Abstract
Purpose
Hepatic encephalopathy (HE) is a potential complication of cirrhosis. Magnetic resonance imaging (MRI) may demonstrate hyperintense T1 signal in the globi pallidi. The purpose of this study was to evaluate the performance of MRI-based radiomic features for diagnosing and grading chronic HE in adult patients affected by cirrhosis.
Methods
Adult patients with and without cirrhosis underwent brain MRI with identical imaging protocol on a 3T scanner. Patients without history of chronic liver disease were the control population. HE grading was based on underlying liver disease, severity of clinical manifestation, and number of encephalopathic episodes. Texture analysis was performed on axial T1-weighted images on bilateral lentiform nuclei at the level of the foramina of Monro. Diagnostic performance of texture analysis for the diagnosis and grading of HE was assessed by calculating the area under the receiver operating characteristics (AUROC) with 95% confidence interval (CI).
Results
The final study population consisted of 124 patients, 70 cirrhotic patients, and 54 non-cirrhotic controls. Thirty-eight patients had history of HE with 22 having an HE grade > 1. The radiomic features predicted the presence of HE with an AUROC of 0.82 (95% CI: 0.73, 0.90; P < .0001; 82% sensitivity, 66% specificity). Radiomic features predicted grade 1 HE (AUROC 0.75; 95% CI: 0.61, 0.89; P < .0001; 94% sensitivity, 60% specificity) and grade ≥ 2 HE (AUROC 0.82; 95% CI: 0.71, 0.93; P < .0001, 95% sensitivity, 57% specificity).
Conclusion
In cirrhotic patients, MR radiomic is effective in predicting the presence of chronic HE and in grading its severity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatic encephalopathy (HE) is a cerebral disorder that may occur in the setting of end-stage chronic liver disease [1], presenting with a wide spectrum of neuropsychiatric manifestations. HE is widely considered one of the most serious complications of cirrhosis, representing the initial decompensating event in approximately 20% of these patients and its presence carries a 5-year survival rate of about 20% [2]. HE is the most common cause of protracted hospitalization and readmission in cirrhotic patients and thus poses an enormous financial burden on health care costs [1, 2]. The 5-year incidence of overt HE in cirrhotic patients ranges between 5 and 25%, depending on the underlying cause of chronic liver disease and the presence of additional complications, increasing up to 50% in cirrhotic patient who have undergone transjugular intrahepatic portosystemic shunt (TIPS) for treatment of complications of portal hypertension, including refractory ascites and/or prophylaxis of variceal rebleeding [1,2,3,4,5,6].
In patients with cirrhosis and HE, a common magnetic resonance imaging (MRI) finding in chronic HE is bilateral symmetric T1 hyperintense signal in the globi pallidi and substantiae nigrae (reported in 80%–90% of patients with chronic liver failure), probably a sequela of chronic accumulation of manganese from failed hepatobiliary excretion. However, this imaging finding does not have a direct or linear correlation with serum ammonia levels and/or patient’s symptoms and it is observed in chronic stages [7]. Furthermore, bilateral T1-weighted bright signal intensity within the globi pallidi may be observed in other disorders that are not necessarily linked to elevated manganese levels [8]. Additional and more complex imaging findings may be seen in cirrhotic patients presenting with an acute encephalopathy, often triggered by a sudden exacerbation of liver dysfunction which leads to an acute elevation of serum ammonia level. These patients’ imaging studies will often demonstrate features specific to acute hyperammonemic encephalopathy superimposed on background imaging findings of chronic HE. Imaging findings of acute HE may include gyriform hyperintense FLAIR signal and corresponding restricted diffusion affecting bilateral cerebral cortices, favoring involvement of the insular cortex and the cingulate gyri and often sparing perirolandic and occipital cortex; these aspects of acute hyperammonemic cortical injury are often superimposed on a background of imaging features of chronic HE, in particular, hyperintense T1 signal in the globi pallidi [7].
Radiomics is emerging as a new quantitative imaging-based method that allows extracting from radiological images some mathematical features that cannot be perceived by human eyes [9,10,11]. Radiomics and texture-based features reflect the distribution and heterogeneity of signal intensities within a defined region of interest [12]. The extracted features can be used to create clinical decision support systems for prediction of histopathological features, prognosis, and response to therapy, thus explaining the considerable interest that precision medicine has shown in the utility of this emerging methodology [11]. Recent studies in neuroimaging have explored the potential of radiomic and MRI-based texture analysis for the assessment of brain tumors [13, 14], multiple sclerosis lesions [15, 16], and other neurodegenerative disorders [17].
The aim of this study was to evaluate the performance of MRI-based radiomic features for diagnosing the presence of chronic HE and grading its severity, in a population of adult cirrhotic patients.
Materials and methods
This retrospective study was reviewed and approved by the Institutional Research Review Board of our institution; informed consent form was waived. Informed written consent for magnetic resonance (MR) study was obtained in all subjects who underwent brain MR for clinical purposes.
Patients population
Eligible patients were identified through a retrospective search of the electronic database of our tertiary transplant center, identifying adult patients who, between October 2018 and February 2020, underwent brain MR exams, all performed with identical imaging protocol.
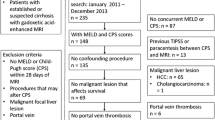
Cirrhotic patients were selected in the study group if they met the following inclusion criteria (Fig. 1): (1) diagnosis of cirrhosis; (2) brain MRI acquired on a 3T MR scanner using the same scanning protocol; and (3) available clinical information for the assessment of chronic hepatic encephalopathy. Patients were excluded from the study group if they had (1) motion artifact on axial T1-weighted images and (2) presence of transjugular intrahepatic portosystemic shunt (TIPS).
Patients without history of chronic liver disease or chronic hepatic encephalopathy undergoing brain MRI with the same MR scanner and imaging protocol were selected as the control group.
The final study population consisted of 124 patients, 70 cirrhotic patients (74% men, mean age 66 ± 8 years, range 40–86 years), and 54 non-cirrhotic controls (69% men, mean age 62 ± 13 years, range 28–81 years).
The diagnosis of cirrhosis was established by liver biopsy or by unequivocal clinical, laboratory, and morphological characteristics of the liver at computed tomography and/or magnetic resonance imaging. The clinical diagnosis of chronic hepatic encephalopathy was established by a hepatologist (with 15 year of post fellowship experience) who categorized each case according to the number and severity of encephalopathic episodes and the etiology of liver disease [18]. The severity of chronic hepatic encephalopathy was graded according to the Association of the Study of Liver Disease–European Association for the Study of the Liver Practice Guideline using the West Haven criteria (WHC) [1].
MR imaging acquisition
MR examinations were acquired with a 3T MR scanner (Discovery 750w, General Electric Healthcare, Milwaukee, WI, USA) with a 32-channel dedicated head coil. Axial, sagittal, and coronal non-enhanced and contrast-enhanced (0.1 mmol/kg gadobutrol – Gadovist, Bayer, Germany) fast-spin echo (FSE) T1-weighted (600/20 [TR ms / TE ms]) MR images with a field-of-view (FOV) of 240 mm, matrix 320 × 320, slice thickness 4 mm, intersection gap 1 mm, number of excitations 1 were acquired as a part of standard MR protocol along with axial and sagittal FSE T2-weighted (8000/90 [TR ms/TE ms]) images, and axial fluid attenuated inversion-recovery (FLAIR) (9000/150/2250 [TR ms/TE ms/TI ms]) images.
Imaging and texture analysis
MRI examinations were evaluated by a neuroradiologist (with 20 years of experience in neuroimaging) in order to further stratify cirrhotic patients according to the presence of hyperintense signal in the basal ganglia on non-contrast T1-weighted images (supplemental material 1).
Texture analysis was performed independently by a second radiologist (6 years of experience in neuroimaging and 3 years of experience with texture analysis), blinded to any patients’ clinical data. For each patient, the axial non-contrast T1-weighted images were anonymized and exported in DICOM format into a dedicated workstation for texture analysis. Images were analyzed in a random order using a freely available texture analysis software (LIFEx, version 5.10, www.lifexsoft.org). Using a single slice, axial T1-weighted image aligned to a similar orientation, the radiologist manually drew a polygonal region of interest (ROI) in bilateral lentiform nuclei at the level of the foramina of Monro. The ROI was placed within the margins of the lentiform nuclei including the largest possible area but carefully avoiding calcifications. None of the subjects presented basal ganglia structural lesion on MRI, apart from incidental mineralization.
A total of 43 texture features (supplemental material 2) were automatically extracted from the software, including first-order features calculated from analysis of the gray level distribution within a defined ROI without considering spatial relations among pixels and second-order features that considered the spatial relationship among pixels. In particular, first-order parameters included conventional (mean, standard deviation) and histogram (reflecting the histogram distribution of pixel intensities). Second-order features included gray level co-occurrence matrix (GLCM) that took into account the arrangements of pairs of pixels to calculate textural indices; gray-level run length matrix (GLRLM); quantified consecutive pixels with the same intensity along specific directions; neighborhood gray-level different matrix (NGLDM), accounting for the difference of gray levels between one pixel and its eight neighbors; and grey-level zone length matrix (GLZLM), providing information on the size of homogeneous zones for each grey-level in different dimensions.
The detailed formula of each texture features has been extensively described in the freely available LifeX online manual [19].
Computational statistical analysis
Data summarized as continuous variables were expressed as mean and standard deviation (SD), and categorical variables were expressed as numbers and percentages.
Correction for multiple comparisons to achieve a false discovery rate of 5% was performed using the Benjamini–Hochberg procedure [20] and the corrMatching function under MatLab software package version 2019b (The MathWorks Inc., Natick, MA, USA) that allowed one to obtain a maximally normalized Fischer Z-transformation cross-correlation texture analysis parameters with each other in both directions [21]. This procedure allows to select the most discriminative texture features, avoiding repetition of multiple features with high similarity or with incidental statistical significance. Correlation matrix was used in order to identify the combination of the most discriminative features able to differentiate each of the following condition: (1) cirrhotic patients versus controls; (2) the presence of HE in cirrhotic patients; (3) the grade of HE in cirrhotic patients (grade 1 vs grade > 2); and (4) the value of the T1-weighted hyperintensity in the globi pallidi in predicting the presence of HE in cirrhotic patients.
Multiparametric logistic regression models and classifiers were tested using as the dependent variable the presence of each condition, as covariates the combination of the most discriminative texture analysis features previously selected. The area under the receiver operating characteristics (AUROC) with 95% confidence interval (CI) and p value were calculated. Cutoff values for the identification of each condition with the combined features were calculated by means of the highest Youden index with the relative sensitivity, specificity, accuracy, positive predictive value (PPV), and negative predictive value (NPV) values.
Statistical analysis was conducted using SPSS Statistics software package version 25 (SPSS, Chicago, USA) and the ROC curve fitting function version 2 under MatLab [22].
Results
Patients’ characteristics
Baseline characteristics of the patients included are summarized in Table 1
Clinical indications for brain MRI in cirrhotic patients included pre-transplant evaluations in 52 (74%) patients, assessment of changes in the SI in the globi pallidi related to chronic HE in 8 (12%) patients, and other neurological manifestations in 10 patients (14%). At clinical examination, chronic HE was confirmed in 38 (54%) of cirrhotic patients. HE was classified as grade 1 in 16 (23%) patients, while 22 (32%) had HE grade ≥ 2. Episodic, recurrent, and persistent HE was present in 14 (20%), 11 (16%), and 13 (19%) patients, respectively.
Clinical indications for brain MRI in patients without history of chronic liver disease or hepatic encephalopathy included memory disturbance in 6 (11%) patients, migraine in 11 (20%) patients, chronic hypoxic-ischemic encephalopathy in 31 (57%) patients, optic neuropathy in 2 (4%) patients, and cortical laminar necrosis in 4 (8%) patients.
Diagnostic performance of T1-weighted hyperintensity in basal ganglia
Hyperintense signal in the basal ganglia (globus pallidus) on T1-weighted imaging was qualitatively reported in 34 (49%) patients with cirrhosis. The hyperintense signal on T1-weighted imaging demonstrated a fair diagnostic performance for the identification of cirrhotic patients with an AUROC of 0.75 (95% CI: 0.65, 0.85; P < 0.0001; sensitivity 66%; specificity 78%) (Fig. 2a). However, the hyperintense signal on T1-weighted imaging was not a statistically significant discriminator for the presence of HE in cirrhotic patients (AUROC 0.65; 95% CI: 0.53, 0.75; P > 0.05) (Fig. 2b).
(a) Graph representing the AUROC for the T1-weighted hyperintense signal of the globi pallidi as predictor of cirrhotic patients. The T1-weighted hyperintense signal of the globi pallidi was statistically significant discriminator for identification of cirrhotic patients (AUROC = 0.75; 95% CI: 0.65, 0.85; P < .0001). (b) Graph represents the AUROC for the T1-weighted hyperintense signal of the globi pallidi in discriminating the presence of chronic HE in cirrhotic patients. The T1-weighted hyperintense signal of the globi pallidi was not statistically significant discriminator for the presence of chronic HE in cirrhotic patients (AUROC 0.65; 95% CI: 0.53, 0.75; P > .05). AUROC, area under the receiver operator characteristics curve; CI, confidence intervals
Diagnostic performance of texture-based models
Eight uncorrelated texture-based features (GLRLM_GLNU, GLRLM_LGRE, GLRLM_SRHGE, GLRLM_SRLGE, GLZLM_GLNU, GLZLM_LGZE, GLZLM_SZLGE, GLZLM_ZP) were selected with the method described which avoids repetition of multiple features with high similarity or with incidental statistical significance. The combination of these eight texture-based features were significant predictors of cirrhosis and hepatic encephalopathy.
Performance of multiparametric logistic regression models is reported in Table 2. Radiomic-based model demonstrated an excellent diagnostic performance for the identification of cirrhotic patients with an AUROC of 0.97 (95% CI: 0.94, 0.1, P < 0.0001) (Fig. 3a). A cutoff value > 0.5 selected by means of the highest Youden index had a sensitivity of 94% and a specificity of 91% for the prediction of HE. The radiomic model provided a good performance for the diagnosis of HE in cirrhotic patients with an AUROC of 0.82 (95% CI: 0.73, 0.90; P < 0.0001) (Fig. 3b). A cutoff value > 0.37 had a sensitivity of 82% and a specificity of 66% for the prediction of HE.
(a) Graph representing the AUROC for radiomics-based model for prediction of cirrhotic patients having a statistically significant high prediction value (AUROC = 0.97; 95% CI: 0.94, 0.1, P < .0001). (b) Graph representing the AUROC for radiomics-based model for the prediction of chronic HE cirrhotic patients. The radiomic-based model showed a significant prediction value in discriminating the presence of chronic HE in cirrhotic patients (AUROC of 0.82; 95% CI: 0.73, 0.90; P < .0001). AUROC, area under the receiver operator characteristics curve; CI, confidence intervals
When considering the HE grades, the performance of radiomic-based model was fair for the diagnosis of grade 1 HE (AUROC 0.75; 95% CI: 0.61, 0.90; P < 0.0001) and good for grade ≥ 2 HE (AUROC 0.82; 95% CI: 0.71, 0.93; P < 0.0001) (Fig. 4). The sensitivity and specificity for the diagnosis of grade 1 HE were 94% and 60%, respectively, using the optimal cutoff value > 0.13; while for the diagnosis of grade ≥ 2 HE, the sensitivity and specificity were 95% and 57% respectively using the optimal cutoff value > 0.08.
Graph representing the AUROC of radiomics-based model for the diagnosis of grade 1 chronic HE (red curve) and grade ≥ 2 chronic HE (blue curve) in cirrhotic patients. The performance of radiomics-based model for the diagnosis of grade 1 chronic HE had an AUROC of 0.75 (95% CI: 0.61, 0.90; P < .0001) and an AUROC of 0.82 (95% CI: 0.71, 0.93; P < .0001) for grade ≥ 2 chronic HE. Radiomics-based model was significantly predictive for both grade 1 chronic HE and grade ≥ 2 chronic HE in cirrhotic patients. AUROC, area under the receiver operator characteristics curve; CI, confidence intervals; HE, hepatic encephalopathy
When considering the patients Model for End-Stage Liver Disease (MELD) score, the radiomic model performed well with an AUROC 0.94 (95% CI: 0.9, 0.1; P < 0.0001) (Fig. 5) with a sensitivity of 91% and a specificity of 81% when using the optimal cutoff of > 9.7.
Graph representing the AUROC of radiomics-based model as predictor of MELD score. The radiomics-based model provided significant performance as predictor of MELD score in cirrhotic patients with an AUROC of 0.94 (95% CI: 0.9, 0.98; P < .0001). AUROC, area under the receiver operator characteristics curve; CI, confidence intervals; MELD, Model for End-Stage Liver Disease
Discussion
Our study demonstrates that MRI-based texture-analysis radiomic models may provide clinically valuable information for the diagnosis and staging of chronic HE in cirrhotic patients. The combination of texture features extracted on T1-weighted imaging was highly accurate in predicting the presence of cirrhosis (AUROC of 0.97) and moderately accurate for predicting the presence of chronic HE (AUROC of 0.82). To the best of our knowledge, this is the first study investigating the potential use of brain MR radiomics for predicting the presence of HE in cirrhotic patients.
The pathological basis of HE in cirrhosis are not fully understood. In the setting of significant liver dysfunction, increased hepatic resistance to the normal enterohepatic circulation forces gut-derived toxins, including ammonia and inflammatory cytokines, into the systemic circulation via spontaneous portosystemic shunts. Subsequently, ammonia is taken up by the brain, a process that has been shown to be associated with edematous changes affecting astrocytes and neurons. Chronic cerebral ammonia exposure leads to irreversible structural changes in astrocytes [5]. The mechanism for astrocyte swelling in acute liver failure remains uncertain but is likely to include excessive generation of osmolytes, mainly glutamine, within the astrocytes as a result of ammonia detoxification through the action of glutamine synthetase. Additionally, abnormalities in intracellular pH and membrane potential can disrupt ion homeostasis and lead to cell swelling [23]. The relatively more rapid evolution of the HE in fulminant hepatic failure, compared to the chronic liver failure, may explain why the homeostatic compensatory metabolic changes are not allowed to counteract the osmotic unbalance induced by intra-astrocytic glutamine accumulation, while in chronic liver failure there is enough time for activation of effective compensatory mechanisms of cellular adaptation to the osmotic change [24]. In patients with cirrhosis subjected to ammonia load, this mechanism of osmolar adaptation is clearly reflected in proton MR spectroscopy studies, which consistently show increases in the glutamine/glutamate signal intensity accompanied by myo-inositol depletion and decreases in the choline signal intensity [25]. This osmoregulatory mechanism is activated after liver failure and accounts for the protection against massive edema in chronic liver failure [26]. Other factors including inflammation, oxidative stress, increased bile acids, and lactate contribute to the progression and severity of HE [6].
In patients with cirrhosis and HE, a common magnetic resonance imaging (MRI) finding in chronic HE is bilateral symmetric T1 hyperintense signal in the globi pallidi and substantiae nigrae (reported in 80%–90% of patients with chronic liver failure), probably a sequela of chronic accumulation of manganese from failed hepatobiliary excretion then transferred to the brain through the blood–brain barrier by several transport systems. This increase in brain manganese has a neurotoxic effect, inducing selective neuronal loss in basal ganglia structures and reactive gliosis. These effects are more prominent in the globus pallidus; the substantia nigra reticulata; and, to a lesser extent, the striatum, with sparing of the substantia nigra pars compacta neurons and striatal dopamine [26]. However, T1 hyperintense signal-intensity alterations in the globi pallidi are not closely linked to the presence of HE. Patients with cirrhosis and no clinical signs of HE can also show signal-intensity alterations, whereas others with HE may present slight signal-intensity alterations [27]. Moreover, studies have shown quick regression of HE after liver transplantation, whereas T1 signal intensity abnormalities need up to 1 year to resolve [28]. This clinical-MR imaging discrepancy may explain why the T1 high signal intensity in the globi pallidi cannot be used as a quantitative measure of tissue manganese deposition because it represents solely a semiquantitative measurement of abnormal manganese deposition. Thus, it is possible that manganese accumulation participates in the pathogenesis of HE only after reaching a certain degree, which may not be clearly identified by MR imaging.
Hepatic encephalopathy classification relies on 2 scales: the International Society for Hepatic Encephalopathy and Nitrogen Metabolism (ISHEN) and the West Haven Criteria (WHC) [1]. The ISHEN makes a distinction between covert HE and overt HE. Patients with covert HE shows little to no clinical symptoms and usually do not require hospitalization, while overt HE is characterized by temporal and spatial disorientations or the presence of asterixis and usually require hospitalization [3]. The WHC classification, used to define the grade of HE in this study, includes 6 stages: unimpaired, minimal, and grades I through IV HE. From a clinical standpoint, differentiation between minimal HE and grade I HE is intangible while grades II through IV describe overt HE, and range in severity from asterixis in grade II, to coma in grade IV [1].
We speculated that radiomics of globi pallidi may be able to capture the regional heterogeneity in the affected brain areas and, therefore, provide good-to-excellent performances for the identification of cirrhotic patients and chronic HE.
In our study, the radiomic models provided higher sensitivity for the prediction of cirrhosis and chronic HE (sensitivities of 94% and 82%, respectively) compared to the hyperintensity on T1-weighted images (sensitivity of 66% for prediction of cirrhosis status), which is classically considered the MR imaging features of chronic HE in cirrhotic patients.
The radiomic models provided a fair-to-good performance for the diagnosis of grade 1 HE (AUROC of 0.75) and grade ≥ 2 HE (AUROC 0.83). The cutoff values provided by radiomic models have demonstrated high sensitivity for the diagnosis of HE grades. In particular, a cutoff > 0.13 had a sensitivity of 94% for grade 1 HE, while a cutoff > 0.08 had a sensitivity of 95% for grade ≥ 2 HE, while the specificity remained limited (60–57%, respectively).
In this setting, the radiomic-based models may be used in clinical practice to maximize the sensitivity for the MRI diagnosis of chronic HE especially in cirrhotic patients undergoing MR imaging for surveillance and with minimal neurological symptoms, which could be further confirmed at clinical evaluation. The possibility of discriminating between cirrhotic and non-cirrhotic patients through brain MR radiomic features could assist in establishing the underlying etiology of chronic liver disease in patients who undergo brain MRI for other causes.
The preliminary findings of our study may stimulate additional research projects including, for instance, the search for radiomic features that may be predictive of post TIPS HE, in order to better stratify patients before TIPS procedure. Post-TIPS HE is the major drawback of TIPS, with a reported incidence of 30–50%; while symptoms are mild in most patients, they can be severe in some post TIPS patients, even requiring hospitalization [16]. Post TIPS HE poses an enormous financial burden for healthcare systems; in a large recent study, performed in a cohort of about 28,000 hospitalized patients who underwent TIPS procedure, the rate of 30-day readmissions was 28%, and the most common reason for readmissions was related to HE, with or without coma, in at least one-third of the patients [17]. Several clinical factors such as older age, history of HE, higher Child–Pugh/MELD score, poor nutritional status, and low serum sodium, are associated to post TIPS HE [16]; yet, it remains challenging to correctly identify patients at risks prior to performing TIPS. The potential for developing post TIPS HE is a leading cause for hepatologists’ hesitation in recommending TIPS, especially in patients with refractory ascites in whom post TIPS survival is lower than in patients with variceal bleeding as their main indication for a TIPS. The potential benefit of adding brain radiomic features as an additional tool for optimized patients’ selection for TIPS could theoretically lead to lower incidence of post TIPS HE. Another potential future study might involve applying this methodology to evaluate its performance in the setting of acute encephalopathies. In particular, one could evaluate the performance of this technique in detecting the presence of underlying HE in patients presenting with an acute, non-specific encephalopathic status, to establish its ability to separate the acutely ill patients who are affected by chronic HE from those without underlying HE.
Our study presents some limitations that must be acknowledged. First, this was a retrospective study with a limited number of cirrhotic patients and controls and we did not investigate the inter-reader agreement for texture features evaluation. Second, all subjects were imaged using a 3T MR scanner. Therefore, these results may be not generalizable to 1.5 T MR scanners, which are still frequently employed for the clinical assessment of cirrhotic patients. Additionally, radiomic model was based on only axial non-contrast T1-weighted images. We did not explore the performance of radiomics in other sequences such as FLAIR or DWI, which may demonstrate more subtle changes related to chronic HE. Given the findings of our study, radiomics applied to sequence other than T1 alone, including FLAIR and DWI, might make for a very interesting subject of future research. Finally, our sample of cirrhotic patients had a mean MELD score of 14 indicating a severe liver disease; these results should therefore be confirmed in patients with less advanced liver disease.
Further studies are needed to validate the proposed results in large multicentric cohorts including control groups undergoing MRI for different clinical indications and by evaluating radiomic changes related to chronic HE in other sequences such as FLAIR or DWI.
In conclusion, MRI-based texture analysis proved to be highly accurate in predicting the presence of cirrhosis and chronic hepatic encephalopathy and effective in correctly staging HE grade in cirrhotic patients.
Availability of data and material (data transparency)
Yes, if requested (by GitHub).
Code availability (software application or custom code)
Not applicable.
Change history
18 July 2022
Missing Open Access funding information has been added in the Funding Note.
Abbreviations
- AUROC:
-
Area under the ROC curve
- FSE:
-
Fast-spin echo
- FOV:
-
Field-of-view
- MELD:
-
Model for End-Stage Liver Disease
- MR:
-
Magnetic resonance
- MRI:
-
Magnetic resonance imaging
- ROI:
-
Region of interest
- ROC:
-
Receiver operating characteristics
- SD:
-
Standard deviation
- TA:
-
Texture analysis
References
Vilstrup H, Amodio P, Bajaj J et al (2014) Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology 60:715–735. https://doi.org/10.1002/hep.27210
D’Amico G, Pasta L, Morabito A, D’Amico M, Caltagirone M, Malizia G et al (2014) Competing risks and prognostic stages in cirrhosis: a 25-year inception cohort study of 494 patients. Aliment Pharmacol Ther 39:1180–1193
Amodio P (2018) Hepatic encephalopathy: diagnosis and management. Liver Int 8:966–975
Elsaid MI, Rustgi VK (2020) Epidemiology of Hepatic Encephalopathy. Clin Liver Dis 24:157–174. https://doi.org/10.1016/j.cld.2020.01.001
Bosoi CR, Rose CF (2013) Brain edema in acute liver failure and chronic liver disease: similarities and differences. Neurochem Int 62:446–457
Ochoa-Sanchez R, Rose CF (2018) Pathogenesis of Hepatic Encephalopathy in Chronic Liver Disease. J Clin Exp Hepatol 8:262–271. https://doi.org/10.1016/j.jceh.2018.08.001
McKinney AM, Lohman BD, Sarikaya B et al (2010) Acute Hepatic encephalopathy: diffusion-weighted and fluid-attenuated inversion recovery findings, and correlation with plasma ammonia level and clinical outcome. Am J Neuroradiol 31:1471–1479
Lai PH, Chen C, Liang HL et al (1999) Hyperintense basal ganglia on T1-weighted MR imaging. AJR Am J Roentgenol 72:1109–1115
Gillies RJ, Kinahan PE, Hricak H (2016) Radiomics: images are more than pictures, they are data. Radiology 278:563–577
Li Wen Y, Leech M (2020) Review of the role of radiomics in tumour risk classification and prognosis of cancer. Anticancer Res 40:3605–3618
Vernuccio F, Cannella R, Comelli A, Salvaggio G, Lagalla R, Midiri M (2020) Radiomics and artificial intelligence: new frontiers in medicine. Recenti Prog Med 111:130–135
Davnall F, Yip CS, Ljungqvist G et al (2012) Assessment of tumor heterogeneity: an emerging imaging tool for clinical practice? Insights Imaging 3:573–589
Zhang S, Chiang GC, Magge RS et al (2019) Texture analysis on conventional MRI images accurately predicts early malignant transformation of low-grade gliomas. Eur Radiol 29:2751–2759
Liu Y, Xu X, Yin L, Zhang X, Li L, Lu H (2017) Relationship between Glioblastoma Heterogeneity and Survival Time: An MR Imaging Texture Analysis. AJNR Am J Neuroradiol 38:1695–1701
Caruana G, Pessini LM, Cannella R et al (2020) Texture analysis in susceptibility-weighted imaging may be useful to differentiate acute from chronic multiple sclerosis lesions. Eur Radiol 30:6348–6356. https://doi.org/10.1007/s00330-020-06995-3
Loizou CP, Pantzaris M, Pattichis CS (2020) Normal appearing brain white matter changes in relapsing multiple sclerosis: texture image and classification analysis in serial MRI scans. Magn Reson Imaging 73:192–202
Betrouni N, Lopes R, Defebvre L, Leentjens AFG, Dujardin K (2020) Texture features of magnetic resonance images: a marker of slight cognitive deficits in Parkinsonʼs disease. Mov Disord 35:486–494
Bajaj JS, Wade JB, Sanyal AJ (2009) Spectrum of neurocognitive impairment in cirrhosis: Implications for the assessment of hepatic encephalopathy. Hepatology 50:2014–2021
Orlhac F, Nioche C, Buvat I (2019) LIFEx Texture User Guide. https://www.lifexsoft.org/images/phocagallery/documentation/ProtocolTexture/UserGuide/TextureUserGuide.pdf
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple hypothesis testing. J R Stat Soc B 57:289–300
Wue Y (2021) Template Matching using Correlation Coefficients. (https://www.mathworks.com/matlabcentral/fileexchange/28590-template-matching-using-correlation-coefficients), MATLAB Central File Exchange
Cardillo G. ROC curve. (https://github.com/dnafinder/roc), GitHub
Rovira A, Alonso J, Córdoba J (2008) MR imaging findings in hepatic encephalopathy. AJNR Am J Neuroradiol 29:1612–1621
Butterworth RF, Giguere JF, Michaud J et al (1987) Ammonia: key factor in the pathogenesis of hepatic encephalopathy. Neurochem Pathol 6:1–12
Ross BD, Jacobson S, Villamil F et al (1994) Subclinical hepatic encephalopathy: proton MR spectroscopic abnormalities. Radiology 193:457–463
Butterworth RF, Spahr L, Fontaine S et al (1995) Manganese toxicity, dopaminergic dysfunction and hepatic encephalopathy. Metab Brain Dis 10:259–267
Weissenborn K, Ehrenheim C, Hori A et al (1995) Pallidal lesions in patients with liver cirrhosis: clinical and MRI evaluation. Metab Brain Dis 10:219–231
Naegele T, Grodd W, Viebahn R et al (2000) MR imaging and 1H spectroscopy of brain metabolites in hepatic encephalopathy: time-course of renormalization after liver transplantation. Radiology 216:683–691
Funding
Open access funding provided by Università degli Studi di Palermo within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Contributions
Gianvincenzo Sparacia: Conceptualization, methodology, investigation.
Roberto Cannella, Giuseppe Mamone, Ioannis Petridis, Luigi Maruzzelli, Vincenzina Lo Re, Albert Comelli: Data curation, investigation.
Gianvincenzo Sparacia, Roberto Miraglia: Writing—original draft preparation.
Giuseppe Parla: Software, validation.
Mona Shahriari, Alberto Iaia: Writing—reviewing and editing.
Angelo Luca: Supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Ethics approval (include appropriate approvals or waivers)
Institutional Review Board approval was obtained.
Consent to participate (include appropriate statements)
This study was reviewed and approved by the institutional research review board (IRRB) of our institution, and informed consent form was waived; however, informed written consent to the MR was obtained in all subjects.
Consent for publication (include appropriate statements)
Informed written consent to the MR was obtained in all subjects and their MR images and data were anonymized.
Additional statements
• We confirm that this work is original and has not been published elsewhere, nor is it currently under consideration for publication elsewhere.
• No overlap with previously published works.
• This paper was submitted solely to Neuroradiology.
• Study subjects or cohorts overlap: None.
• Methodology: retrospective, diagnostic or prognostic study, performed at one institution.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sparacia, G., Parla, G., Cannella, R. et al. Brain magnetic resonance imaging radiomics features associated with hepatic encephalopathy in adult cirrhotic patients. Neuroradiology 64, 1969–1978 (2022). https://doi.org/10.1007/s00234-022-02949-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-022-02949-2