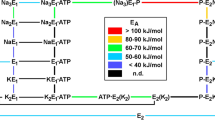
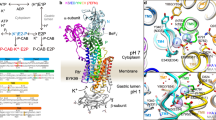
Abstract
The interactions of divalent cations with the adenosine triphosphatase (ATPase) and para-nitrophenyl phosphatase (pNPPase) activity of the purified dog kidney Na pump and the fluorescence of fluorescein isothiocyanate (FITC)-labeled pump were determined. Sr2+ and Ba2+ did not compete with K+ for ATPase (an extracellular K+ effect). Sr2+ and Ba2+ did compete with Na+ for ATPase (an intracellular Na+ effect) and with K+ for pNPPase (an intracellular K+ effect). These results suggest that Ba2+ or Sr2+ can bind to the intracellular transport site, yet neither Ba2+ nor Sr2+ was able to activate pNPPase activity; we confirmed that Ca2+ and Mn2+ did activate. As another measure of cation binding, we observed that Ca2+ and Mn2+, but not Ba2+, decreased the fluorescence of the FITC-labeled pump; we confirmed that K+ substantially decreased the fluorescence. Interestingly, Ba2+ did shift the K+ dose-response curve. Ethane diamine inhibited Mn2+ stimulation of pNPPase (as well as K+ and Mg2+ stimulation) but did not shift the 50% inhibitory concentration (IC50) for the Mn2+-induced fluorescence change of FITC, though it did shift the IC50 for the K+-induced change. These results suggest that the Mn2+-induced fluorescence change is not due to Mn2+ binding at the transport site. The drawbacks of models in which Mn2+ stimulates pNPPase by binding solely to the catalytic site vs. those in which Mn2+ stimulates by binding to both the catalytic and transport sites are presented. Our results provide new insights into the pNPPase kinetic mechanism as well as how divalent cations interact with the Na pump.











Similar content being viewed by others
References
Albers RW (1967) Biochemical aspects of active transport. Annu Rev Biochem 36:727–756
Apell HJ (2004) How do P-type ATPases transport ions? Bioelectrochemistry 63:149–156
Apell HJ (2003) Structure-function relationship in P-type ATPases–a biophysical approach. Rev Physiol Biochem Pharmacol 150:1–35
Beauge L, Campos MA (1983) Calcium inhibition of the ATPase and phosphatase activities of Na+,K+-ATPase. Biochim Biophys Acta 729:137–149
Beauge L, Campos MA (1986) Effects of mono and divalent cations on total and partial reactions catalysed by pig kidney Na,K-ATPase. J Physiol 375:1–25
Blostein R (1999) Structure-function studies of the sodium pump. Biochem Cell Biol 77:1–10
Blostein R, Chu L (1977) Sidedness of (sodium, potassium)-adenosine triphosphate of inside-out red cell membrane vesicles. Interactions with potassium. J Biol Chem 252:3035–3043
Campos M, Beauge L (1988) Binding of manganese ions to the Na+,K+-ATPase during phosphorylation by ATP. Biochim Biophys Acta 944:242–248
Campos M, Berberian G, Beauge L (1988) Phosphatase activity of Na+,K+-ATPase. Enzyme conformations from ligands interactions and Rb occlusion experiments. Biochim Biophys Acta 940:43–50
Drapeau P, Blostein R (1980) Interactions of K+ with (Na,K)-ATPase orientation of K+- phosphatase sites studied with inside-out red cell membrane vesicles. J Biol Chem 255:827–834
Farley RA, Tran CM, Carilli CT, Hawke D, Shively JE (1984) The amino acid sequence of a fluorescein-labeled peptide from the active site of (Na,K)-ATPase. J Biol Chem 259:9532–9535
Forbush B 3rd (1988) Rapid release of 45Ca from an occluded state of the Na,K-pump. J Biol Chem 263:7970–7978
Gatto C, Helms JB, Prasse MC, Arnett KL, Milanick MA (2005) Kinetic characterization of tetrapropylammonium inhibition reveals how ATP and Pi alter access to the Na+-K+-ATPase transport site. Am J Physiol 289:C302–C311
Gatto C, Helms JB, Prasse MC, Huang SY, Zou X, Arnett KL, Milanick MA (2006) Similarities and differences between organic cation inhibition of the Na,K-ATPase and PMCA. Biochemistry 45:13331–13345
Gonzalez-Lebrero RM, Kaufman SB, Garrahan PJ, Rossi RC (2002) The occlusion of Rb+ in the Na+/K+-ATPase. II. The effects of Rb+, Na+, Mg2+, or ATP on the equilibrium between free and occluded Rb+. J Biol Chem 277:5922–5928
Horisberger JD (2004) Recent insights into the structure and mechanism of the sodium pump. Physiology (Bethesda) 19:377–387
Huang W, Askari A (1975) Na+/K+-activated adenosinetriphosphatase: fluorimetric determination of the associated K+ -dependent 3-O-methylfluorescein phosphatase and its use for the assay of enzyme samples with low activities. Anal Biochem 66:265–271
Huang WH, Askari A (1984) Interaction of Ca2+ with Na+,K+-ATPase: properties of the Ca2+-stimulated phosphatase activity. Arch Biochem Biophys 231:287–292
Jorgensen PL (1974) Purification and characterization of Na+/K+-ATPase. IV. Estimation of the purity and of the molecular weight and polypeptide content per enzyme unit in preparations from the outer medulla of rabbit kidney. Biochim Biophys Acta 356:53–67
Jorgensen PL, Hakansson KO, Karlish SJD (2003) Structure and mechanism of Na,K-ATPase: functional sites and their interactions. Annu Rev Physiol 65:817–849
Jorgensen PL, Petersen J (1985) Chymotryptic cleavage of alpha-subunit in E1-forms of renal Na+/K+-ATPase: effects on enzymatic properties, ligand binding and cation exchange. Biochim Biophys Acta 821:319–333
Kaplan JH (2002) Biochemistry of Na,K-ATPase. Annu Rev Biochem 71:511–535
Karlish SJ (1980) Characterization of conformational changes in (Na,K) ATPase labeled with fluorescein at the active site. J Bioenerg Biomembr 12:111–136
Karlish SJ (2003) Investigating the energy transduction mechanism of P-type ATPases with Fe2+-catalyzed oxidative cleavage. Ann N Y Acad Sci 986:39–49
Lin SH, Faller LD (2000) Preparation of Na,K-ATPase specifically modified on the anti-fluorescein antibody-inaccessible site by fluorescein 5’-isothiocyanate. Anal Biochem 287:303–312
Martin DW (2005) Structure-function relationships in the Na+/K+-pump. Semin Nephrol 25:282–291
Moller JV, Juul B, le Maire M (1996) Structural organization, ion transport, and energy transduction of P-type ATPases. Biochim Biophys Acta 1286:1–51
Robinson JD (1981) Substituting manganese for magnesium alters certain reaction properties of the Na+/K+-ATPase. Biochim Biophys Acta 642:405–417
Robinson JD (1985) Divalent cations and the phosphatase activity of the Na+/K+-dependent ATPase. J Bioenerg Biomembr 17:183–200
Robinson JD (1989) Modification of ligand binding to the Na+/K+-activated ATPase. Biochim Biophys Acta 997:41–48
Robinson JD, Levine GM, Robinson LJ (1983) A model for the reaction pathways of the K+-dependent phosphatase activity of the Na+/K+-dependent ATPase. Biochim Biophys Acta 731:406–414
Robinson JD, Pratap PR (1993) Indicators of conformational changes in the Na+/K+-ATPase and their interpretation. Biochim Biophys Acta 1154:83–104
Schneeberger A, Apell HJ (2001) Ion selectivity of the cytoplasmic binding sites of the Na,K-ATPase: II. Competition of various cations. J Membr Biol 179:263–273
Swann AC, Albers RW (1980) Na,K-ATPase of mammalian brain: differential effects on cation affinities of phosphorylation by ATP and acetylphosphate. Arch Biochem Biophys 203:422–427
Toyoshima C, Inesi G (2004) Structural basis of ion pumping by Ca2+-ATPase of the sarcoplasmic reticulum. Annu Rev Biochem 73:269–292
Toyoshima C, Nomura H, Sugita Y (2003) Structural basis of ion pumping by Ca2+-ATPase of sarcoplasmic reticulum. FEBS Lett 555:106–110
Vasallo PM, Post RL (1986) Calcium ion as a probe of the monovalent cation center of sodium, potassium ATPase. J Biol Chem 261:16957–16962
Vilsen B (1999) Mutant Phe788→Leu of the Na+/K+-ATPase is inhibited by micromolar concentrations of potassium and exhibits high Na+-ATPase activity at low sodium concentrations. Biochemistry 38:11389–11400
Acknowledgement
This work was supported by National Institutes of Health grants GM061583 (to C. G.) and DK37512 (to M. A. M.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gatto, C., Arnett, K.L. & Milanick, M.A. Divalent Cation Interactions with Na,K-ATPase Cytoplasmic Cation Sites: Implications for the para-Nitrophenyl Phosphatase Reaction Mechanism. J Membrane Biol 216, 49–59 (2007). https://doi.org/10.1007/s00232-007-9028-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00232-007-9028-x