Abstract
Purpose
The artery of Adamkiewicz (AKA) provides the major blood supply to the anterior thoracolumbar spinal cord and iatrogenic injury or inadequate reconstruction of this vessel during vascular and endovascular surgery can result in postoperative neurological deficit due to spinal cord ischemia. The aim of this study was to provide comprehensive data on the prevalence and anatomical characteristics of the AKA.
Methods
An extensive search was conducted through the major electronic databases to identify eligible articles. Data extracted included study type, prevalence of the AKA, gender, number of AKA per patient, laterality, origin based on vertebral level, side of origin, morphometric data, and ethnicity subgroups.
Results
A total of 60 studies (n = 5437 subjects) were included in the meta-analysis. Our main findings revealed that the AKA was present in 84.6% of the population, and patients most frequently had a single AKA (87.4%) on the left side (76.6%) originating between T8 and L1 (89%).
Conclusion
As an AKA is present in the majority of the population, caution should be taken during vascular and endovascular surgical procedures to avoid injury or ensure proper reconstruction. All surgeons operating in the thoracolumbar spinal cord should have a thorough understanding of the anatomical characteristics and surgical implications of an AKA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
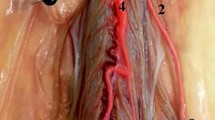
The artery of Adamkiewicz (AKA), also known as the great anterior radiculomedullary artery, is a major artery that joins the anterior spinal artery in the lower one-third of the spinal cord (Fig. 1) [1]. Because of its large role in feeding the spinal cord, many reports have stressed the importance of reattaching the intercostal or lumbar arteries to the AKA in the event of spinal cord ischemia following vascular and endovascular surgery (Fig. 2). Identification of the AKA preoperatively helps surgeons to determine the appropriate range of aortic lesions that require graft replacement [2]. Therefore, accurate localization and detailed anatomical knowledge of the AKA are important when planning surgical and interventional radiological treatments of thoracoabdominal diseases and spinal lesions in order to help reduce the risk of postoperative ischemic spinal complications and paraplegia.
The AKA is the most dominant anterior radiculomedullary artery and is responsible for the arterial blood supply to the spinal cord from T8 to the conus medullaris [3]. Its origin is highly variable and extends from the mid-thoracic level to the lumbar levels, including the bilateral T3-T12 intercostal arteries [4] and L1-L4 lumbar arteries [5]. It typically arises from the T8–L1 neural foramina [6] from the left intercostal or lumbar arteries [7]. The AKA has a diameter of 0.8–1.3 mm, and the distal portion of this artery, together with the anterior spinal artery, forms a characteristic “hairpin” turn [8] (Fig. 3). Various techniques have been devised to preoperatively identify the location and anatomy of this artery. Such techniques include computed tomography angiography (CTA), magnetic resonance angiography (MRA), and digital subtraction angiography (DSA), with the latter considered the gold standard [9].
The most important cause of injury to the AKA is iatrogenic, and in part, this is a factor of the high degree of variability in the anatomical location of this artery [10]. Preoperative AKA identification and its subsequent reconstruction or preservation may aid in reducing the incidence of postoperative neurological deficits and improving the outcomes of thoracolumbar surgical procedures. To this end, the aim of this study was to provide comprehensive data on the prevalence and anatomical characteristics of the AKA.
Materials and methods
Search strategy
A search of all major electronic databases (PubMed, EMBASE, ScienceDirect, China National Knowledge Infrastructure (CNKI), SciELO, BIOSIS, and Web of Science) was performed in order to identify potential articles. The following search terms were employed: artery of Adamkiewicz, arteria radicularis magna (ARM), radicularis magna, great radicular artery of Adamkiewicz, major anterior segmental medullary artery, great anterior segmental medullary artery, artery of the lumbar enlargement, arteria radicularis anterior magna, and great anterior radiculomedullary artery. A search through the references of the initially selected articles was conducted to identify any potential studies that were omitted. The authors adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines throughout this meta-analysis (Supplement 1).
Eligibility assessment
An eligibility assessment was conducted by two independent reviewers. Studies were included in this meta-analysis if they (1) provided complete data on the prevalence of the AKA or (2) provided data on the anatomy of AKA. The following exclusion criteria were employed: case, case-series, conference abstracts, letters to editors, and studies not published in peer-reviewed journals. Studies that were originally published in languages other than English were translated by medical professionals who are fluent both in English and the original language of the manuscript. All differences of opinion among the reviewers concerning the eligibility of the studies were resolved by consensus through consultation with the author of the respective study.
Data extraction
Two reviewers carried out data extraction independently. The following data was extracted: publication year, country of origin, study type (cadaveric, CTA, MRA, DSA), prevalence data of AKA, number of AKAs per patient, laterality of the AKA, origin of the AKA based on the vertebral level, side of origin, and morphometric data. In cases of incomplete data, the authors of the original articles were contacted for clarification.
Quality assessment
The AQUA tool [11] was used by the reviewers to evaluate quality and reliability of the included studies. In brief, the tool was devised to probe for potential risk of bias. Five domains were evaluated in the analysis: (1) objective(s) and subject characteristics, (2) study design, (3) methodology characterization, (4) descriptive anatomy, and (5) reporting of results; and each domain was categorized as either of “Low,” “High,” or “Unclear” risk of bias. Decision was made that a “No” answer in whichever signaling question within each of the categories arbitrated the domain to be of “High” risk of bias, whereas all answers “Yes” suggested that it presented a “Low” risk of bias. “Unclear” option was chosen when the study with incoherent data did not permit for a clear scrutiny.
Statistical analysis
The prevalence analysis was conducted using MetaXL version 5.8 by EpiGear Pty Ltd. (Wilston, Queensland, Australia). Morphometric analysis using Comprehensive Meta-Analysis version 3.3 yielded the pooled mean diameter of the AKA. Single and multi-categorical pooled prevalence rates were calculated using a random effects model. Heterogeneity was assessed using a chi-squared test and the I2 statistic. For the I2 statistic, the values of 0–40% indicated that heterogeneity might not be important; values of 30–60% could indicate moderate heterogeneity; values of 50–90% could indicate substantial heterogeneity; and values of 75–100% indicated considerable heterogeneity. A p value below 0.10 for Cochran’s Q suggested significant heterogeneity [12].
An analysis of the subgroups was conducted to determine the source of heterogeneity. The difference between the groups was considered to be insignificant if the confidence intervals (CIs) of specific rates overlapped [13]. Subgroups according to study type, gender, and geographical location were analyzed.
Results
Study identification and characteristics of included studies
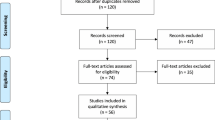
The study identification process is presented in Fig. 4. An initial search yielded 747 entries. After thorough analysis, 627 entries were excluded. In total, 120 articles were analyzed, and 60 studies were included in this meta-analysis.
The characteristics of the included studies are presented in Table 1. A total of 60 studies (4317 subjects with AKA) published between 1989 [14] and 2017 [15] were included [1,2,3,4,5,6,7,8,9,10, 14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62]. The studies originated from North America, Asia and Europe, and from ten different countries.
Quality assessment
The majority of studies included in this meta-analysis, evaluated by the AQUA tool, revealed domain one (objective(s) and subject characteristics) and domain three (methodology characterization) to be at “High” risk of bias, owing to missing demographic data of the research group and no information regarding experience of the researchers. All studies had a “Low” risk of bias found in domain two (study design) and domain five (reporting of results), and almost all studies had a “Low” risk of bias found in domain four (descriptive anatomy). The AQUA tool evaluation can be found in Table 2.
Prevalence of the artery of Adamkiewicz
A total of 60 studies (n = 5437 subjects) reported data on the prevalence of the AKA. The pooled prevalence estimate (PPE) of the AKA was 84.6% (95% CI 79.7–89.0) (Table 3).
The subgroup analysis of gender differences showed that the AKA was slightly more prevalent in males (93.7% [95% CI 83.3–100.0]) than females (90.4% [95% CI 68.9–100.0]), although not significantly.
Seven cadaveric studies (n = 300) yielded the highest PPE of the AKA (97.5% [95% CI 92.4–100.0]) among the different study types. This was followed by MRA, CTA, and DSA studies with PPEs of 88.3%, 88.1%, and 75.4%, respectively (Table 3).
The subgroup analysis of geographical origin showed that the AKA was most prevalent in the Netherlands, with a PPE of 99.4% (95% CI 98.2–100.0); France with a PPE of 89.8% (95% CI 83.8–94.6); and Japan, with a PPE of 85.3 (95% CI 81.0–89.2). It was least prevalent in the USA, with a PPE of 79.5% (95% CI 57.0–95.7).
Number of arteries of Adamkiewicz per patient
An analysis of 20 studies (n = 1329 subjects with AKAs) showed that the majority of patients (87.4% [95% CI 83.4–91.9]) had one AKA. Patients presented with two AKAs in 11.3% (95% CI 7.5–15.8) of cases, three AKAs in 0.8% (95% CI 0.0–2.5) of cases, and four AKAs in 0.5% (95% CI 0.0–1.6) of cases.
In patients with two AKAs, the majority (73.3% [95% CI 47.3–93.4]) presented unilaterally as duplications. A total of 26.7% (95% CI 6.6–52.7; I2 66.2%, 95% CI 12.0–87.0; p = 0.019) of patients with two AKAs had bilateral configuration.
Origin of the artery of Adamkiewicz
A total of 56 studies (n = 3316 patients with AKA) analyzed the side of origin of AKA. The results showed that 76.6% (95% CI 73.2–79.9) of AKAs originated from the left side, while 23.4% (95% CI 20.1–26.8; I2 78.5%, 95% CI 72.5–83.2; p < 0.001) from the right side. The analysis of 43 studies (n = 2834 patients with AKA) showed that 89% of arteries originated between T8 and L1 (Table 4). AKA most frequently originated at the level of T9 with PPE of 22.2% (95% CI 18.9–25.4), followed by T10 and T11 with PPE of 21.7% (95% CI 18.5–25.0) and 18.7% (95% CI 15.6–21.8), respectively.
Continuity of the artery of Adamkiewicz
A total of seven studies (n = 375 patients with AKAs) were included in an analysis of the continuity of the AKA from the aorta to the anterior spinal artery. The results showed that AKA continued from the aorta to the anterior spinal artery in 71.3% of patients (95% CI 45.8–91.6; I2 95.6%, 95% CI 92.8–97.2; p < 0.001).
Morphometric analysis of the artery of Adamkiewicz
Five studies (n = 324 patients with AKA) analyzed the morphometric data of the AKA. The analysis showed a pooled mean diameter of 1.09 mm (95% CI 0.69–1.50; I2 36.2%; p < 0.001).
Discussion
Because the AKA originates from the lumbar arteries, it may be prudent to preserve the blood flow from the lumbar arteries when a thoracoabdominal aortic repair is planned [5, 63]. Concomitant or previous abdominal aortic repair and extensive thoracic aorta exclusion by means of multiple stent grafts are associated with a significantly higher risk of paraplegia [64]. After the interruption of most of the intercostal and lumbar arteries, the residual collateral blood supply is marginal, and in some cases, the spinal cord may become extremely prone to injury due to arterial hypotension or low cardiac output from any cause [65]. During aortic repair, preservation, reattachment, or reconstruction of the intercostal or lumbar arteries can maintain the blood supply to the spinal cord [66, 67]. Depending on the number of intercostal or lumbar arteries that require reconstruction, the ischemic duration may be prolonged during reconstruction. In our study, in patients with an AKA present, 11.3% had two AKAs, with bilateral AKAs present in 26.7% of these patients. The preoperative identification of the AKA and its anatomical characteristics allows for superior surgical planning, such that the surgical time and postoperative spinal complication risk are decreased [31]. Therefore, AKA identification is of interest for surgeons aiming to reconstruct intercostal or lumbar arteries in order to prevent postoperative spinal ischemic complications [3].
With respect to the continuity between the radicular arteries (including the AKA) and the anterior spinal arteries, the AKA continued from the aorta to the anterior spinal artery in 71.3% of the patients in our study. When this continuity is present, blood may drain away from the spinal cord through the anterior spinal arteries and the radicular arteries, acting as stealing channels by rerouting the blood to be distal to an aortic obstruction [5]. During aortic cross-clamping, back-bleeding from the ostia of the posterior intercostal and lumbar arteries may be a clinical manifestation of such rerouting of blood when continuity between the AKA and the anterior spinal arteries is present. This steal phenomenon may further worsen spinal cord ischemia, causing irreversible neurological injuries if the ischemia time is longer than 20 to 30 min [68].
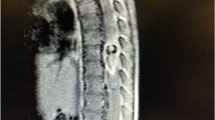
The detection of the AKA can be difficult because of the various possible levels of origins of the artery, its small size, the amount of time needed to obtain the angiogram, and complications that can occur during surgical procedures [14, 26]. In our study, the pooled mean diameter of the AKA was 1.09 mm. Various techniques have been devised to preoperatively identify the location and anatomy of the AKA, such as CTA [55], MRA [7], and DSA [9]. These techniques can be used to identify both the level and the laterality of the artery, which can affect a surgeon’s approach to an aneurysm or spinal lesion. We have included three DSA images with injected contrast into left radicular artery at the level of T4 (Fig. 5), T8 (Fig. 6), and T11 (Fig. 7). In our meta-analysis, cadaveric studies had the highest prevalence of an AKA (97.5%), and among the different imaging modalities, MRA and CTA had the highest prevalence rates (88.3% and 88.1%, respectively), while DSA had the lowest prevalence rate (75.4%). In spite of its apparent success in detecting an AKA, MRA has been shown to be inferior to DSA in terms of evaluating vessel continuity, sharpness, and background homogeneity [7]. Furthermore, compared with CTA, a more limited field of view is a major disadvantage of MRA [61]. As a result, MRA may fail to depict the clinically important collateral vessels to the AKA in some patients, when a collateral source is the internal thoracic artery or the thoracodorsal artery [69]. Despite DSA studies reporting a lower prevalence rate of the AKA than MRA and CTA in our meta-analysis, DSA remains the “gold standard” for identifying spinal cord vasculature as it is both safe and efficient [9]. A possible reason for this discrepancy could be the small number of patients included in our DSA analysis as compared to the number of patients included in our MRA and CTA analyses.
Future studies should examine the blood supply and the collateral circulation of the spinal cord in the presence of degenerative atherosclerotic or dissecting aneurysm, or after a surgical or endovascular aortic procedure. In these patients, the disease and the surgical procedure may occlude several segmental arteries and promote collateral vessels enlargement, significantly altering the normal patterns of blood supply to the spinal cord [5].
Our meta-analysis was limited by the high amount of heterogeneity between the studies. However, the number of included studies and their large sample sizes mitigate this limitation. As cadaveric dissection is the gold standard for anatomical considerations, more cadaveric studies should assess prevalence of AKA, especially performed on subjects poorly represented in our meta-analysis, such as Africa, South America, and Oceania.
Because of the lower prevalence of AKA in radiological studies, surgeons should keep in mind that these results might be false negative. In this case, the risk of iatrogenic injury to the AKA during thoracolumbar surgical procedures is increased. More accurate imaging methods should be developed to assess the true prevalence of AKA.
To ensure spinal cord safety, preoperative AKA identification and its subsequent reconstruction or preservation are effective adjuncts for more secure protection of the spinal cord, along with other adequate management strategies.
Conclusions
Our main findings revealed that the AKA was found to be present in the vast majority of the general population (84.6%), most often as a single vessel (87.4%) originating between T8 and L1 (89%) on the left side (76.6%). Based on our anatomical findings, we recommend that efforts should be made to identify and subsequently reconstruct or preserve the AKA to prevent postoperative neurological deficit due to spinal cord ischemia in vascular and endovascular surgical procedures in the thoracolumbar spinal cord.
References
Sukeeyamanon W, Siriapisith T, Wasinrat J (2010) Preoperative localization of Adamkiewicz arteries and their origins by using MDCT angiography. J Med Assoc Thail 93:1430–1436
Ogino H, Sasaki H, Minatoya K, Matsuda H, Yamada N, Kitamura S (2006) Combined use of Adamkiewicz artery demonstration and motor-evoked potentials in descending and thoracoabdominal repair. Ann Thorac Surg 82:592–596. https://doi.org/10.1016/j.athoracsur.2006.03.041
Takagi H, Ota H, Natsuaki Y, Komori Y, Ito K, Saiki Y, Takase K (2015) Identifying the Adamkiewicz artery using 3-T time-resolved magnetic resonance angiography: its role in addition to multidetector computed tomography angiography. Jpn J Radiol 33:749–756. https://doi.org/10.1007/s11604-015-0490-6
Gailloud P (2013) The artery of von Haller: a constant anterior radiculomedullary artery at the upper thoracic level. Neurosurgery 73:1034–1043. https://doi.org/10.1227/NEU.0000000000000163
Biglioli P, Roberto M, Cannata A, Parolari A, Fumero A, Grillo F, Maggioni M, Coggi G, Spirito R (2004) Upper and lower spinal cord blood supply: the continuity of the anterior spinal artery and the relevance of the lumbar arteries. J Thorac Cardiovasc Surg 127:1188–1192. https://doi.org/10.1016/j.jtcvs.2003.11.038
Koshino T, Murakami G, Morishita K, Mawatari T, Abe T (1999) Does the Adamkiewicz artery originate from the larger segmental arteries? J Thorac Cardiovasc Surg 117:898–905. https://doi.org/10.1016/S0022-5223(99)70369-7
Yamada N, Okita Y, Minatoya K, Tagusari O, Ando M, Takamiya M, Kitamura S (2000) Preoperative demonstration of the Adamkiewicz artery by magnetic resonance angiography in patients with descending or thoracoabdominal aortic aneurysms. Eur J Cardiothorac Surg 18:104–111
Yoshioka K, Niinuma H, Ohira A, Nasu K, Kawakami T, Sasaki M, Kawazoe K (2003) MR angiography and CT angiography of the artery of Adamkiewicz: noninvasive preoperative assessment of thoracoabdominal aortic aneurysm. RadioGraphics 23:1215–1225. https://doi.org/10.1148/rg.235025031
Fanous AA, Lipinski LJ, Krishna C, Roger EP, Siddiqui AH, Levy EI, Leonardo J, Pollina J (2015) The impact of preoperative angiographic identification of the artery of Adamkiewicz on surgical decision making in patients undergoing thoracolumbar corpectomy. Spine (Phila Pa 1976) 40:1194–1199. https://doi.org/10.1097/BRS.0000000000000909
Nijenhuis RJ, Leiner T, Cornips EMJ, Wilmink JT, Jacobs MJ, van Engelshoven JMA, Backes WH (2004) Spinal cord feeding arteries at MR angiography for thoracoscopic spinal surgery: feasibility study and implications for surgical approach. Radiology 233:541–547. https://doi.org/10.1148/radiol.2331031672
Henry BM, Tomaszewski KA, Ramakrishnan PK, Roy J, Vikse J, Loukas M, Tubbs RS, Walocha JA (2017) Development of the anatomical quality assessment (AQUA) tool for the quality assessment of anatomical studies included in meta-analyses and systematic reviews. Clin Anat 30:6–13. https://doi.org/10.1002/ca.22799
Higgins J, Green S (eds) (2011) Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]
Henry BM, Tomaszewski KA, Walocha JA (2016) Methods of evidence-based anatomy: a guide to conducting systematic reviews and meta-analysis of anatomical studies. Ann Anat 205:16–21. https://doi.org/10.1016/j.aanat.2015.12.002
Fereshetian A, Kadir S, Kaufman SL, Mitchell SE, Murray RR, Kinnison ML, Williams GM (1989) Digital subtraction spinal cord angiography in patients undergoing thoracic aneurysm surgery. Cardiovasc Intervent Radiol 12:7–9
Guziński M, Bryl M, Ziemińska K, Wolny K, Sąsiadek M, Garcarek J (2017) Detection of the Adamkiewiczartery in computed tomography of the thorax and abdomen. Adv Clin Exp Med 26:31–37. https://doi.org/10.17219/acem/62788
Alleyne CH, Cawley CM, Shengelaia GG, Barrow DL (1998) Microsurgical anatomy of the artery of Adamkiewicz and its segmental artery. J Neurosurg 89:791–795. https://doi.org/10.3171/jns.1998.89.5.0791
Amako M, Yamamoto Y, Nakamura K et al (2011) Preoperative visualization of the artery of Adamkiewicz by dual-phase CT angiography in patients with aortic aneurysm. Kurume Med J 58:117–125
Bachet J, Guilmet D, Rosier J et al (1996) Protection of the spinal cord during surgery of thoraco-abdominal aortic aneurysms. Eur J Cardiothorac Surg 10:817–825
Backes WH, Nijenhuis RJ, Mess WH, Wilmink FA, Schurink GWH, Jacobs MJ (2008) Magnetic resonance angiography of collateral blood supply to spinal cord in thoracic and thoracoabdominal aortic aneurysm patients. J Vasc Surg 48:261–271. https://doi.org/10.1016/j.jvs.2008.03.015
Bley TA, Duffek CC, François CJ, Schiebler ML, Acher CW, Mell M, Grist TM, Reeder SB (2010) Presurgical localization of the artery of Adamkiewicz with time-resolved 3.0-T MR angiography. Radiology 255:873–881. https://doi.org/10.1148/radiol.10091304
Boll DT, Bulow H, Blackham KA, Aschoff AJ, Schmitz BL (2006) MDCT angiography of the spinal vasculature and the artery of Adamkiewicz. AJR Am J Roentgenol 187:1054–1060. https://doi.org/10.2214/AJR.05.0562
Bowen BC, DePrima S, Pattany PM, Marcillo A, Madsen P, Quencer RM (1996) MR angiography of normal intradural vessels of the thoracolumbar spine. AJNR Am J Neuroradiol 17:483–494
Champlin AM, Rael J, Benzel EC, Kesterson L, King JN, Orrison WW, Mirfakhraee M (1994) Preoperative spinal angiography for lateral extracavitary approach to thoracic and lumbar spine. AJNR Am J Neuroradiol 15:73–77
Charles YP, Barbe B, Beaujeux R, Boujan F, Steib JP (2011) Relevance of the anatomical location of the Adamkiewicz artery in spine surgery. Surg Radiol Anat 33:3–9. https://doi.org/10.1007/s00276-010-0654-0
Furukawa K, Kamohara K, Nojiri J, Egashira Y, Okazaki Y, Kudo S, Morita S (2010) Operative strategy for descending and thoracoabdominal aneurysm repair with preoperative demonstration of the Adamkiewicz artery. Ann Thorac Surg 90:1840–1846. https://doi.org/10.1016/j.athoracsur.2010.07.056
Heinemann MK, Brassel F, Herzog T et al (1998) The role of spinal angiography in operations on the thoracic aorta: myth or reality? Ann Thorac Surg 65:346–351
Hyodoh H, Kawaharada N, Akiba H, Tamakawa M, Hyodoh K, Fukada J, Morishita K, Hareyama M (2005) Usefulness of preoperative detection of artery of Adamkiewicz with dynamic contrast-enhanced MR angiography. Radiology 236:1004–1009. https://doi.org/10.1148/radiol.2363040911
Hyodoh H, Shirase R, Akiba H, Tamakawa M, Hyodoh K, Yama N, Shonai T, Hareyama M (2007) Double-subtraction maximum intensity projection MR angiography for detecting the artery of Adamkiewicz and differentiating it from the drainage vein. J Magn Reson Imaging 26:359–365. https://doi.org/10.1002/jmri.21024
Hyodoh H, Shirase R, Kawaharada N et al (2009) MR angiography for detecting the artery of Adamkiewicz and its branching level from the aorta. Magn Reson Med Sci 8:159–164
Jaspers K, Nijenhuis RJ, Backes WH (2007) Differentiation of spinal cord arteries and veins by time-resolved MR angiography. J Magn Reson Imaging 26:31–40. https://doi.org/10.1002/jmri.20940
Kawaharada N, Morishita K, Fukada J, Yamada A, Muraki S, Hyodoh H, Abe T (2002) Thoracoabdominal or descending aortic aneurysm repair after preoperative demonstration of the Adamkiewicz artery by magnetic resonance angiography. Eur J Cardiothorac Surg 21:970–974
Hachiro Y, Kawaharada N, Morishita K, Fukada J, Fujisawa Y, Kurimoto Y, Abe T (2004) Thoracoabdominal aortic aneurysm repair after detection of the Adamkiewicz artery by magnetic resonance angiography; a way to shorten operating time and improve outcome. Kyobu Geka 57:280–283
Kawaharada N, Morishita K, Kurimoto Y et al (2007) Spinal cord ischemia after elective endovascular stent-graft repair of the thoracic aorta. Eur J Cardiothorac Surg 31:998–1003. https://doi.org/10.1016/j.ejcts.2007.01.069
Kieffer E, Richard T, Chiras J, Godet G, Cormier E (1989) Preoperative spinal cord arteriography in aneurysmal disease of the descending thoracic and thoracoabdominal aorta: preliminary results in 45 patients. Ann Vasc Surg 3:34–46. https://doi.org/10.1016/S0890-5096(06)62382-0
Kieffer E, Fukui S, Chiras J, Koskas F, Bahnini A, Cormier E (2002) Spinal cord arteriography: a safe adjunct before descending thoracic or thoracoabdominal aortic aneurysmectomy. J Vasc Surg 35:262–268
Kovács A, Schiller W, Gerhards HM, Welz A, Willinek W, Schild H, Urbach H, Flacke S (2009) Visualization of the Adamkiewicz artery in patients with acute Stanford A dissections: a prospective 64-row multi-detector CT study. Rofo 181:870–874. https://doi.org/10.1055/s-0028-1109441
Kroszczynski AC, Kohan K, Kurowski M, Olson TR, Downie SA (2013) Intraforaminal location of thoracolumbar anterior medullary arteries. Pain Med 14:808–812. https://doi.org/10.1111/pme.12056
Kudo K, Terae S, Asano T, Oka M, Kaneko K, Ushikoshi S, Miyasaka K (2003) Anterior spinal artery and artery of Adamkiewicz detected by using multi-detector row CT. AJNR Am J Neuroradiol 24:13–17
Matsuda H, Fukuda T, Iritani O, Nakazawa T, Tanaka H, Sasaki H, Minatoya K, Ogino H (2010) Spinal cord injury is not negligible after TEVAR for lower descending aorta. Eur J Vasc Endovasc Surg 39:179–186. https://doi.org/10.1016/j.ejvs.2009.11.014
Matsuda H, Ogino H, Fukuda T, Iritani O, Sato S, Iba Y, Tanaka H, Sasaki H, Minatoya K, Kobayashi J, Yagihara T (2010) Multidisciplinary approach to prevent spinal cord ischemia after thoracic endovascular aneurysm repair for distal descending aorta. Ann Thorac Surg 90:561–565. https://doi.org/10.1016/j.athoracsur.2010.04.067
Melissano G, Bertoglio L, Civelli V, Moraes Amato AC, Coppi G, Civilini E, Calori G, de Cobelli F, del Maschio A, Chiesa R (2009) Demonstration of the Adamkiewicz artery by multidetector computed tomography angiography analysed with the open-source software OsiriX. Eur J Vasc Endovasc Surg 37:395–400. https://doi.org/10.1016/j.ejvs.2008.12.022
Mordasini P, El-Koussy M, Schmidli J et al (2012) Preoperative mapping of arterial spinal supply using 3.0-T MR angiography with an intravasal contrast medium and high-spatial-resolution steady-state. Eur J Radiol 81:979–984. https://doi.org/10.1016/J.EJRAD.2011.02.025
Morishita K, Murakami G, Fujisawa Y, Kawaharada N, Fukada J, Saito T, Abe T (2003) Anatomical study of blood supply to the spinal cord. Ann Thorac Surg 76:1967–1971
Murthy NS, Maus TP, Behrns CL (2010) Intraforaminal location of the great anterior radiculomedullary artery (artery of Adamkiewicz): a retrospective review. Pain Med 11:1756–1764. https://doi.org/10.1111/j.1526-4637.2010.00948.x
Nakayama Y, Awai K, Yanaga Y, Nakaura T, Funama Y, Hirai T, Yamashita Y (2008) Optimal contrast medium injection protocols for the depiction of the Adamkiewicz artery using 64-detector CT angiography. Clin Radiol 63:880–887. https://doi.org/10.1016/j.crad.2008.01.009
Nijenhuis RJ, Jacobs MJ, Schurink GW, Kessels AGH, van Engelshoven JMA, Backes WH (2007) Magnetic resonance angiography and neuromonitoring to assess spinal cord blood supply in thoracic and thoracoabdominal aortic aneurysm surgery. J Vasc Surg 45:71–77; discussion 77-8. https://doi.org/10.1016/j.jvs.2006.08.085
Nijenhuis RJ, Jacobs MJ, Jaspers K, Reijnders M, van Engelshoven JMA, Leiner T, Backes WH (2007) Comparison of magnetic resonance with computed tomography angiography for preoperative localization of the Adamkiewicz artery in thoracoabdominal aortic aneurysm patients. J Vasc Surg 45:677–685. https://doi.org/10.1016/j.jvs.2006.11.046
Nishida J, Kitagawa K, Nagata M, Yamazaki A, Nagasawa N, Sakuma H (2014) Model-based iterative reconstruction for multi-detector row CT assessment of the Adamkiewicz artery. Radiology 270:282–291. https://doi.org/10.1148/radiol.13122019
Nishii T, Kono AK, Negi N, Hashimura H, Uotani K, Okita Y, Sugimura K (2013) The feasibility of a 64-slice MDCT for detection of the Adamkiewicz artery: comparison of the detection rate of intravenous injection CT angiography using a 64-slice MDCT versus intra-arterial and intravenous injection CT angiography using a 16-slice MDCT. Int J Cardiovasc Imaging 29(Suppl 2):127–133. https://doi.org/10.1007/s10554-013-0301-z
Nojiri J, Matsumoto K, Kato A, Miho T, Furukawa K, Ohtsubo S, Itoh T, Kudo S (2007) The Adamkiewicz artery: demonstration by intra-arterial computed tomographic angiography. Eur J Cardiothorac Surg 31:249–255. https://doi.org/10.1016/j.ejcts.2006.11.024
Ou P, Schmit P, Layouss W, Sidi D, Bonnet D, Brunelle F (2007) CT angiography of the artery of Adamkiewicz with 64-section technology: first experience in children. AJNR Am J Neuroradiol 28:216–219
Polaczek M, Maslanka M, Skadorwa T, Ciszek B (2014) How does Adamkiewicz artery influence blood supply to the fetal spinal cord? Ital J Anat Embryol 119:255–262
Rodriguez-Baeza A, Muset-Lara A, Rodriguez-Pazos M, Domenech-Mateu JM (1991) The arterial supply of the human spinal cord: a new approach to the arteria radicularis magna of Adamkiewicz. Acta Neurochir 109:57–62
Schurink GWH, Nijenhuis RJ, Backes WH, Mess W, de Haan MW, Mochtar B, Jacobs MJ (2007) Assessment of spinal cord circulation and function in endovascular treatment of thoracic aortic aneurysms. Ann Thorac Surg 83:S877–S881. https://doi.org/10.1016/j.athoracsur.2006.11.028
Takase K, Sawamura Y, Igarashi K, Chiba Y, Haga K, Saito H, Takahashi S (2002) Demonstration of the artery of Adamkiewicz at multi- detector row helical CT. Radiology 223:39–45. https://doi.org/10.1148/radiol.2231010513
Takase K, Akasaka J, Sawamura Y (2007) Preoperative MDCT evaluation of the artery of Adamkiewicz and its origin. J Vasc Surg 45:1086. https://doi.org/10.1016/j.jvs.2007.03.005
Tanaka H, Ogino H, Minatoya K, Matsui Y, Higami T, Okabayashi H, Saiki Y, Aomi S, Shiiya N, Sawa Y, Okita Y, Sueda T, Akashi H, Kuniyoshi Y, Katsumata T (2016) The impact of preoperative identification of the Adamkiewicz artery on descending and thoracoabdominal aortic repair. J Thorac Cardiovasc Surg 151:122–128. https://doi.org/10.1016/j.jtcvs.2015.07.079
Uotani K, Yamada N, Kono AK, Taniguchi T, Sugimoto K, Fujii M, Kitagawa A, Okita Y, Naito H, Sugimura K (2008) Preoperative visualization of the artery of Adamkiewicz by intra-arterial CT angiography. AJNR Am J Neuroradiol 29:314–318. https://doi.org/10.3174/ajnr.A0812
Williams GM, Perler BA, Burdick JF, Osterman FA Jr, Mitchell S, Merine D, Drenger B, Parker SD, Beattie C, Reitz BA (1991) Angiographic localization of spinal cord blood supply and its relationship to postoperative paraplegia. J Vasc Surg 13:23–33 discussion 33–5
Yingbin J, Jiefei M, Jian L, Yonghui S, Haiyan P, Baimeng Z, Weigoo F (2013) Evaluation of the thoracic aortic dissection treated by endografts covering a longer distance of aorta according to the location of the Adamkiewicz artery. Thorac Cardiovasc Surg 61:569–574. https://doi.org/10.1055/s-0032-1322629
Yoshioka K, Niinuma H, Ehara S, Nakajima T, Nakamura M, Kawazoe K (2006) MR angiography and CT angiography of the artery of Adamkiewicz: state of the art. Radiographics 26(Suppl 1):S63–S73. https://doi.org/10.1148/rg.26si065506
Zhao S, Logan L, Schraedley P, Rubin GD (2009) Assessment of the anterior spinal artery and the artery of Adamkiewicz using multi-detector CT angiography. Chin Med J 122:145–149
Griepp RB, Ergin MA, Galla JD, Lansman S, Khan N, Quintana C, McCollough J, Bodian C (1996) Looking for the artery of Adamkiewicz: a quest to minimize paraplegia after operations for aneurysms of the descending thoracic and thoracoabdominal aorta. J Thorac Cardiovasc Surg 112:1202–1213 discussion 1213–5
Buffolo E, da Fonseca JHP, de Souza JAM, Alves CMR (2002) Revolutionary treatment of aneurysms and dissections of descending aorta: the endovascular approach. Ann Thorac Surg 74:S1815–S1817 discussion S1825-32
Ishimaru S, Kawaguchi S, Koizumi N, Obitsu Y, Ishikawa M (1998) Preliminary report on prediction of spinal cord ischemia in endovascular stent graft repair of thoracic aortic aneurysm by retrievable stent graft. J Thorac Cardiovasc Surg 115:811–818. https://doi.org/10.1016/S0022-5223(98)70360-5
Safi HJ, Miller CC, Carr C, Iliopoulos DC, Dorsay DA, Baldwin JC (1998) Importance of intercostal artery reattachment during thoracoabdominal aortic aneurysm repair. J Vasc Surg 27:58–66 discussion 66–8
Wadouh F, Wadouh R, Hartmann M, Crisp-Lindgren N (1990) Prevention of paraplegia during aortic operations. Ann Thorac Surg 50:543–552
Taira Y, Marsala M (1996) Effect of proximal arterial perfusion pressure on function, spinal cord blood flow, and histopathologic changes after increasing intervals of aortic occlusion in the rat. Stroke 27:1850–1858
Yoshioka K, Niinuma H, Kawazoe K, Ehara S (2005) Three-dimensional demonstration of the collateral circulation to the artery of Adamkiewicz via internal thoracic artery with 16-row multi-slice CT. Eur J Cardiothorac Surg 28:492. https://doi.org/10.1016/j.ejcts.2005.04.043
Acknowledgements
KAT was supported by the Polish Ministry of Higher Education grant for young scientists.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
For this type of meta-analysis study formal consent is not required.
Informed consent
NA
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOC 64 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Taterra, D., Skinningsrud, B., Pękala, P.A. et al. Artery of Adamkiewicz: a meta-analysis of anatomical characteristics. Neuroradiology 61, 869–880 (2019). https://doi.org/10.1007/s00234-019-02207-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-019-02207-y