Abstract
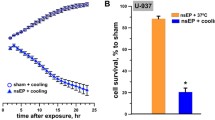
Electric pulses of nanosecond duration (nsEP) are emerging as a new modality for tissue ablation. Plasma membrane permeabilization by nsEP may cause osmotic imbalance, water uptake, cell swelling, and eventual membrane rupture. The present study was aimed to increase the cytotoxicity of nsEP by fostering water uptake and cell swelling. This aim was accomplished by lowering temperature after nsEP application, which delayed the membrane resealing and/or suppressed the cell volume mechanisms. The cell diameter in U-937 monocytes exposed to a train of 50, 300-ns pulses (100 Hz, 7 kV/cm) at room temperature and then incubated on ice for 30 min increased by 5.6 +/− 0.7 μm (40–50%), which contrasted little or no changes (1 +/− 0.3 μm, <10%) if the incubation was at 37 °C. Neither this nsEP dose nor the 30-min cooling caused cell death when applied separately; however, their combination reduced cell survival to about 60% in 1.5–3 h. Isosmotic addition of a pore-impermeable solute (sucrose) to the extracellular medium blocked cell swelling and rescued the cells, thereby pointing to swelling as a primary cause of membrane rupture and cell death. Cooling after nsEP exposure can potentially be employed in medical practice to assist tissue and tumor ablation.





Similar content being viewed by others
References
Andre FM, Rassokhin MA, Bowman AM, Pakhomov AG (2010) Gadolinium blocks membrane permeabilization induced by nanosecond electric pulses and reduces cell death. Bioelectrochemistry 79:95–100
Andreason GL, Evans GA (1989) Optimization of electroporation for transfection of mammalian cell lines. Anal Biochem 180:269–275
Babiychuk EB, Monastyrskaya K, Potez S, Draeger A (2011) Blebbing confers resistance against cell lysis. Cell Death Differ 18:80–89. doi:10.1038/cdd.2010.81
Beebe SJ, Blackmore PF, White J, Joshi RP, Schoenbach KH (2004) Nanosecond pulsed electric fields modulate cell function through intracellular signal transduction mechanisms. Physiol Meas 25:1077–1093
Beebe SJ, Fox PM, Rec LJ, Willis EL, Schoenbach KH (2003) Nanosecond, high-intensity pulsed electric fields induce apoptosis in human cells. FASEB J 17:1493–1495. doi:10.1096/fj.02-0859fje
Bier M, Hammer SM, Canaday DJ, Lee RC (1999) Kinetics of sealing for transient electropores in isolated mammalian skeletal muscle cells. Bioelectromagnetics 20:194–201
Charras GT, Hu CK, Coughlin M, Mitchison TJ (2006) Reassembly of contractile actin cortex in cell blebs. J Cell Biol 175:477–490. doi:10.1083/jcb.200602085
Chernomordik LV, Sukharev SI, Popov SV, Pastushenko VF, Sokirko AV, Abidor IG, Chizmadzhev YA (1987) The electrical breakdown of cell and lipid membranes: the similarity of phenomenologies. Biochim Biophys Acta 902:360–373
Chu G, Hayakawa H, Berg P (1987) Electroporation for the efficient transfection of mammalian cells with DNA. Nucleic Acids Res 15:1311–1326
Deng J, Schoenbach KH, Buescher ES, Hair PS, Fox PM, Beebe SJ (2003) The effects of intense submicrosecond electrical pulses on cells. Biophys J 84:2709–2714
Deutsch C, Lee SC (1988) Cell volume regulation in lymphocytes. Ren Physiol Biochem 11:260–276
Dunn WA, Hubbard AL, Aronson NN Jr (1980) Low temperature selectively inhibits fusion between pinocytic vesicles and lysosomes during heterophagy of 125I-asialofetuin by the perfused rat liver. J Biol Chem 255:5971–5978
Escande-Geraud ML, Rols MP, Dupont MA, Gas N, Teissie J (1988) Reversible plasma membrane ultrastructural changes correlated with electropermeabilization in Chinese hamster ovary cells. Biochim Biophys Acta 939:247–259
Galluzzi L et al (2015) Essential versus accessory aspects of cell death: recommendations of the NCCD 2015. Cell Death Differ 22:58–73. doi:10.1038/cdd.2014.137
Gehl J, Skovsgaard T, Mir LM (2002) Vascular reactions to in vivo electroporation: characterization and consequences for drug and gene delivery. Biochim Biophys Acta 1569:51–58
Glaser RW, Leikin SL, Chernomordik LV, Pastushenko VF, Sokirko AI (1988) Reversible electrical breakdown of lipid bilayers: formation and evolution of pores. Biochim Biophys Acta 940:275–287
Hall EH, Schoenbach KH, Beebe SJ (2005) Nanosecond pulsed electric fields (nsPEF) induce direct electric field effects and biological effects on human colon carcinoma cells. DNA Cell Biol 24:283–291
Haylett T, Thilo L (1991) Endosome-lysosome fusion at low temperature. J Biol Chem 266:8322–8327
Hoffmann EK, Pedersen SF (1998) Sensors and signal transduction in the activation of cell volume regulatory ion transport systems. Contrib Nephrol 123:50–78
Ibey BL et al (2010) Selective cytotoxicity of intense nanosecond-duration electric pulses in mammalian cells. Biochim Biophys Acta 1800:1210–1219. doi:10.1016/j.bbagen.2010.07.008
Ibey BL, Roth CC, Pakhomov AG, Bernhard JA, Wilmink GJ, Pakhomova ON (2011) Dose-dependent thresholds of 10-ns electric pulse induced plasma membrane disruption and cytotoxicity in multiple cell lines. PLoS One 6:e15642. doi:10.1371/journal.pone.0015642
Idone V, Tam C, Goss JW, Toomre D, Pypaert M, Andrews NW (2008) Repair of injured plasma membrane by rapid Ca2+-dependent endocytosis. J Cell Biol 180:905–914. doi:10.1083/jcb.200708010
Jaiswal JK, Andrews NW, Simon SM (2002) Membrane proximal lysosomes are the major vesicles responsible for calcium-dependent exocytosis in nonsecretory cells. J Cell Biol 159:625–635. doi:10.1083/jcb.200208154
Kinosita K Jr, Tsong TT (1977a) Hemolysis of human erythrocytes by transient electric field. Proc Natl Acad Sci USA 74:1923–1927
Kinosita K Jr, Tsong TY (1977b) Formation and resealing of pores of controlled sizes in human erythrocyte membrane. Nature 268:438–441
Knight DE, Baker PF (1982) Calcium-dependence of catecholamine release from bovine adrenal medullary cells after exposure to intense electric fields. J Membr Biol 68:107–140
Lariccia V, Fine M, Magi S, Lin MJ, Yaradanakul A, Llaguno MC, Hilgemann DW (2011) Massive calcium-activated endocytosis without involvement of classical endocytic proteins. J Gen Physiol 137:111–132. doi:10.1085/jgp.201010468
Lee B, McKenna K, Bramhall J (1985) Kinetic studies of human erythrocyte membrane resealing. Biochim Biophys Acta 815:128–134
Lee SC, Price M, Prystowsky MB, Deutsch C (1988) Volume response of quiescent and interleukin 2-stimulated T-lymphocytes to hypotonicity. Am J Physiol 254:C286–C296
Lieber MR, Steck TL (1982) Dynamics of the holes in human erythrocyte membrane ghosts. J Biol Chem 257:11660–11666
McNeil PL, Steinhardt RA (1997) Loss, restoration, and maintenance of plasma membrane integrity. J Cell Biol 137:1–4
Miyake K, McNeil PL (1995) Vesicle accumulation and exocytosis at sites of plasma membrane disruption. J Cell Biol 131:1737–1745
Nesin OM, Pakhomova ON, Xiao S, Pakhomov AG (2011) Manipulation of cell volume and membrane pore comparison following single cell permeabilization with 60- and 600-ns electric pulses. Biochim Biophys Acta 3:792–801
Nuccitelli R et al (2009) A new pulsed electric field therapy for melanoma disrupts the tumor’s blood supply and causes complete remission without recurrence. Int J Cancer 125:438–445
Nuccitelli R et al (2006) Nanosecond pulsed electric fields cause melanomas to self-destruct. Biochem Biophys Res Commun 343:351–360
Nuccitelli R, Tran K, Sheikh S, Athos B, Kreis M, Nuccitelli P (2010) Optimized nanosecond pulsed electric field therapy can cause murine malignant melanomas to self-destruct with a single treatment. Int J Cancer 127:1727–1736. doi:10.1002/ijc.25364
Okada Y, Maeno E, Shimizu T, Dezaki K, Wang J, Morishima S (2001) Receptor-mediated control of regulatory volume decrease (RVD) and apoptotic volume decrease (AVD). J Physiol 532:3–16
Pahapill PA, Schlichter LC (1990) Modulation of potassium channels in human T lymphocytes: effects of temperature. J Physiol 422:103–126
Pakhomov AG, Kolb JF, White JA, Joshi RP, Xiao S, Schoenbach KH (2007a) Long-lasting plasma membrane permeabilization in mammalian cells by nanosecond pulsed electric field (nsPEF). Bioelectromagnetics 28:655–663
Pakhomov AG, Pakhomova ON (2010) Nanopores: A distinct transmembrane passageway in electroporated cells. In: Pakhomov AG, Miklavcic D, Markov MS (eds) Advanced electroporation techniques in biology in medicine. CRC Press, Boca Raton, pp 178–194
Pakhomov AG et al (2004) Characterization of the cytotoxic effect of high-intensity, 10-ns duration electrical pulses IEEE transactions on Plasma. Science 32:1579–1585
Pakhomov AG, Shevin R, White JA, Kolb JF, Pakhomova ON, Joshi RP, Schoenbach KH (2007b) Membrane permeabilization and cell damage by ultrashort electric field shocks. Arch Biochem Biophys 465:109–118
Pakhomova ON, Gregory B, Semenov I, Pakhomov AG (2014) Calcium-mediated pore expansion and cell death following nanoelectroporation. Biochim Biophys Acta 1838:2547–2554. doi:10.1016/j.bbamem.2014.06.015
Pakhomova ON, Gregory BW, Khorokhorina VA, Bowman AM, Xiao S, Pakhomov AG (2011) Electroporation-induced electrosensitization. PLoS ONE 6:e17100. doi:10.1371/journal.pone.0017100
Pakhomova ON, Gregory BW, Semenov I, Pakhomov AG (2013) Two modes of cell death caused by exposure to nanosecond pulsed electric field. PLoS ONE 8:e70278. doi:10.1371/journal.pone.0070278
Potter H (1993) Application of electroporation in recombinant DNA technology. Methods Enzymol 217:461–478
Rassokhin MA, Pakhomov AG (2012) Electric field exposure triggers and guides formation of pseudopod-like blebs in U937 monocytes. J Membr Biol 245:521–529. doi:10.1007/s00232-012-9433-7
Rassokhin MA, Pakhomov AG (2014) Cellular regulation of extension and retraction of pseudopod-like blebs produced by nanosecond pulsed electric field (nsPEF). Cell Biochem Biophys 69:555–566. doi:10.1007/s12013-014-9831-9
Ren W, Sain NM, Beebe SJ (2012) Nanosecond pulsed electric fields (nsPEFs) activate intrinsic caspase-dependent and caspase-independent cell death in Jurkat cells. Biochem Biophys Res Commun 421:808–812. doi:10.1016/j.bbrc.2012.04.094
Rols MP, Dahhou F, Mishra KP, Teissie J (1990) Control of electric field induced cell membrane permeabilization by membrane order. BioChemistry 29:2960–2966
Rols MP, Delteil C, Serin G, Teissie J (1994) Temperature effects on electrotransfection of mammalian cells. Nucleic Acids Res 22:540
Saulis G (1999) Cell electroporation: estimation of the number of pores and their sizes. Biomed Sci Instrum 35:291–296
Saulis G (2005) The loading of human erythrocytes with small molecules by electroporation. Cell Mol Biol Lett 10:23–35
Saulis G, Venslauskas MS, Naktinis J (1991) Kinetics of pore resealing in cell membranes after electroporation. Bioelectrochem Bioenerg 26:1–13
Schoenbach KS et al (2007) Bioelectric effects of nanosecond pulses. IEEE Trans on Dielectr Electr Insulation 14:1088–1109
Serpersu EH, Kinosita K Jr, Tsong TY (1985) Reversible and irreversible modification of erythrocyte membrane permeability by electric field. Biochim Biophys Acta 812:779–785
Sowers AE, Lieber MR (1986) Electropore diameters, lifetimes, numbers, and locations in individual erythrocyte ghosts. FEBS Lett 205:179–184
Stacey M, Stickley J, Fox P, Statler V, Schoenbach K, Beebe SJ, Buescher S (2003) Differential effects in cells exposed to ultra-short, high intensity electric fields: cell survival, DNA damage, and cell cycle analysis. Mutat Res 542:65–75
Tam C et al (2010) Exocytosis of acid sphingomyelinase by wounded cells promotes endocytosis and plasma membrane repair. J Cell Biol 189:1027–1038. doi:10.1083/jcb.201003053
Teissie J, Ramos C (1998) Correlation between electric field pulse induced long-lived permeabilization and fusogenicity in cell membranes. Biophys J 74:1889–1898. doi:10.1016/S0006-3495(98)77898-1
Teissie J, Tsong TY (1980) Evidence of voltage-induced channel opening in Na/K ATPase of human erythrocyte membrane. J Membr Biol 55:133–140
Walker K 3rd, Pakhomova ON, Kolb J, Schoenbach KS, Stuck BE, Murphy MR, Pakhomov AG (2006) Oxygen enhances lethal effect of high-intensity, ultrashort electrical pulses. Bioelectromagnetics 27:221–225
Wang J et al (2012) Synergistic effects of nanosecond pulsed electric fields combined with low concentration of gemcitabine on human oral squamous cell carcinoma in vitro. PLoS ONE 7:e43213. doi:10.1371/journal.pone.0043213
Yin D et al (2012) Cutaneous papilloma and squamous cell carcinoma therapy utilizing nanosecond pulsed electric fields (nsPEF). PLoS ONE 7:e43891. doi:10.1371/journal.pone.0043891
Acknowledgements
This work was supported by a 2015 AFOSR MURI grant (to AGP) on Nanoelectropulse-Induced Electromechanical Signaling and Control of Biological Systems, administered through Old Dominion University and by a grant from Pulse Biosciences, Inc. (to O.N.P.)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors confirm that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Muratori, C., Pakhomov, A.G. & Pakhomova, O.N. Effect of Cooling On Cell Volume and Viability After Nanoelectroporation. J Membrane Biol 250, 217–224 (2017). https://doi.org/10.1007/s00232-017-9952-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00232-017-9952-3