP, which during sleep is associated with 71% of patients affected by cLSS with nocturnal cramps [11]; SIT, which relieves the pain partially; and FLEX, which relieves pain as much as possible. Furthermore, psoas relaxed SUP is the standard positioning for MRI investigations due to lumbar stenosis. Second, we analyzed the influence of postural changes on the prevalence of RNR (Fig. 1).
RNR are coiled, elongated, and thickened cauda nerve roots (CNR) associated with severe stenosis [12]. In conventional supine MRI, the CNR are stretched on one side of the stenotic level and serpentine or loop-shaped on the other. Frequently, RNR are shown cranially to the stenotic level, but they can appear caudally or both [13–15]. Patients who show RNR on preoperative MRI have a longer history, more severe symptoms, a smaller DCSA, and a lower postoperative recovery rate than patients without RNR [16, 17]. Therefore, we considered RNR as a negative prognostic factor. It is of interest to evaluate which posture provides “RNR-free” time, and to what extent. This positional MRI study aimed to answer four questions:
-
1.
Does the transition from the extended lumbar spinal canal in the STA to the inflected spinal canal in the FLEX affect the prevalence and direction of redundant nerve roots?
-
2.
Does the stenotic lumbar spine behave differently than the non-stenotic spine in terms of LL, LSC, and APD?
-
3.
Does the change in body posture influence the APD of the stenotic level differently from that of the non-stenotic adjacent levels?
-
4.
How reliable is the ASED-classification of RNR [18] when applied to the imaging of a 0.6-T MRI?
Methods
Study design
This study was a retrospective and observational with a repeated measures design. Files between June 2011 and May 2021 of the same Upright-MRI database were selected according to the following inclusion criteria: single-level degenerative cLSS causing RNR in STA and positional MRI study in at least two further postures (FLEX, SUP, and/or SIT) in T2-weighted (T2WI) modality. The control group consisted of individuals who underwent the investigation because of nonspecific lower back pain and had undergone a positional MRI scan as mentioned above. The exclusion criteria included single-level stenosis without evidence of RNR, two- or multi-level stenosis, previous surgery at the index level, previous fixation devices in the lumbar spine, and acute trauma. The files were anonymized for the purposes of this study.
The Ethics Committee of the Federal State of Hamburg does not require informed consent or approval for a retrospective observational study when the data is acquired, saved, and treated anonymously. The authors adhered to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines [19].
Study sample
Sixty-eight patients with single-level cLSS underwent the positional MRI (FONAR Upright™, 0.6 T, FONAR Corp.; Melville, NY 11747, USA) as they were surgical candidates. All patients underwent STA, FLEX (T2WI), and SIT (T1WI) examinations. Forty-one of them were additionally examined in SUP (T2WI, protocol 1). The remaining 27 patients were examined in SIT (T2WI, protocol 2) to shorten the investigation time due to claustrophobia or obesity-related issues. Patients in SUP position lay flat on their backs with a small pillow beneath the knees to relax the psoas muscle. Forty-five controls were investigated according to protocol one (Fig. 1). The whole cohort underwent SIT (T1WI) for completion of diagnostics.
MR protocol
The standard imaging protocol included the following sequences:
-
STA: Sag T2 FSE, TE 110 ms/TR 1140 ms, Matrix 480 × 480, FOV 330; Axi T2 FSE, TE 140 ms/TR 1440 ms, Matrix 512 × 448, FOV 240
-
SUP: Sag T2 FSE, TE 132 ms/TR 1904 ms, Matrix 540 × 480, FOV 310
-
SIT: Sag T2 FSE, TE 132 ms/TR 1904 ms, Matrix 540 × 480, FOV 310; Sag T1 SE, TE 20 ms/TR 500 ms, Matrix 540 × 480, FOV 310; Axi T2 FSE, TE 132 ms/TR 2167 ms, Matrix 420 × 360, FOV 180; Cor STIR, TE 100 ms/TR 2240 ms, Matrix 400 × 340, FOV 350
-
FLEX: Sag T2 FSE, TE 132 ms/TR 1904 ms, Matrix 540 × 480, FOV 310
Mean scanning time was 45 min and slice thickness was 4 mm for each sequence.
Assessment of the study variables
Parameters LL, LSC, and APD L1-L5 are shown in Fig. 2. Instead of measuring along the posterior border of the vertebral bodies, LSC was intentionally measured among the cauda nerve roots, to monitor the change in their course caused by different postures. DCSA was measured on axial STA (T2WI) and SIT (T2WI) slices (Fig. 3). As per Schizas et al. [20], the Lausanne classification was used for qualitative assessment of stenosis severity. In grade B stenosis, cerebrospinal fluid is still visible around the CNR in the axial T2WI MRI image, unlike in grades C and D stenosis. For this study, grade B stenosis was defined as CSF + , and grades C and D were defined as CSF − . All measurements were performed jointly by three authors (LP, NA, KS) using the JiveX DICOM Viewer (VISUS Health IT, Bochum, Germany).
Parameters: a lumbar lordosis (LL): angle between the upper endplate L1 and the upper endplate S1. b Length of the spinal canal (LSC): sum of the segmental lengths parallel to the main bundle of cauda nerve roots between the upper endplate L1 and the upper endplate S1. c Anteroposterior diameter (APD): segmental sagittal diameter of the dural sac at the mid-disk level
Clinical case: a 73-year-old man presented with neurogenic claudication. In the previous 3 weeks, he experienced exacerbation of leg pain and new hypesthesia in the lower limbs. a Standing: RNR (arrowhead) cranial to the pincer stenosis caused by the buckling of disks (slim arrows) and yellow ligament (thick arrow). b No relevant difference in supine posture was observed. c In neutral sitting partial flattening of disks and yellow ligament with increase in the anteroposterior diameters and resolution of RNR was observed. d Enlargement of the stenotic level and complete resolution of the RNR
The ASED classification for RNR [18] was used for sagittal T2WI in the STA, SUP, SIT, and FLEX positions. The images were classified jointly by three authors (LP, KS, NA), and repeated 8 weeks later to circumvent any issue caused by inferior imaging quality of 0.6-T MRI compared with 3-T MRI. Discrepancies were resolved through consensus.
Statistical analysis
Statistical analyses were performed using SPSS for Windows, version 24.0 (SPSS Inc., USA). Metric variables were presented as means and medians, and dispersion measures were presented as standard deviations and quartiles. Two-sided confidence intervals (CI) were set at 95%. Categorical or nominal data were reported as absolute or relative frequencies and analyzed using the chi-square test or Fisher’s exact test. When comparing two independent, normally distributed samples, the Student’s t-test was used. Paired t-test was used when the samples were dependent. Levene’s test was performed to check for homogeneity of variance. Welch’s t-test was performed in the absence of the latter. Two-sided significance tests were performed. A p value less than 0.05 was set as the criterion for statistical significance.
Results
Patients and controls were comparable in terms of mean age (68 ± 10 years; p = 0.88) and sex distribution (31% vs. 42% women; p = 0.22). In Table 1, the changes in LL, LSC, and APD in the four body positions are compared between the patients and controls.
Prevalence and direction of RNR
Sixty-eight patients showed RNR in the STA. The first subgroup of 41 patients underwent a SUP examination. In this body posture, RNR persisted in 20 patients (49%). The second subgroup of 27 patients underwent SIT examination. In this body posture, RNR persisted in seven patients (26%). All patients underwent FLEX examination. In this body posture, RNR persisted in three patients (4%). Only the CSF + in STA variable predicted the resolution of RNR in SUP (p = 0.005) or SIT (p = 0.004), whereas the changes in other parameters such as LL (p = 0.21), LSC (p = 0.77), and APD (p = 0.91) did not. The location of the RNR at the stenotic level is shown in Fig. 4. Notably, in the three body postures subject to axial gravity (STA, SIT, and FLEX), the majority of RNR were located caudal to the stenotic level.
Same patient as in Fig. 1: dural cross-sectional area (DCSA) in standing position at the stenotic level (a) and 10-mm cranial (b) coiled and loop-shaped cauda nerve roots with positive sedimentation sign. c In sitting DCSA trebled, cauda nerve roots run perpendicular to the axial plane and the sedimentation sign became negative
LL
LL was significantly lower in patients in STA (p = 0.005) and SIT (p = 0.015) groups than in control group. A possible explanation for this is the antalgic slightly bent forward posture of the patients affected by cLSS. However, LL did not differ between patients in SUP (p = 0.134) and FLEX (p = 0.228) groups compared to control group. While the SUP position is “standardized” due to the use of a horizontal table, the FLEX seated position is actively maximized by the patients (Fig. 5).
In patients, STA-LL decreased to SUP-LL by 6%, to SIT-LL by 18%, and to FLEX-LL by 81%. In the controls, STA-LL decreased to SUP-LL by 11%, to SIT-LL by 42%, and to FLEX-LL by 79%. Thus, the significant difference in STA-LL between patients and controls (p = 0.005) was equalized in the FLEX group (p = 0.228).
LSC
The comparison of LSC in patients vs. controls in STA, SUP, SIT, and FLEX did not show any significant differences (Fig. 6).
LSC increased in patients from STA to SUP by 1%, SIT by 5%, and FLEX by 8%. LSC increased in controls from STA to SUP by 2%, SIT by 6%, and FLEX by 10%. Hence, the maximum LSC did not differ between the groups (p = 0.934).
APD
APD L1-L5 in patients did not change substantially from STA to SUP (by − 0.4%), increased to SIT by 5%, and to FLEX by 17%. APD L1-L5 increased in controls by 3%, 1.4%, and 14%, respectively. In both groups, the spinal canal expanded with comparable dynamics (p = 0.78), although at a different capacitance (Fig. 7). Interestingly, the APD of the stenotic level increased from STA to SUP by 18% (p = 0.001), to SIT by 34% (p = 0.001), and to FLEX by 67% (p < 0.001) (Fig. 8). The corresponding values for the non-stenotic adjacent levels were 6%, 19%, and 29%, respectively. The prevalence of RNR dropped dramatically during the transition from the extended spinal canal in the STA to the flexed spinal canal in FLEX due to the disproportionate enlargement of the stenotic level, which almost doubled the expansion with each change in posture (Fig. 9).
DCSA
The critical CNR cross-sectional area at the level L1/L2 was 77.4 ± 13.3 mm2 and decreased roughly by 10 mm2 at each further caudal level [21]. Schoenstroem et al. [22, 23] measured an in vitro pressure of 50 mmHg when the area was constricted to 62.2 ± 12.0 mm2. A further reduction of the area to 56.8 ± 10.05 mm2 (− 25%) doubled the pressure to 100 mmHg. These data show that the CNR cross-sectional area is crucial for functioning roots. In STA, the DCSA of the stenotic CSF + levels (101.60 ± 26.98 mm2) exceeded the threshold and caused “fluid-dynamic” RNR, possibly caused by the increased velocity of the CSF through the stenotic area, influence of gravity, increased drag force on the CNR, and post-stenotic drop of the hydrostatic pressure [24]. Conversely, the DCSA of CSF − levels (49.16 ± 21.02 mm2) fell below the threshold and caused “anatomical” RNR due to the mechanical constriction of CNR at the narrowest DCSA.
ASED classification
The challenge of classifying RNR on MRI (0.6 T) was in selecting the most valid sagittal slice, especially in patients with coronal deformity of the lumbar spine. Therefore, consensus among the three raters was recorded. Eight weeks later, the rating process was repeated. The percentage agreement is presented in Table 2.
Discussion
RNR are rarely associated in conventional supine MRI with disk herniation or tumor [25, 26], but it has been reported in 33–43% of patients with spinal canal stenosis [12, 27, 28]. Coiled,elongated, and thickened CNR is clinically relevant as a negative prognostic factor [12, 17, 28]. Patients who show RNR on preoperative MRI have a longer history, more severe symptoms, and a lower postoperative recovery rate than those without RNR [16 17]. The current mechanical squeeze theory of RNR dates to 1992, when Suzuki et al. suggested that at the stenotic spine level, the pulsatile movements of the CNR are restricted or blocked. Repeated flexion of the lumbar spine stretches and elongates the nerve roots, and spine extension causes tortuosity, redundancy, and coiling. A more common location of RNR above the stenotic level seems to support this mechanism [12]. Current knowledge about RNR has mostly been forged by anatomical, myelographic, and supine MRI studies. An earlier positional MRI study [7] on RNR comparing the STA and FLEX positions, but lacking the supine position, showed that the prevalence and location of RNR depends on the body posture. This concept of highly dynamic RNR focuses on the fluid-dynamic aspects beyond the mechanical constriction of the CNR. The present study evaluated a larger number of patients, completed the MRI examination in the supine position, with a cohort of age- and sex-matched control patients suffering from back pain.
First, our study shows that the transition from the extended lumbar spinal canal in the STA to the inflected spinal canal in FLEX affects the prevalence and direction of redundant nerve roots. As a rule of thumb, each postural change in the STA sequence halves the prevalence of RNR. Furthermore, the resolution of RNR from the STA to the SUP correlates strongly with the severity of stenosis. In STA, a mild anatomical stenosis may cause CSF flow turbulence that generates RNR mostly below the stenotic level but resolves in SUP. In severe cases, such as CSF − condition, stenosis traps the traversing CNR in STA as well as in SUP. Additionally, the prevalent location of RNR being caudal to the stenotic level in all body postures exposed to axial gravity (all postures except SUP) confirms the role of gravity on CSF fluid movements.
Second, we find that the stenotic lumbar spine does not behave differently from the non-stenotic spine in terms of LL, LSC, and APD during posture changes. Although the absolute figures are lower in the former, the stenotic spine behaves comparably to the non-stenotic spine.
Third, changing body posture influences the APD of the stenotic level differently from that of the non-stenotic adjacent levels. The stenotic level disproportionally increased the intradural capacitance when compared with the non-stenotic levels in the same spine. Combined stretching of the buckling yellow ligament, usually remarkably thickened, and the flattening of the posterior disk, usually remarkably bulging, during progressive flexion of the spine could anatomically explain the difference between stenotic and non-stenotic levels. More trivial, but noteworthy, is the logic of small numbers: the 100% increase of a stenotic APD from 3 to 6 mm is functionally more relevant than the 27% increase of a non-stenotic APD from 11 to 14 mm at the adjacent level, although in both cases, the difference is 3 mm. In contrast, if in the MRI-SUP investigation of a patient affected by cLSS, stenosis should be maximized to resemble the findings in STA, the comfortable psoas-relaxed position should be substituted with a combination of straightening legs (anterior pelvic tilt) and placing a lumbar pillow underneath the back. Madsen et al. showed that this positioning best resembles STA [29]. We agree with this pragmatic alternative to the STA investigation, which is not currently implemented. However, we are aware of two limitations of this alternative: the influence of gravity is eliminated, and the horizontal table restricts the antalgic posture while standing.
Lastly, the present study tested the reliability of the ASED classification of RNR. The classification is intended to facilitate communication between radiologists and clinicians by referring to imaging details with clinical prognostic relevance. The strong agreement rate confirmed the reliability of the classification, even with reasonable, but not excellent, imaging quality.
The retrospective design of this study was biased by its intrinsic limitations. The selective inclusion criteria led to a relatively small number of patients, even when recruited over a decade. Furthermore, the partial change in the imaging protocol during this time generated two even smaller subgroups (SUP and SIT). Some studies indicate that DCSA measurement is more sensitive than APD for detecting changes in the width of the spinal canal. In this study, the DCSA was measured only in two postures due to time constraints. On CT, the correlation between the DCSA and APD is strong [3]. Nevertheless, our findings support reports in the literature that RNR are a sign of advanced and clinically relevant lumbar stenosis. The sequential decrease of the prevalence of RNR in the four body postures mirrors the spontaneous choice of the patients affected by cLSS during daytime (squatting, flexed sitting, and walking) and at night (sleeping in fetal position, unpublished results) aiming to increase their “RNR-free” time. The temporary effectiveness of flexion exercises or the segmental kyphosis due to interspinous devices indicates similar outcomes. The high dynamics of RNR, which may be based on fluid-dynamic aspects, are the topic of an ongoing study to further decipher details of RNR.
Conclusions
Positional MRI shows that the prevalence of RNR in conventional supine MRI is underestimated by half. Posture changes modify LL, LSC, and APD similarly in patients with cLSS stenotic spines as in patients with non-stenotic low back pain. Stenotic levels compensate for insufficient intradural volume with disproportionate enlargement.
Abbreviations
- ASED:
-
Allocation-Shape-Extension-Direction—classification of RNR
- APD:
-
Segmental antero-posterior diameter
- APD L1-L5:
-
Cumulative antero-posterior diameter L1-L5
- CNR:
-
Cauda nerve roots
- cLSS:
-
Central lumbar spinal stenosis
- DCSA:
-
Dural cross-sectional area
- FSE:
-
Fast spin echo
- FLEX:
-
Flexed seated position
- LL:
-
Lumbar lordosis
- LSC:
-
Length of the spinal canal
- RNR:
-
Redundant nerve roots
- SE:
-
Spin echo
- SIT:
-
Neutral seated position
- STA:
-
Standing position
- SUP:
-
Supine position
- TE:
-
Echo time
- TR:
-
Repetition time
- T1WI:
-
T1-weighted images
- T2WI:
-
T2-weighted images
References
Liyang D, Yinkan X, Wenming Z, Zhihua Z (1989) The effect of flexion-extension motion on the lumbar spine on the capacity of the spinal canal: an experimental study. Spine 14(5):523–525
Teske W, Schwert M, Zirke S, Schulze Pellengahr Ch, Wiese M, Lahner M (2015) Intrathecal volume changes in lumbar spinal canal stenosis following extension and flexion: an experimental cadaver study. Technol Helath Care 23:645–652. https://doi.org/10.3233/THC-1510111
Kanbara S, Yukawa Y, Ito K, Machino M, Kato F (2014) Dynamic changes in the dural sac of patients with lumbar canal stenosis evaluated by multidetector-row computed tomography after myelography. Eur Spine J 23:74–79. https://doi.org/10.1007/s00586-013-2873-7
Gökce E, Beyhan M (2021) Magnetic resonance imaging findings of redundant nerve roots of the cauda equina. World J Radiol 13(1):29–39. https://doi.org/10.4329/wjr.v13.i1.29
Hirasawa Y, Bashir WA, Smith FW, Magnusson ML, Pope MH, Takahashi K (2007) Postural changes of the dural sac in the lumbar spines of asymptomatic individuals using positional stand-up magnetic resonance imaging. Spine 32(4):E136–E140
Eberhardt K, Ganslandt O, Stadlbauer A (2014) Functional and quantitative magnetic resonance myelography of symptomatic stenoses of the lumbar spine. Neuroradiology 56:1069–1078. https://doi.org/10.1007/s00234-014-1433-0
Papavero L, Ebert S, Marques CJ (2020) The prevalence of redundant nerve roots in patients with lumbar stenosis is body position dependent: a retrospective observational study with repeated measures design in an upright MRI scanner. Neuroradiology 62:979–985. https://doi.org/10.1007/s00234-020-02423-x
Muto M, Giurazza F, Guarnieri G, Sense R, Schena E, Zeccolini F, Diano A (2016) Dynamic MRI in patients affected by neurogenical claudication: technique and results from a single center experience. Neuroradiology 58(8):765–770. https://doi.org/10.1007/s00234-016-1697-7
Kubosch D, Vicari M, Siller A, Strohm PC, Kubosch EJ, Knoeller S, Hennig J, Suedkamp NP, Izadpanah K (2015) The lumbar spine as a dynamic structure depicted in upright MRI. Medicine (Baltimore) 94(32):e1299. https://doi.org/10.1097/MD.0000000000001299
Doktor K, Hartvigsen J, Hancock M, Christensen HW, Fredberg U, Boyle E, Kindt M, Brix L, Jensen TS (2022) Reliability of reporting differences in degenerative MRI findings of the lumbar spine from the supine to the upright position. Sleletal Radiol. https://doi.org/10.1007/s00256-022-04060-2
Matsumotu M, Watanabe K, Tsuji T, Ishii K, Takaishi H, Nakamura M, Toyama Y, Chiba K, Michikawa T, Nishiwaki Y (2009) Nocturnal leg cramps: a common complaint in patients with lumbar spinal canal stenosis. Spine 34(5):E189-94. https://doi.org/10.1097/BRS.0b013e31818f953c
Suzuki K, Takatsu T, Inoue H, Teramato T, Ishida Y, Ohmori K (1992) Redundant nerve roots of the cauda equina caused by lumbar spinal canal stenosis. Spine 17(11):1337–1342
Poureisa M, Daghighi MH, Efthekari P, Bookani KR, Fouladi DF (2015) Redundant nerve roots of the cauda equina in lumbar spinal canal stenosis, an MRI study on 500 cases. Eur Spine J 24(10):2315–2320. https://doi.org/10.1007/s00586-015-4059-y
Min JH, Jang JS, Lee SH (2008) Clinical significance of redundant nerve roots of the cauda equina in lumbar spinal stenosis. Clin Neurol Neurosurg 110(1):14–18. https://doi.org/10.1016/j.clineuro.2007.08.05
Duncan AW, Kido DK (1981) Serpentine cauda equina nerve roots. Radiology 139(1):109–111. https://doi.org/10.1148/radiology.139.1.7208911
Marques CJ, Hillebrand H, Papavero L (2018) The clinical significance of redundant nerve roots of the cauda equina in lumbar spinal stenosis patients: a systematic literature review and meta-analysis. Clin Neurol Neurosurg 174:40–47. https://doi.org/10.1016/j.clineuro.2018.09.001
Yoon LJ, Moon BG, Bae IS, Kang HI, Kim JH, Kim DR (2021) Association between redundant nerve root and clinical outcome after fusion for lumbar spinal stenosis. Asian J Pain 7:5. https://doi.org/10.35353/ajp.2021.00038
Papavero L, Marques CJ, Lohmann J, Fitting T, Schawjinski K, Ali N, Hillebrand H, Maas R (2019) Redundant nerve roots in lumbar spinal stenosis: inter- and intra-rater reliability of an MRI-based classification. Neuroradiology 62:223–230. https://doi.org/10.1007/s00234-019-02337-3
Kottner J, Audige L, Brorson S, Donner A, Gejewski BJ, Hrobjartsson A, Roberts C, Shoukri M, Streiner DL (2011) Guidelines for Reporting Reliability and Agreement Studies (GRRAS) were proposed. Int J Nurs Stud 48(6):661–671. https://doi.org/10.1016/j.ijnurstu.2011.01.016
Schizas C, Theumann N, Burn A, Tansey R, Wardlaw D, Smith FW, Kulik G (2010) Qualitative grading of severity of lumbar spinal stenosis based on the morphology of the dural sac on magnetic resonance images. Spine 35(21):1919–1924. https://doi.org/10.1097/BRS.0b013e3181d3559bd
Hogan Q (1996) Size of human lower thoracic and lumbosacral roots. Anesthesiology 85(1):37–42. https://doi.org/10.1097/00000542-199607000-00005
Schoenstroem NS, Bolender NF, Spengler DM (1985) The pathomorphology of spinal stenosis as seen on CT scans of the lumbar spine. Spine 10(9):806–811
Schoenstroem N, Hansson T (1988) Pressure changes following constriction of the cauda equina: an experimental study in situ. Spine 13(4):385–388
Martin BA (2009) Loth F (2009) The influence of coughing on cerebrospinal fluid pressure in an in vitro syringomyelia model with spinal subarachnoid space stenosis. Cerebrospinal Fluid Res 6:17. https://doi.org/10.1186/1743-8454-6-17
Marin-Sanabria EA, Sih IM, Tan KK, Tan JSH (2007) Mobile cauda equina schwannomas. Singapore Med J 48(2):e53–e56
Yang SM, Park HK, Cho SJ, Chang JC (2013) Redundant nerve roots of cauda equina mimicking intradural disc herniation: a case report. Korean J Spine 10(1):41–43. https://doi.org/10.14245/kjs.2013.10.1.41
Hur JW, Hur JK, Kwon TH, Park YK, Chung HS, Kim JH (2012) Radiological significance of ligamentum flavum hypertrophy in the occurrence of redundant nerve roots of central lumbar stenosis. J Korean Neurosurg Soc 52(3):215–220
Chen J, Wang J, Wang B, Xu H, Lin S, Zhang H (2016) Post-surgical functional recovery, lumbar lordosis, and range of motion associated with MRI-detectable redundant nerve roots in lumbar spinal stenosis. Clin Neurol Neurosurg 140:79–84. https://doi.org/10.1016/j.clineuro.2015.11.016
Madsen R, Jensen TS, Pope M, Sørensen JS, Benidx T (2008) The effect of body position and axial load on spinal canal morphology. Spine 33(1):61–67
Acknowledgements
The authors thank former patient Mr. Dieter Witt for sponsoring Open Access and the English editing of the manuscript performed by Editage (www. editage.com). The authors acknowledge the support of Holger Frey.
Funding
The authors did not receive support from any organization for the submitted work.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by SE, LP, NA, KS, AH, and RM. The first draft of the manuscript was written by LP and NA. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
We declare that we have no conflict of interest.
Ethics approval
According to the Ethics Committee of the Federal State of Hamburg, Germany, retrospective observational studies do not require approval and patient informed consent whenever the data are required, saved, and treated anonymously (WF-198/20). This applies to the present study.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Luca Papavero and Nawar Ali are shared first authors.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Papavero, L., Ali, N., Schawjinski, K. et al. The prevalence of redundant nerve roots in standing positional MRI decreases by half in supine and almost to zero in flexed seated position: a retrospective cross-sectional cohort study. Neuroradiology 64, 2191–2201 (2022). https://doi.org/10.1007/s00234-022-03047-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-022-03047-z