Abstract
Purpose
The neurotropism of SARS-CoV-2 and the consequential damage to the olfactory system have been proposed as one of the possible underlying causes of olfactory dysfunction in COVID-19. We aimed to aggregate the results of the studies which reported imaging of the olfactory system of patients with COVID-19 versus controls.
Methods
PubMed and EMBASE were searched to identify relevant literature reporting the structural imaging characteristics of the olfactory bulb (OB), olfactory cleft, olfactory sulcus (OS), or olfactory tract in COVID-19 patients. Hedge’s g and weighted mean difference were used as a measure of effect size. Quality assessment, subgroup analyses, meta-regression, and sensitivity analysis were also conducted.
Results
Ten studies were included in the qualitative synthesis, out of which seven studies with 183 cases with COVID-19 and 308 controls without COVID-19 were enrolled in the quantitative synthesis. No significant differences were detected in analyses of right OB volume and left OB volume. Likewise, right OS depth and left OS depth were also not significantly different in COVID-19 cases compared to non-COVID-19 controls. Also, we performed subgroup analysis, meta-regression, and sensitivity analysis to investigate the potential effect of confounding moderators.
Conclusion
The findings of this review did not confirm alterations in structural imaging of the olfactory system, including OB volume and OS depth by Covid-19 which is consistent with the results of recent histopathological evaluations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neurotropism is reported to be a distinct feature of coronaviruses, including severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which causes coronavirus disease 2019 (COVID-19) first emerged in Wuhan, China, in December 2019. COVID-19 became a global pandemic over several months, causing considerable morbidity and mortality worldwide [1, 2]. Olfactory dysfunction (OD) presenting as hyposmia or anosmia is one of the distinguishing symptoms of COVID-19, which may even play a diagnostic role in cases with an isolated sudden loss of smell without other known etiologies [3]. OD in COVID-19 is often accompanied by taste disorders. However, unlike other upper respiratory infections (URI), it is not associated with nasal discharge and conductive obstruction of the olfactory cleft (OC) and is rather suggested to have a neurological origin [4]. Different investigations have shown a heterogeneous range of olfactory involvement in various populations, with COVID-19 indicating a 5 to 85% prevalence, mostly in mild cases [5].
The olfaction process is initiated by converting a chemical stimulus, an odorant, into an electrical signal by G-protein coupled receptors on dendrites of first-order olfactory sensory neurons, which lie at the roof of the nasal cavity adjacent to the supporting epithelium. The olfactory nerves send produced action potential through the cribriform plate towards the olfactory bulb (OB) and higher-level areas such as the pyriform cortex and amygdala that interpret olfactory information [6]. It has been proposed that SARS-CoV-2 enters the olfactory sensory neurons in the nasal cleft olfactory mucosa and continues its way through the cribriform plate to reach secondary olfactory neurons in the OB [3, 7]. The two OBs are neural structures that, along with the olfactory tracts (OT), which link the OBs to the brain, are located in the olfactory sulcus (OS) in the inferior medial portion of the frontal lobe adjacent to the gyrus rectus [8]. A recent systematic review showed a significantly higher prevalence of OC opacification in patients with COVID-19 infection than in controls. Several studies have also reported other findings, such as alterations in OB volume, OC volume, OS depth, and olfactory nerve morphology [9].
However, an alternative theory argues against the olfactory route to brain infection. SARS-CoV-2 enters host cells by binding the spike proteins (S) to host cell receptor, i.e., angiotensin-converting enzyme 2 (ACE2), and S protein priming by cellular protease, i.e., transmembrane protease serine 2 (TMPRSS2) [10]. Results of several animal and human histological studies deny the expression of ACE and TMPRSS2 on mature olfactory sensory neurons [11, 12]. These studies stated that SARS-CoV-2 does not infect the sensory neurons, and sustentacular cells are the major target of this virus in the olfactory mucosa.
It is well-documented that magnetic resonance (MR) imaging is reliable for evaluating the olfactory system, especially in the traumatic, infectious, metabolic, psychiatric, and neurodegenerative disorders leading to OD [13,14,15,16,17]. Additionally, it can provide a tool to differentiate with limitations between infectious and neurodegenerative conditions and predict the prognosis [18]. Due to the promising potential of imaging technologies in OD cases which have been implemented in such disorders as multiple sclerosis, Parkinson’s, and Alzheimer’s disease [19,20,21], and the recent advances in the imaging techniques like MR surface-coil scans in the evaluation of olfactory bulb and tract injuries [22], we aimed to aggregate the results of the studies which conducted imaging on COVID-19 cases to evaluate the impact on the olfactory system. By doing so, we hoped to draw possible conclusions that could elucidate the underlying mechanism of OD in these patients.
Methods
We performed this systematic review in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [23]. Two authors developed and submitted the study protocol to the International Prospective Register of Systematic Reviews (PROSPERO) website (Registration No. CRD42021285434).
Search strategy
We primarily searched PubMed and Scopus up to October 15, 2021. The search was updated on March 22, 2022. The search strategy for different databases is provided in Table S1. Two authors independently performed the title/abstract screening. Relevant articles were then retrieved to find included studies. Discrepancies were solved through a mutual discussion with a third reviewer. Publication time, language, modality of imaging, and age group of participants were not restricted. The references of included studies were screened to find additional relevant articles.
Eligibility criteria
Studies reporting the structural imaging characteristics of the olfactory bulb, olfactory cleft, olfactory sulcus, or olfactory tract in SARS-CoV-2 patients, which fulfilled the following criteria, were included in this systematic review and meta-analysis: (1) observational studies, including case–control, cohort, and cross-sectional designs in the human population; (2) included a group of non-SARS-CoV-2 controls; (3) reported the suitable outcomes using mean and standard deviation (SD). Studies with the following criteria were excluded: (1) reviews, opinions, book chapters, commentaries, animal studies, and conference papers; (2) reported irrelevant outcomes; (3) not original (4) lacking a control group. Two reviewers independently assessed and selected the studies according to the aforementioned criteria. Discrepancies were solved through discussion with a third reviewer.
Data collection and extraction
Two reviewers extracted data on main outcomes, including the first author’s name, year of publication, study location, study design, inclusion and exclusion criteria for cases and controls, matching of cases and controls, modality of imaging, the strength of magnetic field (for MR imaging studies), olfactory function test used to assess the olfactory dysfunction, characteristics of study population including sample size, race, mean age, percentage of males, and percentage of anosmic and hyposmic participants, and mean disease duration of cases. A third reviewer assessed the quality of data extraction. Authors of included studies were contacted in the case of any missing data.
Quality assessment
Two authors separately assessed the methodological quality of studies using the Newcastle–Ottawa scale (NOS), which is recommended by the Cochrane Collaboration [24, 25]. The NOS allocates stars to three main aspects of selection, comparability, and exposure as a means for quality assessment. A maximum of eight stars (four stars for selection, two for comparability, and two for exposure) can be allocated to case–control studies. Discrepancies were solved through discussion with a third reviewer.
Statistical analysis
We performed the quantitative data analysis using Stata version 16 software (StataCorp, College Station, TX) on the structural imaging characteristics of the olfactory bulb, olfactory sulcus, olfactory cleft, and olfactory tract in SARS-CoV-2 patients. Studies with at least 5 participants in each study group were included in the quantitative synthesis. We calculated the effect sizes using the mean and standard deviation levels of outcomes. We reported weighted mean difference (WMD) and Hedge’s g, as a measure of standardized mean difference (SMD), with the corresponding confidence interval (CI). Subgroup meta-analysis and meta-regression were performed for variables that could potentially affect the overall effect sizes and were reported in a sufficient number of studies. The random-effects model and fixed-effects model were interchangeably used to calculate the effect sizes. We used Higgin’s I2 test and Cochran’s Q test to measure between-study heterogeneity. Cochrane manual suggests that the I2 of less than 40% corresponds to a low level of heterogeneity. Therefore, we used the fixed-effects model in the case that the between-study heterogeneity measured by I2 was below 40% and the random-effects model if I2 was over 40%. A P-value of less than 0.05 was considered to be statistically significant.
Also, we used funnel plots and Egger’s test to explore the publication bias in included studies. The funnel plot is used as a qualitative and visual measure of publication bias, and the asymmetry of this plot implies publication bias, while Egger’s test is the quantitative measure of publication bias [26]. In the case that publication bias was proved using these two methods, we used the trim-and-fill method to adjust the effect sizes.
Results
Study selection
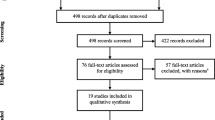
As presented in Fig. 1, the study selection process began with the identification of 914 articles in the electronic databases (PubMed = 172, Scopus = 742), from which 126 duplicate records were removed. The remaining 788 studies underwent screening on the basis of their title and abstract, resulting in the exclusion of 745 articles. After the exclusion of two studies for which full-text manuscripts were not available, 41 possibly relevant articles were further evaluated thoroughly based upon full-text manuscripts leading to the exclusion of 31 other studies for the following reasons: irrelevant to the subject (n = 4), non-original studies (n = 8), case reports (n = 5), not reporting the desired variables (n = 6), and lack of a control group (n = 8). Discrepancies occurred during the study selection process for six studies [27,28,29,30,31,32]. After discussing with a third reviewer, one of these studies was included [31], and the rest were excluded. Ten studies were included for the narrative synthesis of the current review [4, 5, 31, 33,34,35,36,37,38,39]. Two of the included studies were excluded from the quantitative analysis due to the low number of participants in the control group [31, 38]. Also, the only variable measured in the study of Tsivgoulis et al. was only reported in one study, and thus, we were not able to include this study in the meta-analysis [36]. Therefore, seven studies with 491 participants (183 cases with COVID-19 and 308 controls without COVID-19) were included in the meta-analysis [4, 33,34,35,36,37, 39].
Study characteristics and quality assessment
The summary of demographic and methodologic characteristics for each included study can be found in Table 1. The most frequently reported structural measurements were right OB volume and left OB volume with six studies [4, 33,34,35, 37, 39], right OS depth and left OS depth with five studies [4, 33, 34, 37, 39], and signal intensity of OB with three studies [31, 38, 39]. Quality assessment of the included studies by the NOS scale indicated a fair status for most of the articles, with the total score ranging from two to seven (Table 2).
Olfactory bulb
Six studies with 175 COVID-19 cases and 300 non-COVID-19 controls were analyzed for the comparison of mean right and mean left OB volumes between the mentioned groups [4, 33,34,35, 37, 39]. These showed that mean right OB volume was not significantly different between those with COVID-19 and those without (SMD, − 0.494; 95% CI, − 1.372 to 0.383; P = 0.2698; I2 = 94.56%; PQ < 0.0001; Fig. 2; Table 3). According to Egger’s test, there was no evidence of publication bias among the studies (P = 0.8480). The analysis of the mean left OB volume also demonstrated no significant difference between COVID-19 patients with OD and non-COVID-19 controls (SMD, − 0.535; 95% CI, − 1.437 to 0.367; P = 0.2451; I2 = 94.82%; PQ < 0.0001; Fig. 3; Table 3). The result of Egger’s test for this analysis was not indicative of any publication bias (P = 0.8332).
Forrest plot of the meta-analysis for right olfactory bulb volume between COVID-19 cases and non-COVID-19 controls. The meta-analysis was conducted with a random-effects model. The size of the square for each article demonstrates the attributed weight, and the horizontal line indicates the 95% confidence interval (CI). The diamonds show the standardized mean difference, and their width represents the 95% CI. Abbreviations: N, number of subjects; SD, standard deviation
Forrest plot of the meta-analysis for left olfactory bulb volume between COVID-19 cases and non-COVID-19 controls. The meta-analysis was conducted with a random-effects model. The size of the square for each article demonstrates the attributed weight, and the horizontal line indicates the 95% confidence interval (CI). The diamonds show the standardized mean difference, and their width represents the 95% CI. Abbreviations: N, number of subjects; SD, standard deviation
Only the study by Tsivgoulis et al. reported mean bilateral OB height [36]. They stated that mean bilateral OB height was significantly lower in patients with persistent OD attributable to COVID-19 than in healthy controls (P = 0.004).
Chetrit et al. revealed that the OB signal intensity ratio on coronal T2/FLAIR images was significantly higher in the COVID-19 cases with OD than in the normosmic patients with COVID-19 (P < 0.001) [38]. Likewise, Aragão et al. reported five cases of COVID-19 scanned by MR imaging with the post-contrast enhancement of both OB in three of them (two of which had documented anosmia), post-contrast enhancement of left OB in one patient (without anosmia), and finally pre- and post-contrast hyperintensity suggestive of microbleeding (methemoglobin) in the left OB in another patient which had anosmia [31]. In the study by Yildirim et al., increased signal intensity was detected in 51.6% of patients with COVID-19-related OD (n = 16) and 46.4% of patients with OD due to other URIs (n = 45), but there was no significant difference between these groups [39]
Olfactory sulcus
Mean right and mean left OS depths have been evaluated in five studies with pooled population of 436 individuals (155 COVID-19 cases and 281 non-COVID-19 controls) [4, 33, 34, 37, 39]. There was no statistically significant difference in right OS depth (SMD, − 0.237; 95% CI, − 0.697 to 0.223; P = 0.3122; I2 = 80.12%; PQ = 0.005; Fig. 4; Table 3) between COVID-19 cases and non-COVID-19 controls. Hedge’s analysis on left OS depth showed a statistical insignificance for the difference between two mentioned groups (SMD, − 0.360; 95% CI, − 0.798 to 0.079; P = 0.1078; I2 = 64.22%; PQ = 0.0126; Fig. 5; Table 3) and the result of WMD was also indicative of a borderline insignificance reduced left OS depth in COVID-19 group (WMD, − 0.405 mm; 95% CI, − 0.812 to 0.002 P = 0.0514; Table 3).
Forrest plots of the meta-analysis for right olfactory sulcus depth between COVID-19 cases and non-COVID-19 controls. The meta-analysis was conducted with a random-effects model. The size of the square for each article demonstrates the attributed weight, and the horizontal line indicates the 95% confidence interval (CI). The diamonds show the standardized mean difference, and their width represents the 95% CI. Abbreviations: N, number of subjects; SD, standard deviation
Forrest plots of the meta-analysis for left olfactory sulcus depth between COVID-19 cases and non-COVID-19 controls. The meta-analysis was conducted with a random-effects model. The size of the square for each article demonstrates the attributed weight, and the horizontal line indicates the 95% confidence interval (CI). The diamonds show the standardized mean difference, and their width represents the 95% CI. Abbreviations: N, number of subjects; SD, standard deviation
Olfactory cleft
In the study by Altundag et al. [4], mean right and mean left OC widths and total bilateral OC volume were measured and compared between three distinct groups of patients comprising (1) OD cases due to COVID-19, (2) OD cases due to other URI, and (3) normosmic healthy controls. Findings of this study denoted that the mean widths of the right and left OC and the total bilateral volume of OC were significantly increased in groups 1 and 2 compared to healthy controls (all P values < 0.001). This study also revealed that the mean T2 signal intensity of the OC area (i.e., OC mucosa along the height extending from the cleft top to 10 mm inferior) was significantly higher than healthy controls (P = 0.001). Furthermore, another study using CT scan imaging found that mean bilateral OC width and total bilateral OC volume were significantly increased in COVID-19 patients with OD in comparison to COVID-19 patients without OD (both P values < 0.001) [5].
Olfactory tract
Altunisik et al. demonstrated that both mean right and mean left OT lengths were significantly lower in patients diagnosed with COVID-19 and persistent OD than in normosmic healthy controls (P < 0.001 and P = 0.003, respectively) [34].
Meta-regression
Univariate meta-regression was conducted on the OB volume and OS depth measurements to assess the influence of probable confounding factors on the results of meta-analyses. As presented in Table 4, the number of participants in the case groups, i.e., the patients with COVID-19, had a significantly negative impact on the analysis results of mean right OB volume (β, − 0.0954477; P = 0.044), mean left OB volume (β, − 0.1033219; P = 0.025), mean right OS depth (β, − 0.0637904; P < 0.001), and mean left OS depth (β, − 0.0534206; P = 0.023). Likewise, the mean age of individuals in the case groups was also found to be significantly related to the effect sizes derived for differences in mean right OB volume (β, − 0.4169846; P < 0.001), mean left OB volume (β, − 0.4641772; P < 0.001), mean right OS depth (β, − 0.2169808; P = 0.010), and mean left OS depth (β, − 0,207,627; P = 0.002) between cases and controls.
Subgroup analysis
Subgroup analyses were done in search of the suspected effect of interstudy variabilities on analyzed measurements, including mean right and mean left OB volumes and OS depths.
Trait of cases and controls
In the subgroup analysis based on the trait of case groups, no significant difference was detected between the results of the study, which included patients with COVID-19 disregarding the presence of OD with the results of studies that included patients with OD due to COVID-19 (Fig. S1). Moreover, the results of analyses according to the trait of control groups demonstrated that in the studies which recruited individuals without COVID-19 and OD (P = 0.03) unlike studies with OD due to URI other than COVID-19 as controls, patients with COVID-19 had significantly lower right OB volume than controls (Fig. 6). Moreover, left OB volume was significantly increased in cases compared to controls in the study that had patients with OD due to URI other than COVID-19 as controls (P = 0.002; Fig. 7). Nonetheless, there were no differences in the results of right and left OS depths between studies based on the trait of controls (Fig. S2).
Subgroup analysis of the difference in the mean right olfactory bulb volume between patients with Covid-19 and non-Covid-19 controls according to trait of controls. The meta-analysis was conducted with a random-effects model. The size of the square for each article demonstrates the attributed weight, and the horizontal line indicates the 95% confidence interval (CI). The diamonds show the standardized mean difference, and their width represents the 95% CI. Abbreviations: N, number of subjects; SD, standard deviation
Subgroup analysis of the difference in the mean left olfactory bulb volume between patients with Covid-19 and non-Covid-19 controls according to trait of controls. The meta-analysis was conducted with a random-effects model. The size of the square for each article demonstrates the attributed weight, and the horizontal line indicates the 95% confidence interval (CI). The diamonds show the standardized mean difference, and their width represents the 95% CI. Abbreviations: N, number of subjects; SD, standard deviation
MR imaging field strength
There were no significant differences in OB volumes and OS depths between studies that used 3 T MR imaging devices and studies that applied 1.5 T MR imaging devices except for left OB volume (Fig. 8). Unlike the overall result and 3 T subgroup, studies with 1.5 T MR imaging devices showed significantly decreased left OB volume in COVID-19 cases compared to controls (P = 0.03; Fig. S3).
Subgroup analysis of the difference in the mean left olfactory bulb volume between patients with Covid-19 and non-Covid-19 controls according to strength of magnetic field. The meta-analysis was conducted with a random-effects model. The size of the square for each article demonstrates the attributed weight, and the horizontal line indicates the 95% confidence interval (CI). The diamonds show the standardized mean difference, and their width represents the 95% CI. Abbreviations: N, number of subjects; SD, standard deviation
Study location
No significant difference was detected for right and left OB volumes in the studies with different locations (Fig. S4).
Adjustment for age
Adjustment for the age did not make any significant difference between the result of studies reporting right and left OB volumes (Fig. S5).
Sensitivity analysis
Sensitivity analysis was performed for each measurement to explore the potential source of heterogeneity by excluding studies one at a time and redoing the corresponding analysis. No change was found in the results of mean right and mean left OB volumes and mean right and mean left OS depths, as differences were still insignificant (Tables S2 and S3).
Discussion
Along with the classic signs and symptoms of a viral URI, such as cough, fever, sore throat, and headache, SARS-CoV-2 has been shown to cause unconventional symptoms like anosmia and ageusia [3]. Primary studies on COVID-19 patients were in favor of SARS-CoV-2 neurotropism, and detection of this virus and its receptors, i.e., ACE2 and TMPRSS2, in the CNS inspired researchers to consider the probable passage of the virus through olfactory neurons and consequential damage to the olfactory system as the main underlying cause of OD in COVID-19 [33, 40]. However, later evidence did not support this scenario and suggested that olfactory neurons, which provide a direct pathway to the CNS via anterograde axonal transport, lack the essential entry proteins for SARS-CoV-2 [12]. A recent study on the hamster olfactory epithelium and a postmortem investigation on olfactory mucosae of COVID-19 patients failed to detect the infection of olfactory neurons by the virus [11, 41]. Likewise, the results of the current study, which pooled the findings of studies reporting OB volume and OS depth (for which at least two studies gave the related measurements), revealed no significant difference between COVID-19 cases vs. non-COVID-19 controls in analyses of mean right OB volume and mean left OB volume as well as mean right OS depth and mean. Only a single study has published results for each of the other variables, including OC width and volume, OB height, and OT length; thus, no meta-analysis was conducted for them.
Our results did not indicate any significant difference between OB volume and OS depth. However, the study by Güney et al. evaluating the patients with OD due to COVID-19 in the chronic period (10–12 months) and the study by Altunisik et al. on the patients with COVID-19-associated OD, which persisted after improvements in the COVID-19’s typical symptoms both reported significantly decreased mean right and mean left OB volumes and decreased mean right and mean left OS depths. These two studies ascribed the findings to the direct damage of the SARS-CoV-2 to the OB and adjacent structures as well as neuroinflammation secondary to neuroglial response [33, 34]. In the study by Kandemirli et al., which compared OB volume and OS depth values of 23 COVID-19-associated OD cases with the standard cut-offs in healthy individuals, 43.5% of cases had a decrease in OB volume, and 60.9% had a decrease in OS depth [42]. On the other hand, Laurendon et al. reported an increase in OB volumes secondary to inflammation and edema in a case with COVID-19-related anosmia. It was demonstrated that altered signal intensities and volumes of the same returned to normal levels 24 days later [43].
One study showed hyperintensity in OB of five patients with COVID-19 on the thin slices of pre and/or postgadolinium fat-suppressed T1WI that may suggest olfactory damage [31]. In another study investigating cerebral microstructural changes in COVID-19 patients with a 3-month follow-up, it was suggested that the volume alterations in the central olfactory areas are in favor of an OB-mediated neuronal retrograde pathway for SARS-CoV-2 [29]. In a recent study, Chetrit et al. found that the OB signal intensity on coronal 3D-FLAIR sequence images was significantly higher in COVID-19 cases with OD than in normosmic patients with COVID-19 [38]. Furthermore, increased T2 FLAIR signal intensity of OB has been detected in COVID-19-related OD compared to non-COVID-19 anosmic controls by a recent study [29]. A recent meta-analysis showed that 88% of patients with OD due to COVID-19 had signal abnormalities in OB, which were not significantly different from controls (94%) [9]. It can be speculated that diversity in the results stems from the methodological differences in the studies, especially the applied field of strength and the time of imaging regarding the disease phase [44].
OC lined with olfactory epithelium is located in the upper part of the nasal cavity between the medial turbinate and nasal septum. Smell recognition initiates with the activation of G-protein cascades in the bipolar olfactory neurons of OC contacting odor molecules [45, 46]. Both the study of Altundag et al. comparing OD cases due to COVID-19 or other URIs with healthy controls and the study of Tekcan Sanli et al. comparing COVID-19 cases with OD and COVID-19 cases without OD revealed that patients with OD had significantly higher values for mean right and mean left OC widths and total bilateral OC volume which interestingly had inverse correlations with TDI scores of sniffin’ sticks test [4, 5]. Previous studies have demonstrated the high presence of ACE2 and TMPRSS2 in the OC region, especially supporting (sustentacular) cells of the olfactory epithelium rather than olfactory neurons [10]. A recent systematic review of 30 studies showed a pooled prevalence of 63% (95% CI, 0.38 to 0.82) for OC opacification in CT or MR imaging of COVID-19 patients with OD, which was significantly higher than the controls with 4% (95% CI, 0.01 to 0.13) [9].
It was proposed in the literature that the harmful effect of the virus on the olfactory epithelium along with damage secondary to the inflammatory response and “nasal cytokine storm “ might be the behind the development of OD in patients with COVID-19 [5]. Later histopathological evaluation of COVID-19 animal models and deceased patients found that sustentacular cells, triggering OD by insufficient support of olfactory neurons, are the major target for this virus in olfactory mucosa, and the olfactory sensory neurons and the OB are spared [11, 41]. Pulling the facts together, it can be hypothesized that olfactory system alterations in COVID-19 detected by imaging may be due to virus-induced systemic and vascular inflammation rather than direct damage of the virus to olfactory neurons [12, 31, 47, 48].
There were several limitations to the current study, mainly arising from the novelty of the subject. Small study populations along with demographic (e.g., age and nationality) and methodologic (e.g., the trait of controls and chronicity of OD) interstudy differences caused significant heterogeneity in the results of the analysis. The other limitation was the lack of comparability between cases and controls, which may bring about significant biases in the included studies as only two studies had age-matched participants [35, 36].
Conclusion
Since the recognition of OD as one of the frequent symptoms of COVID-19, several studies have used imaging tools such as MR and CT to evaluate the different structures of the olfactory system in patients with COVID-19. Even fewer have compared results to control subjects to determine possible alterations in these regions. Consistent with recent histopathological evaluations, the findings of this review did not confirm alterations in structural imaging of the olfactory system, including OB volume and OS depth by Covid-19. This may diminish the possibility of using these variables as diagnostic or prognostic indices. However, future studies of olfactory anatomy with longitudinal designs conducted with standardized protocols controlling possible confounders and biases may reveal new insights. Also, the combined application of imaging modalities with endoscopic techniques and histopathological findings of biopsy specimens of the components of the olfactory system in the COVID-19 patients might provide a better understanding of the pathophysiology of the COVID-19-mediated olfactory dysfunction.
References
Saleki K, Yaribash S, Banazadeh M et al (2021) Interferon therapy in patients with SARS, MERS, and COVID-19: a systematic review and meta-analysis of clinical studies. Eur J Pharmacol 906:174248. https://doi.org/10.1016/j.ejphar.2021.174248
Li Y-C, Bai W-Z, Hirano N et al (2012) Coronavirus infection of rat dorsal root ganglia: ultrastructural characterization of viral replication, transfer, and the early response of satellite cells. Virus Res 163:628–635. https://doi.org/10.1016/j.virusres.2011.12.021
Mehraeen E, Behnezhad F, Salehi MA et al (2021) Olfactory and gustatory dysfunctions due to the coronavirus disease (COVID-19): a review of current evidence. Eur Arch Oto-Rhino-Laryngology 278:307–312. https://doi.org/10.1007/s00405-020-06120-6
Altundag A, Yıldırım D, Tekcan Sanli DE et al (2021) Olfactory cleft measurements and COVID-19-related anosmia. Otolaryngol neck Surg Off J Am Acad Otolaryngol Neck Surg 164:1337–1344. https://doi.org/10.1177/0194599820965920
Tekcan Sanli DE, Altundag A, Yıldırım D et al (2021) Comparison of olfactory cleft width and volumes in patients with COVID-19 anosmia and COVID-19 cases without anosmia. ORL J Otorhinolaryngol Relat Spec 1–9.https://doi.org/10.1159/000518672
Pinto JM (2011) Olfaction. Proc Am Thorac Soc 8:46–52. https://doi.org/10.1513/pats.201005-035RN
van Riel D, Verdijk R, Kuiken T (2015) The olfactory nerve: a shortcut for influenza and other viral diseases into the central nervous system. J Pathol 235:277–287. https://doi.org/10.1002/path.4461
Yousem DM, Geckle RJ, Bilker WB, Doty RL (1998) Olfactory bulb and tract and temporal lobe volumes. Normative data across decades. Ann N Y Acad Sci 855:546–555. https://doi.org/10.1111/j.1749-6632.1998.tb10624.x
Tan CJ, Tan BKJ, Tan XY et al (2022) Neuroradiological basis of COVID-19 olfactory dysfunction: a systematic review and meta-analysis. Laryngoscope. https://doi.org/10.1002/lary.30078
Hoffmann M, Kleine-Weber H, Schroeder S et al (2020) SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181:271-280.e8. https://doi.org/10.1016/j.cell.2020.02.052
Khan M, Yoo S-J, Clijsters M et al (2021) Visualizing in deceased COVID-19 patients how SARS-CoV-2 attacks the respiratory and olfactory mucosae but spares the olfactory bulb. Cell 184:5932-5949.e15. https://doi.org/10.1016/j.cell.2021.10.027
Butowt R, von Bartheld CS (2021) Anosmia in COVID-19: Underlying mechanisms and assessment of an olfactory route to brain infection. Neurosci a Rev J bringing Neurobiol Neurol psychiatry 27:582–603. https://doi.org/10.1177/1073858420956905
Rombaux P, Duprez T, Hummel T (2009) Olfactory bulb volume in the clinical assessment of olfactory dysfunction. Rhinology 47:3–9
Doty RL, Yousem DM, Pham LT et al (1997) Olfactory dysfunction in patients with head trauma. Arch Neurol 54:1131–1140. https://doi.org/10.1001/archneur.1997.00550210061014
Good KP, Tourbier IA, Moberg P et al (2017) Unilateral olfactory sensitivity in multiple sclerosis. Physiol Behav 168:24–30. https://doi.org/10.1016/j.physbeh.2016.10.017
Turetsky BI, Moberg PJ, Yousem DM et al (2000) Reduced olfactory bulb volume in patients with schizophrenia. Am J Psychiatry 157:828–830. https://doi.org/10.1176/appi.ajp.157.5.828
Yousem DM, Geckle RJ, Bilker WB et al (1999) Posttraumatic smell loss: relationship of psychophysical tests and volumes of the olfactory bulbs and tracts and the temporal lobes. Acad Radiol 6:264–272. https://doi.org/10.1016/s1076-6332(99)80449-8
Duprez TP, Rombaux P (2010) Imaging the olfactory tract (cranial nerve #1). Eur J Radiol 74:288–298. https://doi.org/10.1016/j.ejrad.2009.05.065
Çullu N, Yeniçeri İÖ, Güney B et al (2020) Evaluation of olfactory bulbus volume and olfactory sulcus depth by 3 T MR. Surg Radiol Anat 42:1113–1118. https://doi.org/10.1007/s00276-020-02484-w
Doty RL, Li C, Mannon LJ, Yousem DM (1999) Olfactory dysfunction in multiple sclerosis: relation to longitudinal changes in plaque numbers in central olfactory structures. Neurology 53:880–882. https://doi.org/10.1212/wnl.53.4.880
Milardi D, Cacciola A, Calamuneri A et al (2017) The olfactory system revealed: non-invasive mapping by using constrained spherical deconvolution tractography in healthy humans. Front Neuroanat 11:32. https://doi.org/10.3389/fnana.2017.00032
Yousem DM, Geckle RJ, Bilker WB et al (1996) Posttraumatic olfactory dysfunction: MR and clinical evaluation. AJNR Am J Neuroradiol 17:1171–1179
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6:e1000097. https://doi.org/10.1371/journal.pmed.1000097
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ WV (eds) (2020) Cochrane handbook for systematic reviews of interventions version 6.1 (updated September 2020). Cochrane
Stang A (2010) Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 25:603–605. https://doi.org/10.1007/s10654-010-9491-z
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315:629–634. https://doi.org/10.1136/bmj.315.7109.629
Douaud G, Lee S, Alfaro-Almagro F et al (2022) SARS-CoV-2 is associated with changes in brain structure in UK Biobank. Nature 604:697–707. https://doi.org/10.1038/s41586-022-04569-5
Iravani B, Peter MG, Arshamian A et al (2021) Acquired olfactory loss alters functional connectivity and morphology. Sci Rep 11:16422. https://doi.org/10.1038/s41598-021-95968-7
Lu Y, Li X, Geng D et al (2020) Cerebral micro-structural changes in COVID-19 patients — an MRI-based 3-month follow-up study. EClinicalMedicine 25:100484. https://doi.org/10.1016/j.eclinm.2020.100484
Niesen M, Trotta N, Noel A et al (2021) Structural and metabolic brain abnormalities in COVID-19 patients with sudden loss of smell. Eur J Nucl Med Mol Imaging 48:1890–1901. https://doi.org/10.1007/s00259-020-05154-6
Aragão MFVV, Leal MC, Cartaxo Filho OQ et al (2020) Anosmia in COVID-19 associated with injury to the olfactory bulbs evident on MRI. AJNR Am J Neuroradiol 41:1703–1706. https://doi.org/10.3174/ajnr.A6675
Miao X, Paez AG, Rajan S et al (2021) Functional activities detected in the olfactory bulb and associated olfactory regions in the human brain using T2-prepared BOLD functional MRI at 7T. Front Neurosci 15:723441. https://doi.org/10.3389/fnins.2021.723441
Güney B, Bacaksızlar Sarı F, Özdemir MY et al (2021) Changes in olfactory bulbus volume and olfactory sulcus depth in the chronic period after COVID-19 infection. Acta Otolaryngol 141:786–790. https://doi.org/10.1080/00016489.2021.1946138
Altunisik E, Baykan AH, Sahin S et al (2021) Quantitative analysis of the olfactory system in COVID-19: an MR imaging study. AJNR Am J Neuroradiol. https://doi.org/10.3174/ajnr.A7278
Eliezer M, Hamel A-L, Houdart E et al (2020) Loss of smell in patients with COVID-19: MRI data reveal a transient edema of the olfactory clefts. Neurology 95:e3145–e3152. https://doi.org/10.1212/WNL.0000000000010806
Tsivgoulis G, Fragkou PC, Lachanis S et al (2021) Olfactory bulb and mucosa abnormalities in persistent COVID-19-induced anosmia: a magnetic resonance imaging study. Eur J Neurol 28:e6–e8
Burulday V, Sayar MS, Bayar Muluk N (2021) Peripheral and central smell regions in COVID-19 positive patients: an MRI evaluation. Acta Radiol 2841851211034043.https://doi.org/10.1177/02841851211034043
Chetrit A, Lechien JR, Ammar A et al (2020) Magnetic resonance imaging of COVID-19 anosmic patients reveals abnormalities of the olfactory bulb: Preliminary prospective study. J Infect 81:816–846
Yildirim D, Kandemirli SG, Tekcan Sanli DE et al (2022) A comparative olfactory MRI, DTI and fMRI study of COVID-19 related anosmia and post viral olfactory Ddsfunction. Acad Radiol 29:31–41. https://doi.org/10.1016/j.acra.2021.10.019
Meinhardt J, Radke J, Dittmayer C et al (2021) Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat Neurosci 24:168–175. https://doi.org/10.1038/s41593-020-00758-5
Bryche B, St Albin A, Murri S et al (2020) Massive transient damage of the olfactory epithelium associated with infection of sustentacular cells by SARS-CoV-2 in golden Syrian hamsters. Brain Behav Immun 89:579–586. https://doi.org/10.1016/j.bbi.2020.06.032
Kandemirli SG, Altundag A, Yildirim D et al (2021) Olfactory bulb MRI and paranasal sinus CT findings in persistent COVID-19 anosmia. Acad Radiol 28:28–35. https://doi.org/10.1016/j.acra.2020.10.006
Laurendon T, Radulesco T, Mugnier J et al (2020) Bilateral transient olfactory bulb edema during COVID-19-related anosmia. Neurology 95:224–225. https://doi.org/10.1212/WNL.0000000000009850
Strauss SB, Lantos JE, Heier LA et al (2020) Olfactory bulb signal abnormality in patients with COVID-19 who present with neurologic symptoms. AJNR Am J Neuroradiol 41:1882–1887. https://doi.org/10.3174/ajnr.A6751
Ao R, Wang R, Yang M et al (2018) Altered retinal nerve fiber layer thickness and choroid thickness in patients with migraine. Eur Neurol 80:130–137. https://doi.org/10.1159/000494671
Hummel T, Whitcroft KL, Andrews P et al (2017) Position paper on olfactory dysfunction. Rhinol Suppl 54:1–30. https://doi.org/10.4193/Rhino16.248
Cooper KW, Brann DH, Farruggia MC et al (2020) COVID-19 and the chemical senses: supporting players take center stage. Neuron 107:219–233. https://doi.org/10.1016/j.neuron.2020.06.032
Politi LS, Salsano E, Grimaldi M (2020) Magnetic resonance imaging alteration of the brain in a patient with coronavirus disease 2019 (COVID-19) and anosmia. JAMA Neurol 77:1028–1029. https://doi.org/10.1001/jamaneurol.2020.2125
Acknowledgements
None
Funding
Not applicable.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Informed consent
The work did not include humans and animals. Therefore, informed consent was not applicable.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mohammadi, S., Gouravani, M., Salehi, M.A. et al. Olfactory system measurements in COVID-19: a systematic review and meta-analysis. Neuroradiology 65, 25–39 (2023). https://doi.org/10.1007/s00234-022-03014-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-022-03014-8