Abstract
Purpose
Adefovir (as dipivoxil) was selected as a probe drug in a previous transporter cocktail phenotyping study to assess renal organic anion transporter 1 (OAT1), with renal clearance (CLR) as the primary parameter describing renal elimination. An approximately 20% higher systemic exposure of adefovir was observed when combined with other cocktail components (metformin, sitagliptin, pitavastatin, and digoxin) compared to sole administration. The present evaluation applied a population pharmacokinetic (popPK) modeling approach to describe adefovir pharmacokinetics as a cocktail component in more detail.
Methods
Data from 24 healthy subjects were reanalyzed. After establishing a base model, covariate effects, including the impact of co-administered drugs, were assessed using forward inclusion then backward elimination.
Results
A one-compartment model with first-order absorption (including lag time) and a combination of nonlinear renal and linear nonrenal elimination best described the data. A significantly higher apparent bioavailability (73.6% vs. 59.0%) and a lower apparent absorption rate constant (2.29 h−1 vs. 5.18 h−1) were identified in the combined period compared to the sole administration period, while no difference was seen in renal elimination. The population estimate for the Michaelis-Menten constant (Km) of the nonlinear renal elimination was 170 nmol/L, exceeding the observed range of adefovir plasma maximum concentration, while the maximum rate (Vmax) of nonlinear renal elimination was 2.40 µmol/h at the median absolute estimated glomerular filtration rate of 105 mL/min.
Conclusion
The popPK modeling approach indicated that the co-administration primarily affected the apparent absorption and/or prodrug conversion of adefovir dipivoxil, resulting in the minor drug-drug interaction observed for adefovir as a victim. However, renal elimination remained unaffected. The high Km value suggests that assessing renal OAT1 activity by CLR has no relevant misspecification error with the cocktail doses used.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Membrane transporter proteins play a key role in the pharmacokinetics of drugs and are a potential target for transporter-based drug-drug interactions (DDIs) [1]. The “cocktail approach,” which uses a combined administration of selective probe drugs for various transporters, is a valuable approach for the simultaneous investigation of several DDIs in a single clinical trial [2]. Although several cocktails have been established [3,4,5], the specificity of probe substances and the suitability of their pharmacokinetic (PK) parameters for characterizing transporter activities need to be investigated in more detail.
The organic anion transporter 1 (OAT1; gene name SLC22A6), predominantly located in the basolateral membrane of renal proximal tubular cells and mainly responsible for renal disposition of numerous prescribed drugs (e.g., diuretics and antivirals) [6], has been highlighted by regulatory agencies as a key transporter involved in potential DDIs [7, 8]. In order to establish a “more selective” cocktail, according to the suggestions by the FDA guideline [7], adefovir dipivoxil was selected as a specific probe drug for renal OAT1 activity in a previous clinical DDI cocktail study [3], as it is highly selective for OAT1 in vitro [9]. In addition, renal clearance (CLR), which only depends on renal excretion driven by plasma concentrations and is independent of other PK processes (e.g., drug absorption or conversion rate form its prodrug), was selected as the appropriate and practical primary metric to reflect renal OAT1 activity [3]. While the use of renal secretion of adefovir to assess OAT1 activity is considered the gold standard, this metric depends on a precise measurement of glomerular filtration rate (GFR) and fraction unbound (fu), which were unavailable in the study [3]. In the case of adefovir with fu close to 1 [10], the unavailability of this parameter however is of little relevance.
Although the primary metric supported the absence of relevant interactions of adefovir with the other components of the cocktail (including 100 mg sitagliptin, 500 mg metformin, 2 mg pitavastatin, and 0.5 mg digoxin), an approximately 20% increase in systemic exposure for adefovir was observed during concomitant administration [3]. This indicated that there might still be some minor inhibition of OAT1, and/or an effect on other PK processes of adefovir might be present [3]. Extended in vitro analyses, however, did not support an effect of the other cocktail components on OAT1 activity [11]. Unfortunately, the non-compartmental analysis (NCA) applied in this trial could not explain the changes of PK processes in more detail.
Understanding the minor DDI observed for the victim drug adefovir in more detail also needs to take into account that OAT1-mediated secretion of adefovir (almost 60% of renal elimination [2]) might be saturable at studied concentrations. While this hypothesis might not be fully supported by in vitro findings, since adefovir peak plasma concentrations (Cmax) obtained in the clinical trial (5.56–91.0 nmol/L following a single dose of 10 mg adefovir dipivoxil [3]) were well below the Michaelis-Menten constant (Km, mean ± standard deviation [SD] 23.8 ± 4.2 µmol/L) observed in the studies [12, 13], there is evidence that such saturation may exist in vivo. Nonlinearities in adefovir Cmax and/or area under the curve (AUC) have been reported in infants, children infected with HIV, and in adult volunteers [14, 15]. However, a detailed description of the PK processes contributing to the renal elimination of adefovir is currently lacking. If such non-linearities are not properly considered, the reliability of adefovir metrics for detection and quantification of potential DDIs might be affected in the cocktail.
Given these challenges, population pharmacokinetic (popPK) modeling might allow to gain a more mechanistic insight into the potential PK processes involved in the minor DDI observed for adefovir and to identify a possible nonlinear elimination process more precisely.
Materials and methods
Clinical study
Data from 24 healthy subjects in the previous clinical trial were reanalyzed [3]. The trial (ClinicalTrials.gov identifier: NCT02743260) was approved by the Ethics Committee of the Medical Faculty of the University of Cologne, Germany (application number 15-421; approval date: February 19, 2016). Conductance adhered to the pertinent version of the Declaration of Helsinki and to International Conference on Harmonization guidelines for Good Clinical Practice. All subjects gave informed consent. In this trial, a single 10 mg dose of adefovir dipivoxil was given alone and in combination with four aforementioned probe drugs in the reference and test periods, respectively. Plasma and urine concentrations of adefovir were measured up to 24 h using a validated HPLC–MS/MS method [16]. Lower limits of quantification (LLOQs) for adefovir were 0.998 nmol/L in plasma and 0.382 µmol/L in urine, respectively [16]. The PK parameters of adefovir in reference and test periods were initially estimated via NCA using Phoenix WinNonlin™ (Version 7.0, Certara, NJ, USA), based on the concentration profiles in plasma and urine [3].
Basic population pharmacokinetic analysis
A popPK model of adefovir was developed using the nonlinear mixed-effects modeling program NONMEM 7.5.0 (ICON plc, Dublin, Ireland), Perl speaks NONMEM (PsN 5.2.6), and Pirana 3.0.0 (Certara, Princeton, NJ). R version 4.2.1 (R Foundation for Statistical Computing, Vienna, Austria) was used to build figures for model evaluations and for statistical summaries. The base model was developed starting from a one-compartment model, and model complexity was increased step by step. Inter-individual (IIV) and inter-occasion variability (IOV) of PK parameters were estimated assuming log-normally distributed individual PK parameters, and additive, proportional, and combined residual error models were evaluated. To identify a suitable base model, we focus on Bayesian information criterion (BIC)-based comparisons, which penalize model complexity and mitigate the risk of overfitting [17].
The elimination of adefovir was modeled with two components: renal clearance (CLR) and nonrenal clearance (CLNR). Since adefovir exhibits negligible plasma protein binding (< 3.0%) [10] and was not quantified in the study, we assumed that fu = 1 for modeling purposes. Employing a one-compartment model with first-order absorption, two elimination models were considered. “Model 1” assumed linear kinetics for both renal and nonrenal elimination. In “Model 2”, renal elimination followed Michaelis-Menten-type nonlinear kinetics, while nonrenal elimination retained linear kinetics. The details of these models are presented in Table 1.
Covariate evaluation
For covariate evaluations, we utilized objective function value (OFV)-based statistical tests, employing a forward inclusion and backward elimination approach to investigate the influence of co-administered drugs, demographic, and physiological variables. We evaluated the impact of concurrent administration of cocktail components on adefovir PK by separately determining all parameters for both reference and test periods after establishing a reasonable base model. To identify the impact of demographic and physiological factors on PK parameters, we individually added variables like age, sex, body weight, body mass index (BMI), body surface area (BSA), serum creatinine concentration, serum cystatin C concentration, and absolute estimated GFR (AGFR) to the base model. GFR was estimated via the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) 2012 equation [18] and adjusted to AGFR based on individual BSA, determined using the Mosteller formula [19].
Covariates that resulted in a significant decrease (p < 0.05, chi-squared distribution with one degree of freedom) of at least 3.84 in the OFV from the basic model and a reduction in the variability of the PK parameter were included. All significant covariates were simultaneously integrated into a comprehensive “full” model. Subsequently, each covariate was individually removed from the “full” model. If the increase in OFV exceeded 6.64 (p < 0.01, chi-squared distribution with one degree of freedom), indicating significant association with the PK parameter, it was retained in the final model.
Model evaluation
Model validation was based on graphical and statistical criteria. Goodness-of-fit (GOF) plots, which included observed versus population prediction; observed versus individual prediction; conditional weighted residuals (CWRES) versus time and CWRES versus population prediction were initially used for diagnostic purposes [20]. The stability and predictive performance of the final model were further validated by non-parametric bootstrap analysis and visual predictive check (VPC) techniques [21, 22]. For the bootstrap analysis, resampling was repeated 1000 times, and medians and 95% confidence intervals (CIs) for the estimated parameters obtained from the bootstrap procedure were compared with the final model estimates [23]. Regarding the VPC, 1000 datasets were simulated using the final population model parameters, and 95% CIs for 2.5th, median (50th), and 97.5th percentiles of simulated data were calculated and then compared with the observations [21, 22].
Calculation of individual average CLR and individual half-live (t1/2)
The reliability of model parameter estimates were further evaluated by comparing them to previous NCA results [3]. While most parameters could be directly compared, the comparison of CLR between NCA and popPK analysis required special consideration due to its dependence on concentration and changes over time in cases of saturable/nonlinear elimination. In the popPK analysis, individual average CLR values were derived from the empirical Bayes estimates (EBEs) generated by our final model. The EBEs were obtained by integrating the Michaelis-Menten-type nonlinear renal clearance over the observation time period and dividing by the duration of the observation period. Therefore, this provides the average CLR values during the observation period for each subject, accounting for the plasma concentrations of adefovir as described by the model.
Furthermore, individual t1/2 of adefovir was computed using the formula: \({t}_{1/2}= \frac{0.693\times V}{CL}\), where CL represents individual total body clearance derived from CLR + CLNR. Here, CLR represents the aforementioned individual values, while CLNR and the volume of distribution (V) represent the individual EBEs obtained from the final model.
Results
Twenty-four healthy subjects (14 female) with the mean ± SD BMI of 24.5 ± 3.10 kg/m2 and age of 40.4 ± 16.0 years completed the clinical trial [3], resulting in 1101 adefovir plasma and urine concentrations for the popPK analysis. Detailed information on the demographic characteristics of subjects is provided in Supplemental Table 1s. A total of 9 (0.817%) samples with concentrations lower than the LLOQs were removed from the analysis. Supplemental Fig. 1s shows the plasma concentration and urine excretion profiles of adefovir over time.
Population pharmacokinetic analysis
Adefovir base model
Plasma and urine samples were jointly analyzed using a one-compartment model, incorporating first-order absorption (including lag time) and combined renal and nonrenal elimination. This model adequately described adefovir concentrations in both plasma and urine and was selected as the base model. Implementation of nonlinear renal elimination decreased the OFV by 25.2 compared to linear renal elimination. IIV was found to be significant on the maximum rate (Vmax) of nonlinear renal elimination and V. Introduction of IOV for apparent bioavailability, apparent absorption rate constant (Ka), lag time, and V improved the model significantly (OFV reduced by 400, 212, 61.6, 235 points, respectively). The residual unexplained variability of both plasma and urine data was best described by proportional error models.
Covariate model for effects of co-treatment
All potential PK parameters were separately assessed for reference and test periods. As a result, a significantly higher apparent bioavailability was identified in the test period (point estimate 73.6%) compared to the published value of 59.0% [24] which was inputted for the reference period (drop in OFV by 7.39). Additionally, a significant decrease in apparent Ka was observed during the test period (2.29 h−1) compared to the reference period (5.18 h−1), resulting in a decrease of OFV by 9.11. Introducing additional PK parameters for reference and test periods separately did not result in significant model improvement.
Final model with demographic and physiological covariates
Several demographic and clinical parameters (age, body weight, body height, sex, BMI, BSA, serum creatinine and cystatin C concentrations, and AGFR) were tested as potential covariates on PK parameters. After stepwise covariate model building, AGFR on Vmax was the only statistically significant covariate retained in the model (drop in OFV of 12.2 points). The final covariate model on Vmax is therefore represented by \({{\text{TVV}}}_{{\text{max}}}={\theta }_{{\text{Vmax}}}\times ({\text{AGFR}}/105)\), where TVVmax is the typical value of Vmax and 105 mL/min is the median AGFR. Using a power relationship instead of a proportional relationship to describe the effect of AGFR did not improve the model. The key model development steps are summarized in Table 2.
Model evaluation
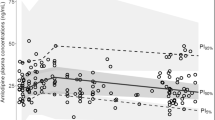
The VPC results (Fig. 1) showed that medians and 2.5th and 97.5th percentiles of the simulated data from the final model incorporating Michaelis-Menten-type nonlinear renal elimination (“Nonlinear model”) were in acceptable agreement with the observations. To facilitate a comparison between models with and without nonlinear renal elimination, an additional VPC is presented. This VPC is based on a linear renal elimination model (“Linear model”), which also incorporates AGFR as a covariate using a proportional equation, this time on CLR, to adjust for individual differences in renal function. Apart from a higher population variability in simulated compared to observed urine excretion amounts with both models, the VPC of the “Nonlinear model” shows no obvious misspecification, while it performs slightly better than the “Linear model,” particularly concerning the initial high adefovir amounts excreted in urine. The GOF plots (Supplemental Fig. 2s) demonstrated that the final model adequately described the observed adefovir plasma concentrations and urinary excretion, exhibiting a satisfactory fit without notable trends. This suggests the absence of systematic deviations in the model. The final point estimates and bootstrap statistics of PK parameters are summarized in Table 3. There are no indications of overparameterization in any of the model diagnostics or the bootstrap results.
Visual predictive check (n = 1000) for the final model stratified by plasma and urine data, and categorized by renal elimination models: linear (“Linear model”) and nonlinear (“Nonlinear model”). Open circles illustrate observed data points. Solid (dashed) black and blue lines represent medians (2.5th and 97.5th percentiles) of observations and simulated data, respectively; blue, gray, and blue areas represent 95% confidence intervals of the 2.5th, median, and 97.5th percentiles of simulated data
Calculation of individual average CLR and individual t1/2
The median (range) individual average CLR and individual t1/2 values for adefovir during the reference and test periods were 12.4 (7.97–34.0) L/h and 6.59 (5.61–8.45) h, and 12.2 (7.82–31.0) L/h and 6.35 (5.07–9.01) h, respectively.
Discussion
The present popPK analysis identified that changes in apparent absorption rate and apparent bioavailability, rather than changes in renal elimination, are the primary causes of the slight DDI that was found for adefovir as a victim when co-administered with other transporter probe substrates. Although the identified nonlinear renal clearance of adefovir is not saturated at the standard dose, further dose reduction in the existing transporter phenotyping cocktail might avoid even minor DDIs.
In this evaluation, plasma and urine data could be reasonably explained by a one-compartment model with first-order absorption (including lag time) and a combination of Michaelis-Menten-type nonlinear renal and linear nonrenal elimination. Despite previous studies reported that adefovir plasma levels declined biexponentially [10, 25, 26], our investigation did not reveal an observable biphasic decline in the semi-log plots (Supplemental Fig. 1s). Introducing a second compartment to the one-compartment base model did not significantly improve the BIC score (drop in BIC by 1.44), residual unexplained variability, or VPCs. Therefore, the one-compartment model was chosen as the final model.
The estimates for PK parameters of adefovir we present here are in line with the previous NCA conducted on the same dataset [3]. According to the final model, the individual apparent volume of distribution (V/F) for adefovir was calculated based on the individual EBEs of V divided by individual EBEs of F. The median of these individual values was 369 L and 308 L in the reference and test periods, respectively. These values are comparable to the geometric mean values obtained from the previous NCA study, which reported 368 L and 307 L for the corresponding periods [3]. Additionally, the median (range) CLR and t1/2 values for adefovir during the reference and test periods in this evaluation are in accordance with those reported in the NCA study [3]. According to the NCA, during the reference period, the geometric mean (95% CI) CLR and the geometric mean (95% CI) t1/2 of adefovir were 12.3 (6.06–24.9) L/h and 6.38 (4.68–8.72) h, respectively. In the test period, these values were 11.3 (7.26–17.8) L/h and 6.58 (5.05–8.57) h, respectively [3].
In our analysis, after normalizing for body weight, median individual values for V/F, CLR, and CL/F are 4.89 L/kg, 0.175 L/h/kg, and 0.493 L/h/kg, respectively. Moreover, the median urinary recovery over 24 h is 46.4%. These findings align with the characteristics reported in the summary of product characteristics for HEPSERA® (adefovir dipivoxil) tablets [23] and are consistent with observations by Sokal et al. [26]. Sokal et al. studied adolescents aged 12–17 years, finding mean ± SD values of 0.739 ± 0.192 L/h/kg for CL/F, 7.16 ± 1.6 L/kg for V/F, and 6.84 ± 0.97 h for t/2 after a single oral dose of 10 mg adefovir dipivoxil [26]. However, our results were slightly lower than those reported by Hughes et al. [15] in infants and children (3 months–18 years) after a 1.5 mg/kg adefovir dipivoxil dose, showing a median CL/F of 1.0 L/h/kg and a median V/F of 8.1 L/kg. Conversely, our estimates of CL/F and V/F were slightly higher than those reported by Shiffman et al. [27] in patients with mild renal impairment (creatinine clearance ≥ 50 to < 80 mL/min), reflecting a mean CL/F of 0.270 L/h/kg and a mean V/F of 2.6 L/kg [27]. These discrepancies may stem from physiological variations due to factors such as growth, development, and disease.
In the study by Cundy et al. [10], a comparable mean ± SD value for CLR was observed at 0.205 ± 0.078 L/h/kg. Nevertheless, they reported a lower V at 0.418 ± 0.076 L/kg, a shorter t1/2 of 1.6 ± 0.5 h, and a reduced CL of 0.223 ± 0.053 L/h/kg compared to our study. This discrepancy was noted following the intravenous administration of adefovir at 1 or 3 mg/kg/day in HIV-infected patients [10]. Considering adefovir dipivoxil, an ester prodrug of adefovir, may rapidly convert to adefovir after administration [28], it is not probable that adefovir dipivoxil pharmacokinetics play an important role in estimating the PK parameters of adefovir. Therefore, the underlying mechanisms of this difference remain unclear.
While Sun et al. [14] and Huang et al. [25] reported 1/2 values similar to those in our study, Sun et al. presented notably low mean values for / (7.0 mL/kg) and CL/ (0.63 mL/h/kg) following a 10 mg oral dose of adefovir dipivoxil [14]. These values diverge from the mean serum concentration–time curves presented in their manuscript, prompting us to consider a potential unit mislabeling in the published results—suggesting “L” may be more appropriate than “mL.” Should this correction prove accurate, it would better align with the results of our current study. In contrast, Huang et al. reported a higher CL (1.00 L/h/kg) and a higher V (10.7 L/kg) after a 10 mg dose of adefovir dipivoxil [25]. We attribute this discrepancy to an error in their article, where they indicate, “open circles represent observed adefovir dipivoxil concentrations” [25]. It appears they inadvertently used adefovir dipivoxil as the moiety for concentrations instead of adefovir, inflating both and CL in their analysis. Overall, published data on adefovir pharmacokinetics show a remarkable variability in clearance and volume of distribution.
To evaluate the influence of co-treatment on PK parameters, we employed two approaches. Firstly, we independently evaluated PK parameters for reference and test periods. Additionally, we integrated co-treatment effect as a covariate on the PK parameters. The relative differences in PK parameters between these periods, as estimated by both methods, are consistent and resulted in an equivalent reduction of the OFV. To allow for greater flexibility in identifying potentially more fundamental distinctions in the description of PK processes between periods, we finally conducted a separate assessment of PK parameters for each of the reference and test periods, respectively. After a detailed assessment of the parameters in various PK processes using the popPK model, a higher apparent bioavailability, but a slower apparent absorption rate of adefovir when co-administered with the cocktail, has been identified. These findings indicate potential changes in the apparent absorption of adefovir dipivoxil or prodrug conversion, contributing to increased systemic exposure during co-administration with the cocktail, as observed in the previous DDI study [3]. Based on sporadic studies, a potential mechanism to explain this result may stem from the co-administered drugs exerting inhibitory effects on the intestinal multidrug resistance-associated protein 2 (MRP2, ABCC2). This protein mediates unidirectional transport of adefovir to the intestinal lumen within enterocytes [29,30,31]; thus, inhibition may enhance the bioavailability of adefovir. Furthermore, the model-based approach did not support the inclusion of Vmax for reference and test periods separately. Additionally, no significant difference was observed in the median CLR between reference and test periods. This suggests that CLR remains unaffected by the other concomitantly administered probe drugs and may reliably reflect changes in the renal OAT1 activity, which is rate limiting for adefovir renal excretion [2, 9, 10].
The final model, incorporating a nonlinear renal elimination, provides an appropriate description of the data, as supported by the supplemental figures. The adefovir renal clearance plot (Supplemental Fig. 3s) indicates a deviation from linearity in urinary excretion at high concentrations, which is well described by the nonlinear renal clearance model. The residual plot (Supplemental Fig. 4s) illustrates clearly biased descriptions of urinary excretion with a linear model at high concentrations, supporting that the linear clearance model is inadequate. Furthermore a model with nonlinear renal elimination offers a physiologically plausible representation for adefovir renal elimination. Adefovir primarily undergoes OAT1-mediated tubular secretion, a process that could potentially saturate and complement kidney filtration. Calculated as CLR – fu*GFR (with a median GFR of 6.28 L/h in this study) [2], adefovir secretion accounts for over 50% of CLR in this study, aligning with findings in previous reports [10, 24]. The population estimate (95% CI) for Km in nonlinear renal elimination is 170 (122–295) nmol/L, which is lower than in vitro studies (mean ± SD 23.8 ± 4.2 µmol/L) [11, 12]. This disparity may stem from challenges in replicating the dynamic in vivo environment in controlled in vitro settings and differences in techniques. Despite this, it exceeds the observed adefovir Cmax range (5.56–91.0 nmol/L). Thus, this mechanism is expected to have little influence on systemic exposure and is considered clinically insignificant at therapeutic doses. However, this result suggests that a reduction of adefovir dose as part of the transporter probe cocktail may be considered in future studies to prevent relevant transporters to be saturated, and to minimize any impact on other cocktail components.
As discussed in the “Introduction” section, indeed there is some prior evidence for nonlinearity on adefovir pharmacokinetics [14, 15], albeit nonlinear renal clearance has not been shown yet [10, 25,26,27, 32,33,34]. The possible reason might be that such finding requires a popPK evaluation to gain a more detailed understanding of the role of individual PK processes for the pharmacokinetics of adefovir, while most of previous studies assessed the PK of adefovir by noncompartmental methods, which may provide insufficient information of individual PK processes [10, 14, 27]. Another possible reason could be that the extent of saturability in adefovir’s elimination, based on the recommended single dose of 10 mg, was relatively small. Therefore, both plasma measurements and urine collection might be necessary for a sound estimation of the nonlinearity. This could explain why Jihan Huang reported first-order elimination for adefovir in their popPK study [25].
The model could not be improved further by incorporating the AGFR as a component of CLR in addition to nonlinear renal clearance. Although it might better reflect the physiological conditions of the renal excretion of adefovir, which involves both active tubular secretion and glomerular filtration, this approach resulted in unstable parameter estimates of Km and Vmax (RSEs > 1000%). As a result, we were unable to estimate the Km value of true renal secretion, which might become more saturated at therapeutic adefovir concentrations.
Another limitation of this study is that it only included healthy adults who received a standard dose. To gain a comprehensive understanding of the nonlinear renal elimination of adefovir, future studies would need to include more dose levels, particularly higher doses, in diverse populations with varying degrees of renal function.
Conclusion
In conclusion, the popPK modeling approach showed that the minor DDI observed for adefovir as a victim when co-administered with other transporter probe substrates is caused by an effect on apparent absorption and/or formation of adefovir from the prodrug, but not by an effect on renal elimination. Renal elimination of adefovir was found to be saturable, which should reflect its active renal secretion. The high Km value suggests that the use of renal elimination of adefovir to assess renal OAT1 activity is not compromised by saturability, while using a lower adefovir dose (e.g., to 50%) may provide an additional safety margin.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Mooij MG, Nies AT, Knibbe CA, Schaeffeler E, Tibboel D, Schwab M, de Wildt SN (2016) Development of human membrane transporters: drug disposition and pharmacogenetics. Clin Pharmacokinet 55(5):507–524. https://doi.org/10.1007/s40262-015-0328-5
Fuhr U, Hsin C-h, Li X, Jabrane W, Sörgel F (2019) Assessment of pharmacokinetic drug–drug interactions in humans: in vivo probe substrates for drug metabolism and drug transport revisited. Annu Rev Pharmacol Toxicol 59:507–536. https://doi.org/10.1146/annurev-pharmtox-010818-021909
Trueck C, Hsin Ch, Scherf-Clavel O, Schaeffeler E, Lenssen R, Gazzaz M, Gersie M, Taubert M, Quasdorff M, Schwab M (2019) A clinical drug-drug interaction study assessing a novel drug transporter phenotyping cocktail with adefovir, sitagliptin, metformin, pitavastatin, and digoxin. Clin Pharmacol Ther 106(6):1398–1407. https://doi.org/10.1002/cpt.1564
Wiebe ST, Giessmann T, Hohl K, Schmidt-Gerets S, Hauel E, Jambrecina A, Bader K, Ishiguro N, Taub ME, Sharma A (2020) Validation of a drug transporter probe cocktail using the prototypical inhibitors rifampin, probenecid, verapamil, and cimetidine. Clin Pharmacokinet 59(12):1627–1639. https://doi.org/10.1007/s40262-020-00907-w
Prueksaritanont T, Tatosian D, Chu X, Railkar R, Evers R, Chavez-Eng C, Lutz R, Zeng W, Yabut J, Chan G (2017) Validation of a microdose probe drug cocktail for clinical drug interaction assessments for drug transporters and CYP3A. Clin Pharmacol Ther 101(4):519–530. https://doi.org/10.1002/cpt.525
Burckhardt G, Burckhardt BC (2011) In vitro and in vivo evidence of the importance of organic anion transporters (OATs) in drug therapy. Handb Exp Pharmacol (201):29-104. https://doi.org/10.1007/978-3-642-14541-4_2
U.S. Food and Drug Administration. Clinical drug interaction studies — cytochrome P450 enzyme- and transporter-mediated drug interactions guidance for industry. Guidance as of January 2020. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/clinical-drug-interaction-studies-cytochrome-p450-enzyme-and-transporter-mediated-drug-interactions
European Medicines Agency. Guideline on the investigation of drug interactions CPMP/EWP/560/95/Rev. 1 Corr. 2**, 21 June 2012. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-investigation-drug-interactions-revision-1_en.pdf
Maeda K, Tian Y, Fujita T, Ikeda Y, Kumagai Y, Kondo T, Tanabe K, Nakayama H, Horita S, Kusuhara H (2014) Inhibitory effects of p-aminohippurate and probenecid on the renal clearance of adefovir and benzylpenicillin as probe drugs for organic anion transporter (OAT) 1 and OAT3 in humans. Eur J Pharm Sci 59:94–103. https://doi.org/10.1016/j.ejps.2014.04.004
Cundy KC, Barditch-Crovo P, Walker RE, Collier AC, Ebeling D, Toole J, Jaffe HS (1995) Clinical pharmacokinetics of adefovir in human immunodeficiency virus type 1-infected patients. Antimicrob Agents Chemother 39(11):2401–2405. https://doi.org/10.1128/AAC.39.11.2401
Hsin C-H, Kuehne A, Gu Y, Jedlitschky G, Hagos Y, Gründemann D, Fuhr U (2023) In vitro validation of an in vivo phenotyping drug cocktail for major drug transporters in humans. Eur J Pharm Sci 186:106459. https://doi.org/10.1016/j.ejps.2023.106459
Cihlar T, LaFlamme G, Fisher R, Carey AC, Vela JE, Mackman R, Ray AS (2009) Novel nucleotide human immunodeficiency virus reverse transcriptase inhibitor GS-9148 with a low nephrotoxic potential: characterization of renal transport and accumulation. Antimicrob Agents Chemother 53(1):150–156. https://doi.org/10.1128/AAC.01183-08
Ho ES, Lin DC, Mendel DB, Cihlar T (2000) Cytotoxicity of antiviral nucleotides adefovir and cidofovir is induced by the expression of human renal organic anion transporter 1. J Am Soc Nephrol 11(3):383–393. https://doi.org/10.1681/ASN.V113383
Sun DQ, Wang HS, Ni MY, Wang BJ, Guo RC (2007) Pharmacokinetics, safety and tolerance of single-and multiple-dose adefovir dipivoxil in healthy Chinese subjects. Br J Clin Pharmacol 63(1):15–23. https://doi.org/10.1111/j.1365-2125.2006.02728.x
Hughes WT, Shenep JL, Rodman JH, Fridland A, Willoughby R, Blanchard S, Purdue L, Coakley DF, Cundy KC, Culnane M (2000) Single-dose pharmacokinetics and safety of the oral antiviral compound adefovir dipivoxil in children infected with human immunodeficiency virus type 1. Antimicrob Agents Chemother 44(4):1041–1046. https://doi.org/10.1128/AAC.44.4.1041-1046.2000
Scherf-Clavel O, Kinzig M, Stoffel MS, Fuhr U, Sörgel F (2019) Quantification of adefovir and pitavastatin in human plasma and urine by LC-MS/MS: a useful tool for drug-drug interaction studies. J Chromatogr B 1125:121718. https://doi.org/10.1016/j.jchromb.2019.121718
Mould DR, Upton RN (2013) Basic concepts in population modeling, simulation, and model-based drug development-part 2: introduction to pharmacokinetic modeling methods. CPT Pharmacometrics Syst Pharmacol 2(4):e38. https://doi.org/10.1038/psp.2013.14
Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL (2012) Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 367(1):20–29. https://doi.org/10.1056/NEJMoa1114248
Mosteller R (1987) Simplified calculation of body surface area. New Engl J Med 317:1098. https://doi.org/10.1056/NEJM198710223171717
Hooker AC, Staatz CE, Karlsson MO (2007) Conditional weighted residuals (CWRES): a model diagnostic for the FOCE method. Pharm Res 24(12):2187–2197. https://doi.org/10.1007/s11095-007-9361-x
Post TM, Freijer JI, Ploeger BA, Danhof M (2008) Extensions to the visual predictive check to facilitate model performance evaluation. J Pharmacokinet Pharmacodyn 35(2):185–202. https://doi.org/10.1007/s10928-007-9081-1
Nguyen T, Ms Mouksassi, Holford N, Al-Huniti N, Freedman V, Hooker AC, John J, Karlsson MO, Mould D, Pérez Ruixo J (2017) Model evaluation of continuous data pharmacometric models: metrics and graphics. CPT Pharmacometrics Syst Pharmacol 6(2):87–109. https://doi.org/10.1002/psp4.12161
Ette EI (1997) Stability and performance of a population pharmacokinetic model. J Clin Pharmacol 37(6):486–495. https://doi.org/10.1002/j.1552-4604.1997.tb04326.x
Gilead Sciences International Limited. Fachinformation für Hepsera® 10 mg Tabletten as of April 2021. In: ed. https://www.fachinfo.de/suche/fi/003016
Huang J, Zhang Y, Huang X, Li L, Li Y, Wang K, Yang J, He Y, Lv Y, Zheng Q (2014) Population pharmacokinetics of adefovir dipivoxil tablets in healthy Chinese volunteers. Int J Clin Pharmacol Ther 52(1):8–14. https://doi.org/10.5414/CP201928
Sokal EM, Kelly D, Wirth S, Mizerski J, Dhawan A, Frederick D (2008) The pharmacokinetics and safety of adefovir dipivoxil in children and adolescents with chronic hepatitis B virus infection. J Clin Pharmacol 48(4):512–517. https://doi.org/10.1177/0091270007313325
Shiffman ML, Pol S, Rostaing L, Schiff E, Thabut D, Zeuzem S, Zong J, Frederick D, Rousseau F (2011) Efficacy and pharmacokinetics of adefovir dipivoxil liquid suspension in patients with chronic hepatitis B and renal impairment. J Clin Pharmacol 51(9):1293–1301. https://doi.org/10.1177/0091270010381385
Noble S, Goa KL (1999) Adefovir dipivoxil. Drugs 58(3):479–487. https://doi.org/10.2165/00003495-199958030-00010
Chen Z, Shi T, Zhang L, Zhu P, Deng M, Huang C, Hu T, Jiang L, Li J (2016) Mammalian drug efflux transporters of the ATP binding cassette (ABC) family in multidrug resistance: a review of the past decade. Cancer Lett 370(1):153–164. https://doi.org/10.1016/j.canlet.2015.10.010
Motoki K, Taniguchi T, Ashizawa N, Sakai M, Chikamatsu N, Yamano K, Iwanaga T (2023) Uricosuric agents affect plasma and kidney concentration of adefovir via inhibition of oat1 and Mrp2 in rats. Biol Pharm Bull 46(2):170–176. https://doi.org/10.1248/bpb.b22-00384
Shin E, Shin N, Oh JH, Lee YJ (2017) High-dose metformin may increase the concentration of atorvastatin in the liver by inhibition of multidrug resistance-associated protein 2. J Pharm Sci 106(4):961–967. https://doi.org/10.1016/j.xphs.2016.11.020
Fok BS, Gardner S, Piscitelli S, Chen S, Chu TT, Chan JC, Tomlinson B (2013) Pharmacokinetic properties of single-dose lamivudine/adefovir dipivoxil fixed-dose combination in healthy Chinese male volunteers. Clin Ther 35(1):68–76. https://doi.org/10.1016/j.clinthera.2012.12.001
Barditch-Crovo P, Toole J, Hendrix C, Cundy K, Ebeling D, Jaffe H, Lietman P (1997) Anti-human immunodeficiency virus (HIV) activity, safety, and pharmacokinetics of adefovir dipivoxil (9-[2-(bis-pivaloyloxymethyl)-phosphonylmethoxyethyl] adenine) in HIV-infected patients. J Infect Dis 176(2):406–413. https://doi.org/10.1086/514057
Deeks SG, Collier A, Lalezari J, Pavia A, Rodrigue D, Drew WL, Toole J, Jaffe HS, Mulato AS, Lamy PD (1997) The safety and efficacy of adefovir dipivoxil, a novel anti-human immunodeficiency virus (HIV) therapy, in HIV-infected adults: a randomized, double-blind, placebo-controlled trial. J Infect Dis 176(6):1517–1523. https://doi.org/10.1086/514150
Funding
Open Access funding enabled and organized by Projekt DEAL. Qian Dong received a scholarship from the China Scholarship Council (CSC) for support of her Ph.D. studies. Dr. Chunli Chen is supported by the 2022 ESI International High Impact Research Article Cooperation Program (No. 212-54900112), the National Natural Science Foundation of Heilongjiang Province (No. YQ2022C017) and the International Postdoctoral Exchange Fellowship Program from the Office of China Postdoctoral Council (No. 2020106 and PC2020013). The Higher Education Commission of Pakistan provided financial support in the form of a Ph.D. scholarship for Muhammad Bilal through the German Academic Exchange Service (DAAD). The study's design, data collection and analysis, and the choice to submit the work for publication were all independent of the sponsor.
Author information
Authors and Affiliations
Contributions
All authors contributed to the conception and design of the research. U.F. provided the data. Q.D., C.C., M.T., and M.B. organized the database and performed the analyses. U.F. supervised the project. Q.D. wrote the first draft of the manuscript. All authors reviewed and approved the submitted version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dong, Q., Chen, C., Taubert, M. et al. Understanding adefovir pharmacokinetics as a component of a transporter phenotyping cocktail. Eur J Clin Pharmacol 80, 1069–1078 (2024). https://doi.org/10.1007/s00228-024-03673-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-024-03673-x