Abstract
Objectives
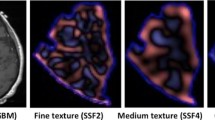
Texture analysis performed on MRI images can provide additional quantitative information that is invisible to human assessment. This study aimed to evaluate the feasibility of texture analysis on preoperative conventional MRI images in predicting early malignant transformation from low- to high-grade glioma and compare its utility to histogram analysis alone.
Methods
A total of 68 patients with low-grade glioma (LGG) were included in this study, 15 of which showed malignant transformation. Patients were randomly divided into training (60%) and testing (40%) sets. Texture analyses were performed to obtain the most discriminant factor (MDF) values for both training and testing data. Receiver operating characteristic (ROC) curve analyses were performed on MDF values and 9 histogram parameters in the training data to obtain cutoff values for determining the correct rates of discrimination between two groups in the testing data.
Results
The ROC analyses on MDF values resulted in an area under the curve (AUC) of 0.90 (sensitivity 85%, specificity 84%) for T2w FLAIR, 0.92 (86%, 94%) for ADC, 0.96 (97%, 84%) for T1w, and 0.82 (78%, 75%) for T1w + Gd and correctly discriminated between the two groups in 93%, 100%, 93%, and 92% of cases in testing data, respectively. In the astrocytoma subgroup, AUCs were 0.92 (88%, 83%) for T2w FLAIR and 0.90 (92%, 74%) for T1w + Gd and correctly discriminated two groups in 100% and 92% of cases. The MDF outperformed all 9 of the histogram parameters.
Conclusion
Texture analysis on conventional preoperative MRI images can accurately predict early malignant transformation of LGGs, which may guide therapeutic planning.
Key Points
• Texture analysis performed on MRI images can provide additional quantitative information that is invisible to human assessment.
• Texture analysis based on conventional preoperative MR images can accurately predict early malignant transformation from low- to high-grade glioma.
• Texture analysis is a clinically feasible technique that may provide an alternative and effective way of determining the likelihood of early malignant transformation and help guide therapeutic decisions.



Similar content being viewed by others
Abbreviations
- AUC:
-
Area under the curve
- LDA:
-
Linear discriminant analysis
- LGG:
-
Low-grade glioma
- MDF:
-
Most discriminant factor
- ROC:
-
Receiver operating characteristic
References
Louis DN, Perry A, Reifenberger G et al (2016) The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol 131:803–820
Claus EB, Walsh KM, Wiencke JK et al (2015) Survival and low-grade glioma: the emergence of genetic information. Neurosurg Focus 38:E6
Okamoto Y, Di Patre PL, Burkhard C et al (2004) Population-based study on incidence, survival rates, and genetic alterations of low-grade diffuse astrocytomas and oligodendrogliomas. Acta Neuropathol 108:49–56
Pignatti F, van den Bent M, Curran D et al (2002) Prognostic factors for survival in adult patients with cerebral low-grade glioma. J Clin Oncol 20:2076–2084
Olar A, Wani KM, Alfaro-Munoz KD et al (2015) IDH mutation status and role of WHO grade and mitotic index in overall survival in grade II-III diffuse gliomas. Acta Neuropathol 129:585–596.
Rotariu D, Gaivas S, Faiyad Z, Haba D, Iliescu B, Poeata I (2010) Malignant transformation of low grade gliomas into glioblastoma a series of 10 cases and review of the literature. Rom Neurosurg 4:403–412
Bogdańska MU, Bodnar M, Piotrowska MJ et al (2017) A mathematical model describes the malignant transformation of low grade gliomas: prognostic implications. PLoS One 12:e0179999
Jiang R, Jiang J, Zhao L et al (2015) Diffusion kurtosis imaging can efficiently assess the glioma grade and cellular proliferation. Oncotarget 6:42380–42393
Arevalo-Perez J, Peck KK, Young RJ, Holodny AI, Karimi S, Lyo JK (2015) Dynamic contrast-enhanced perfusion MRI and diffusion-weighted imaging in grading of gliomas. J Neuroimaging 25:792–798
Bulik M, Jancalek R, Vanicek J, Skoch A, Mechl M (2013) Potential of MR spectroscopy for assessment of glioma grading. Clin Neurol Neurosurg 115:146–153
Kassner A, Thornhill RE (2010) Texture analysis: a review of neurologic MR imaging applications. AJNR Am J Neuroradiol 31:809–816
Gillies RJ, Kinahan PE, Hricak H (2016) Radiomics: images are more than pictures, they are data. Radiology 278:563–577
Xie T, Chen X, Fang J et al (2018) Textural features of dynamic contrast-enhanced MRI derived model-free and model-based parameter maps in glioma grading. J Magn Reson Imaging 47:1099–1111
Skogen K, Schulz A, Dormagen JB, Ganeshan B, Helseth E, Server A (2016) Diagnostic performance of texture analysis on MRI in grading cerebral gliomas. Eur J Radiol 85:824–829
Reza SM, Mays R, Iftekharuddin KM (2015) Multi-fractal Detrended Texture Feature for Brain Tumor Classification. Proc SPIE Int Soc Opt Eng 9414:941410.
Ryu YJ, Choi SH, Park SJ, Yun TJ, Kim JH, Sohn CH (2014) Glioma: application of whole-tumor texture analysis of diffusion-weighted imaging for the evaluation of tumor heterogeneity. PLoS One 9:e108335
Béresová M, Larroza A, Arana E, Varga J, Balkay L, Moratal D (2018) 2D and 3D texture analysis to differentiate brain metastases on MR images: proceed with caution. MAGMA 31:285–294
Li Z, Mao Y, Li H, Yu G, Wan H, Li B (2016) Differentiating brain metastases from different pathological types of lung cancers using texture analysis of T1 postcontrast MR. Magn Reson Med 76:1410–1419
Chaddad A, Tanougast C (2016) Extracted magnetic resonance texture features discriminate between phenotypes and are associated with overall survival in glioblastoma multiforme patients. Med Biol Eng Comput 54:1707–1718
Castellano G, Bonilha L, Li LM, Cendes F (2004) Texture analysis of medical images. Clin Radiol 59:1061–1069
Just N (2014) Improving tumour heterogeneity MRI assessment with histograms. Br J Cancer 111:2205–2213
Aggarwal N, Agrawal R (2012) First and second order statistics features for classification of magnetic resonance brain images. J Signal Inf Process 3:146
Louis DN, Ohgaki H, Wiestler OD et al (2007) The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 114:97–109
Strzelecki M, Szczypinski P, Materka A, Klepaczko A (2013) A software tool for automatic classification and segmentation of 2D/3D medical images. Nucl Inst Methods Phys Res A 702:137–140
Szczypiński PM, Strzelecki M, Materka A, Klepaczko A (2009) MaZda--a software package for image texture analysis. Comput Methods Prog Biomed 94:66–76
Yan PF, Yan L, Hu TT et al (2017) The potential value of preoperative MRI texture and shape analysis in grading meningiomas: a preliminary investigation. Transl Oncol 10:570–577
Collewet G, Strzelecki M, Mariette F (2004) Influence of MRI acquisition protocols and image intensity normalization methods on texture classification. Magn Reson Imaging 22:81–91
Vallières M, Freeman CR, Skamene SR, El Naqa I (2015) A radiomics model from joint FDG-PET and MRI texture features for the prediction of lung metastases in soft-tissue sarcomas of the extremities. Phys Med Biol 60:5471–5496
Brown AM, Nagala S, McLean MA et al (2016) Multi-institutional validation of a novel textural analysis tool for preoperative stratification of suspected thyroid tumors on diffusion-weighted MRI. Magn Reson Med 75:1708–1716
Lisson CS, Lisson CG, Flosdorf K et al (2018) Diagnostic value of MRI-based 3D texture analysis for tissue characterisation and discrimination of low-grade chondrosarcoma from enchondroma: a pilot study. Eur Radiol 28:468–477
Fan M, Cheng H, Zhang P et al (2018) DCE-MRI texture analysis with tumor subregion partitioning for predicting Ki-67 status of estrogen receptor-positive breast cancers. J Magn Reson Imaging 48:237–247
Meyer HJ, Schob S, Hohn AK, Surov A (2017) MRI texture analysis reflects histopathology parameters in thyroid cancer - a first preliminary study. Transl Oncol 10:911–916
Ly KI, Gerstner ER (2018) The role of advanced brain tumor imaging in the care of patients with central nervous system malignancies. Curr Treat Options Oncol 19:40
Wang S, Meng M, Zhang X et al (2018) Texture analysis of diffusion weighted imaging for the evaluation of glioma heterogeneity based on different regions of interest. Oncol Lett 15:7297–7304
Hassan I, Kotrotsou A, Bakhtiari AS et al (2016) Radiomic texture analysis mapping predicts areas of true functional MRI activity. Sci Rep 6:25295
Aerts HJ (2016) The potential of radiomic-based phenotyping in precision medicine: a review. JAMA Oncol 2:1636–1642
Gevaert O, Mitchell LA, Achrol AS et al (2014) Glioblastoma multiforme: exploratory radiogenomic analysis by using quantitative image features. Radiology 273:168–174
Jamshidi N, Diehn M, Bredel M, Kuo MD (2014) Illuminating radiogenomic characteristics of glioblastoma multiforme through integration of MR imaging, messenger RNA expression, and DNA copy number variation. Radiology 270:1–2
Tofts PS, Benton CE, Weil RS et al (2007) Quantitative analysis of whole-tumor Gd enhancement histograms predicts malignant transformation in low-grade gliomas. J Magn Reson Imaging 25:208–214
McKinnon ET, Jensen JH, Glenn GR, Helpern JA (2017) Dependence on b-value of the direction-averaged diffusion-weighted imaging signal in brain. Magn Reson Imaging 36:121–127
Acknowledgements
The authors thank Kelly McCabe Gillen, PhD, for her assistance in manuscript editing.
Funding
This study has received funding by grants from the National Institutes of Health of United States (R01 NS095562, R01 NS090464) and National Natural Science Foundation of China (No. 81730049, 81801666).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantor of this publication is Shun Zhang.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• retrospective
• observational
• performed at one institution
Electronic supplementary material
ESM 1
(DOCX 1194 kb)
Rights and permissions
About this article
Cite this article
Zhang, S., Chiang, G.CY., Magge, R.S. et al. Texture analysis on conventional MRI images accurately predicts early malignant transformation of low-grade gliomas. Eur Radiol 29, 2751–2759 (2019). https://doi.org/10.1007/s00330-018-5921-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-018-5921-1