Abstract
Purpose
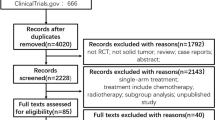
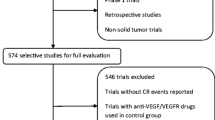
We performed a meta-analysis to systematically review the gastrointestinal (GI) events (diarrhea, nausea, vomiting, anorexia) of five newly approved (after 2011) VEGFR-TKIs in cancer patients.
Methods
The relevant studies of the randomized controlled trials (RCTs) in cancer patients treated with cabozantinib, vandetanib, lenvatinib, regorafenib, and axitinib were retrieved and the systematic evaluation was conducted.
Results
Forty-one randomized controlled trials and 10,860 patients were included. Current analysis suggested that the use of these agents increased the risk of all-grade and high-grade GI events, and the diarrhea was the most common GI events. The risk of all-grade and high-grade GI events varies significantly within drug types, tumor types, and VEGFR-TKIs-based regimens.
Conclusion
The available data suggested that the use of the five newly approved VEGFR-TKIs may increase risk of GI events in cancer patients. Physicians and patients should be aware of these risks and frequent monitoring and careful management should be emphasized when managing these VEGFR-TKIs.




Similar content being viewed by others
References
Folkman J (1971) Tumor angiogenesis: therapeutic implications. N Engl J Med 285(21):1182–1186
Wykosky J, Fenton T, Furnari F et al (2011) Therapeutic targeting of epidermal growth factor receptor in human cancer: successes and limitations. Chin J Cancer 30(1):5–12
Weidner N, Semple JP, Welch WR, Folkman J (1991) Tumor angiogenesis and metastasis—correlation in invasive breast carcinoma. N Engl J Med 324(1):1–8
Carmeliet P, Jain RK (2000) Angiogenesis in cancer and other diseases. Nature 407:249–257
Ferrara N, Hillan KJ, Gerber HP, Novotny W (2004) Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov 3(5):391–400
Santoni M, Conti A, De Giorgi U et al (2014) Risk of gastrointestinal events with sorafenib, sunitinib and pazopanib in patients with solid tumors: a systematic review and meta-analysis of clinical trials. Int J Cancer 35(4):763–773
Moher D, Liberati A, Tetzlaff J et al (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 151:264–269
Guyatt GH, Oxman AD, Vist GE et al (2008) GRADE Working Group GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 336:924–926
Li J, Qin S, Xu R et al (2015) Regorafenib plus best supportive care versus placebo plus best supportive care in Asian patients with previously treated metastatic colorectal cancer (CONCUR): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 16(6):619–629
Grothey A, Van Cutsem E, Sobrero A et al (2013) Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 381(9863):303–312
Demetri GD, Reichardt P, Kang YK et al (2013) Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib: an international, multicentre, prospective, randomised, placebo controlled phase 3 trial (GRID). Lancet 381(9863):295–302
Mir O, Brodowicz T, Italiano A et al (2016) Safety and efficacy of regorafenib in patients with advanced soft tissue sarcoma (REGOSARC): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol 7(12):1732–1742
Pavlakis N, Sjoquist KM, Martin AJ et al (2016) Regorafenib for the treatment of advanced gastric cancer (INTEGRATE): a multinational placebo-controlled phase II trial. J Clin Oncol 34(23):2728–2735
Santoro A, Gebbia V, Pressiani T et al (2015) A randomized, multicenter, phase II study of vandetanib monotherapy versus vandetanib in combination with gemcitabine versus gemcitabine plus placebo in subjects with advanced biliary tract cancer: the VanGogh study. Ann Oncol 26(3):542–547
Heymach JV, Johnson BE, Prager D et al (2007) Randomized, placebo-controlled phase II study of vandetanib plus docetaxel in previously treated non-small-cell lung cancer. J Clin Oncol 25(27):4270–4277
Arnold AM, Seymour L, Smylie M et al (2007) Phase II study of vandetanib or placebo in small-cell lung cancer patients after complete or partial response to induction chemotherapy with or without radiation therapy: National Cancer Institute of Canada Clinical Trials Group Study BR.20. J Clin Oncol 25(25):4278–4284
Heymach JV, Paz-Ares L, De Braud F et al (2008) Randomized phase II study of vandetanib alone or with paclitaxel and carboplatin as first-line treatment for advanced non-small-cell lung cancer. J Clin Oncol Off J Am Soc Clin Oncol 26(33):5407–5415
Herbst RS, Sun Y, Eberhardt WE et al (2010) Vandetanib plus docetaxel versus docetaxel as second-line treatment for patients with advanced non-small-cell lung cancer (ZODIAC): a double-blind, randomised, phase 3 trial. Lancet Oncol 11(7):619–626
de Boer RH, Arrieta Ó, Yang CH et al (2011) Vandetanib plus pemetrexed for the second-line treatment of advanced non-small-cell lung cancer: a randomized, double-blind phase III trial. J Clin Oncol Off J Am Soc Clin Oncol 29(8):1067–1074
Lee JS, Hirsh V, Park K et al (2012) Vandetanib versus placebo in patients with advanced non-small-cell lung cancer after prior therapy with an epidermal growth factor receptor tyrosine kinase inhibitor: a randomized, double-blind phase III trial (ZEPHYR). J Clin Oncol 30(10):1114–1121
Ahn JS, Lee KH, Sun JM et al (2013) A randomized, phase II study of vandetanib maintenance for advanced or metastatic non-small-cell lung cancer following first-line platinum-doublet chemotherapy. Lung Cancer 82(3):455–460
Aisner J, Manola JB, Dakhil SR et al (2013) Vandetanib plus chemotherapy for induction followed by Vandetanib or placebo as maintenance for patients with advanced non-small-cell lung cancer a randomized phase 2 PrECOG study (PrE0501). J Thorac Oncol 8(8):1075–1083
Sanborn RE, Patel JD, Masters GA et al (2017) A randomized, double-blind, phase 2 trial of platinum therapy plus etoposide with or without concurrent vandetanib (ZD6474) in patients with previously untreated extensive-stage small cell lung cancer: Hoosier Cancer Research Network LUN06-113. Cancer 123(2):303–311
Gridelli C, Novello S, Zilembo N et al (2014) Phase II randomized study of vandetanib plus gemcitabine or gemcitabine plus placebo as first-line treatment of advanced non-small-cell lung cancer in elderly patients. J Thorac Oncol 9(5):733–737
Coleman RL, Moon J, Sood AK et al (2014) Randomized phase II study of docetaxel plus vandetanib vs. docetaxel followed by vandetanib in patients with persistent or recurrent epithelial ovarian, fallopian tube or primary peritoneal carcinoma: SWOG S0904. Eur J Cancer 50(9):1638–1648
Hsu C, Yang TS, Huo TI et al (2012) Vandetanib in patients with inoperable hepatocellular carcinoma: a phase II, randomized, double-blind, placebo-controlled study. J Hepatol 56(5):1097–1103
Gupta A, Roberts C, Tysoe F et al (2016) RADVAN: a randomised phase 2 trial of WBRT plus vandetanib for melanoma brain metastases—results and lessons learnt. Brit J Cancer 115(10):1193–1200
Wells SA Jr, Robinson BG, Gagel RF et al (2012) Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind phase III trial. J Clin Oncol 30(2):134–141
Leboulleux S, Bastholt L, Krause T et al (2012) Vandetanib in locally advanced or metastatic diff erentiated thyroid cancer: a randomised, double-blind, phase 2 trial. Lancet Oncol 13(9):897–905
Lee EQ, Kaley TJ, Duda DG et al (2015) A multicenter, phase II, randomized, noncomparative clinical trial of radiation and temozolomide with or without vandetanib in newly diagnosed glioblastoma patients. Clin Cancer Res 21:3610–3618
Choueiri TK, Ross RW, Jacobus S et al (2012) Double-blind, randomized trial of docetaxel plus vandetanib versus docetaxel plus placebo in platinum-pretreated metastatic urothelial cancer. J Clin Oncol 30(5):507–512
Azad AA, Beardsley EK, Hotte SJ et al (2014) A randomized phase II efficacy and safety study of vandetanib (ZD6474) in combination with bicalutamide versus bicalutamide alone in patients with chemotherapy naïve castration-resistant prostate cancer. Invest New Drug 32(4):746–752
Boér K, Láng I, Llombart-Cussac A et al (2012) Vandetanib with docetaxel as second-line treatment for advanced breast cancer: a double-blind, placebo-controlled, randomized phase II study. Invest New Drug 30(2):681–687
Limaye S, Riley S, Zhao S et al (2013) A randomized phase II study of docetaxel with or without vandetanib in recurrent or metastatic squamous cell carcinoma of head and neck (SCCHN). Oral Oncol 49(8):835–841
Elisei R, Schlumberger MJ, Müller SP et al (2013) Cabozantinib in progressive medullary thyroid cancer. J Clin Oncol 31(29):3639–3646
Smith M, De Bono J, Sternberg C et al (2016) Phase III study of cabozantinib in previously treated metastatic castration-resistant prostate cancer: COMET-1. J Clin Oncol 34(25):3005–3013
Choueiri TK, Escudier B, Powles T et al (2016) Cabozantinib versus everolimus in advanced renal cell carcinoma (METEOR): final results from a randomised, open-label, phase 3 trial. Lancet Oncol 17(7):917–927
Schlumberger M, Tahara M, Wirth LJ et al (2015) Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. New Engl J Med 372(7):621–630
Motzer RJ, Hutson TE, Glen H et al (2015) Lenvatinib, everolimus, and the combination in patients with metastatic renal cell carcinoma: a randomised, phase 2, open-label, multicentre trial. Lancet Oncol 16(15):1473–1482
Twelves C, Chmielowska E, Havel L et al (2014) Randomised phase II study of axitinib or bevacizumab combined with paclitaxel/carboplatin as first-line therapy for patients with advanced non-small-cell lung cancer. Ann Oncol 25(1):132–138
Belani CP, Yamamoto N, Bondarenko IM et al (2014) Randomized phase II study of pemetrexed/cisplatin with or without axitinib for non-squamous non-small-cell lung cancer. BMC Cancer 14(1):1–10
Kang YK, Yau T, Park JW et al (2015) Randomized phase II study of axitinib versus placebo plus best supportive care in second-line treatment of advanced hepatocellular carcinoma. Ann Oncol 26(12):2457–2463
Duerinck J, Du Four S, Vandervorst F et al (2016) Randomized phase II study of axitinib versus physicians best alternative choice of therapy in patients with recurrent glioblastoma. J Neuro-Oncol 128(1):147–155
Rugo HS, Stopeck AT, Joy AA et al (2011) Randomized, placebo-controlled, double-blind, phase II study of axitinib plus docetaxel versus docetaxel plus placebo in patients with metastatic breast cancer. J Clin Oncol 29(18):2459–2465
Rini BI, Tomita Y, Melichar B et al (2016) Overall survival analysis from a randomized phase II study of axitinib with or without dose titration in first-line metastatic renal cell carcinoma. Clin Genitourin Cancer 14(6):499–503
Spano JP, Chodkiewicz C, Maurel J et al (2008) Efficacy of gemcitabine plus axitinib compared with gemcitabine alone in patients with advanced pancreatic cancer: an open-label randomised phase II study. Lancet 371(9630):2101–2108
Kindler HL, Ioka T, Richel DJ et al (2011) Axitinib plus gemcitabine versus placebo plus gemcitabine in patients with advanced pancreatic adenocarcinoma: a double-blind randomised phase 3 study. Lancet Oncol 12(3):256–262
Bendell JC, Tournigand C, Swieboda-Sadlej A et al (2013) Axitinib or bevacizumab plus FOLFIRI or modified FOLFOX-6 after failure of first-line therapy for metastatic colorectal cancer: a randomized phase II study. Clin Colorectal Cancer 12(4):239–247
Infante JR, Reid TR, Cohn AL et al (2013) Axitinib and/or bevacizumab with modified FOLFOX-6 as first-line therapy for metastatic colorectal cancer: a randomized phase 2 study. Cancer 119(14):2555–2563
Hopkins TG, Marples M, Stark D (2008) Sunitinib in the management of gastrointestinal stromal tumours (GISTs). Eur J Surg Oncol 34:844–850
Keefe D, Bowen J, Gibson R, Tan T, Okera M, Stringer A (2011) Noncardiac vascular toxicities of vascular endothelial growth factor inhibitors in advanced cancer: a review. Oncologist 16:432–444
Medina PJ, Goodin S (2008) Lapatinib: a dual inhibitor of human epidermal growth factor receptor tyrosine kinases. Clin Therap 30:1426–1447
Acknowledgements
The authors thank Wenxia SUN for English translation.
Author information
Authors and Affiliations
Contributions
Jing Li was responsible for the conception of the work, wrote the manuscript, and study search and selection. Jian Gu contributed to study search and selection and carried out the statistical analyses.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Funding
This study was supported by the Fundamental Research Funds for the Central Universities, Southwest University for Nationalities, 2017NZYQN29.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
For this type of study, formal consent is not required.
Electronic supplementary material
Supplementary Fig. 1
(DOCX 95 kb)
Supplementary Fig. 2
(DOCX 88 kb)
Supplementary Fig. 3
(DOCX 47 kb)
Supplementary Fig. 4
(DOCX 61 kb)
Supplementary Table 1
(DOCX 56 kb)
Supplementary Table 2
(DOCX 23 kb)
ESM 1
(DOCX 23 kb)
ESM 2
(DOC 63 kb)
Rights and permissions
About this article
Cite this article
Li, J., Gu, J. Risk of gastrointestinal events with newly approved (after 2011) vascular endothelial growth factor receptor tyrosine kinase inhibitors in cancer patients: a meta-analysis of randomized controlled trials. Eur J Clin Pharmacol 73, 1209–1217 (2017). https://doi.org/10.1007/s00228-017-2299-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-017-2299-y