Abstract
Objective
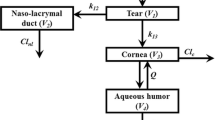
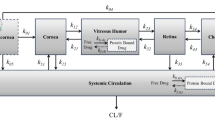
The objective of this evaluation was to model ocular pharmacokinetics of fluorescein administered as conventional eye drops and as lyophilisate to healthy volunteers in order to assess the relative bioavailability of the lyophilisate formulation.
Methods
A total of 44 healthy subjects received equivalent doses of fluorescein as lyophilisate to one eye and as eye drops to the fellow eye in three individual studies. Fluorescein concentrations in the cornea and anterior chamber were measured by fluorophotometry. Data were analyzed by noncompartmental methods (WinNonlin software) and by compartmental population pharmacokinetic methods (NONMEM software).
Results
Compared to eye drops, both maximum fluorescein concentrations (Cmax) and the areas under the concentration-time curve (AUC0-t ) values of fluorescein in the cornea and anterior chamber for lyophilisate were increased in the noncompartmental analysis: mean lyophilisate Cmax in the studies was 6.3- to 14.6-fold higher and mean AUC0–t was 4.7- to 8.9-fold higher for ocular concentrations in the three studies. A three-compartment open model with first-order elimination from the anterior chamber adequately described population data. Estimated fluorescein systemic bioavailability (F) via the ocular route from lyophilisate relative to eye drops was 3.7-fold higher (95% CI 2.6–4.8).
Conclusion
The data clearly show a considerably superior intraocular bioavailability of fluorescein when given as lyophilisate compared to conventional eye drops. There is a clear pharmacokinetic advantage of the lyophilisate preparation.



Similar content being viewed by others
References
Ahmed I, Patton TF (1985) Importance of the noncorneal absorption route in topical ophthalmic drug delivery. Invest Ophthalmol Vis Sci 26(4):584–587
Lee SB, Geroski DH, Prausnitz MR, Edelhauser HF (2004) Drug delivery through the sclera: effects of thickness, hydration, and sustained release systems. Exp Eye Res 78(3):599–607
Schoenwald RD (1990) Ocular drug delivery. Pharmacokinetic considerations. Clin Pharmacokinet 18(4):255–269
Chastain JE (2003) General considerations in ocular drug delivery. In: Mitra AK (ed) Ophthalmic drug delivery system; drugs and the pharmaceutical sciences, 2nd edn. Marcel Dekker, New York, pp 59–107
Mishima S, Gasset A, Klyce SD Jr, Baum JL (1966) Determination of tear volume and tear flow. Invest Ophthalmol 5(3):264–276
Davies NM (2000) Biopharmaceutical considerations in topical ocular drug delivery. Clin Exp Pharmacol Physiol 27(7):558–562
Fraunfelder FT, Meyer SM (1987) Systemic side effects from ophthalmic timolol and their prevention. J Ocul Pharmacol 3(2):177–184
Macdonald EA, Maurice DM (1991) Loss of fluorescein across the conjunctiva. Exp Eye Res 53(4):427–430
Noecker R (2001) Effects of common ophthalmic preservatives on ocular health. Adv Ther 18(5):205–215
Pisella PJ, Pouliquen P, Baudouin C (2002) Prevalence of ocular symptoms and signs with preserved and preservative free glaucoma medication. Br J Ophthalmol 86(4):418–423
Jaenen N, Baudouin C, Pouliquen P, Manni G, Figueiredo A, Zeyen T (2007) Ocular symptoms and signs with preserved and preservative-free glaucoma medications. Eur J Ophthalmol 17(3):341–349
Noecker RJ, Herrygers LA, Anwaruddin R (2004) Corneal and conjunctival changes caused by commonly used glaucoma medications. Cornea 23(5):490–496
Maurice DM, Mishima S (1984) Ocular pharmacokinetics. In: Sears ML (ed) Pharmacology of the eye, handbook of experimental Pharmacology, vol. 69. Springer-Verlag, Berlin, pp 19–116
Patton TF, Franceur M (1978) Ocular bioavailability and systemic loss of topically applied ophthalmic drugs. Am J Ophthalmol 85(2):225–229
Chrai SS, Makoid MC, Eriksen SP, Robinson JR (1974) Drop size and initial dosing frequency problems of topically applied ophthalmic drugs. J Pharm Sci 63(3):333–338
Ahmed I (2003) The noncorneal route in ocular drug delivery. In: Mitra AK (ed) Ophthalmic drug delivery system; drugs and the pharmaceutical sciences, 2nd edn. Marcel Dekker, New York, pp 335–363
Macha S, Hughes PM, Mitra AK (2003) Overview of ocular drug delivery. In: Mitra AK (ed) Ophthalmic drug delivery system; drugs and the pharmaceutical sciences, 2nd edn. Marcel Dekker, New York, pp 1–12
Chang SC, Lee VH (1987) Nasal and conjunctival contributions to the systemic absorption of topical timolol in the pigmented rabbit: implications in the design of strategies to maximize the ratio of ocular to systemic absorption. J Ocul Pharmacol 3(2):159–169
Lee VH, Robinson JR (1986) Topical ocular drug delivery: recent developments and future challenges. J Ocul Pharmacol 2(1):67–108
Lang JC (1995) Ocular drug delivery conventional ocular formulations. Adv Drug Delivery Rev 16:39–43
Urtti A, Pipkin JD, Rork G, Repta AJ (1990) Controlled drug delivery devices for experimental ocular studies with timolol. 1. In vitro release studies. Int J Pharm 61:235−240
Zimmerman TJ, Kooner KS, Kandarakis AS, Ziegler LP (1984) Improving the therapeutic index of topically applied ocular drugs. Arch Ophthalmol 102:551−553
Tang-Liu DD, Liu S, Neff J, Sandri R (1987) Disposition of levobunolol after an ophthalmic dose to rabbits. J Pharm Sci 76(10):780–783
Tang-Liu DD, Liu SS, Weinkam RJ (1984) Ocular and systemic bioavailability of ophthalmic flurbiprofen. J Pharmacokinet Biopharm 12(6):611–626
Dorigo MT, Cerin O, Fracasso G, Altafini R (1990) Cardiovascular effects of befunolol, betaxolol and timolol eye drops. Int J Clin Pharmacol Res 10(3):163–166
Diamond J (1997) Systemic adverse effects of topical ophthalmic agents. Implications for older patiens. Drugs Aging 11:352–36
Hayreh SS, Podhajsky P, Zimmerman MB (1999) Beta-blocker eyedrops and nocturnal arterial hypotension. Am J Ophthalmol 128(3):301–309
Lee VH, Urrea PT, Smith RE, Schanzlin DJ (1985) Ocular drug bioavailability from topically applied liposomes. Surv Ophthalmol 29(5):335–348
Hathout RM, Mansour S, Mortada ND, Guinedi AS (2007) Liposomes as an ocular delivery system for acetazolamide: in vitro and in vivo studies. AAPS PharmSciTech 8(1):1–12
Pontes de Carvalho RA, Krausse ML, Murphree AL, Schmitt EE, Campochiaro PA, Maumenee IH (2006) Delivery from episcleral exoplants. Invest Ophthalmol Vis Sci 47(10):4532–4539
Phinney RB, Schwartz SD, Lee DA, Mondino BJ (1988) Collagen-shield delivery of gentamicin and vancomycin. Arch Ophthalmol 106(11):1599–604
Reidy JJ, Limberg M, Kaufman HE (1990) Delivery of fluorescein to the anterior chamber using the corneal collagen shield. Ophthalmology 97(9):1201–1203
Hornof M, Weyenberg W, Ludwig A, Bernkop-Schnurch A (2003) Mucoadhesive ocular insert based on thiolated poly(acrylic acid): development and in vivo evaluation in humans. J Control Release 89(3):419–428
Davies NM, Farr SJ, Hadgraft J, Kellaway IW (1991) Evaluation of mucoadhesive polymers in ocular drug delivery. I. Viscous solutions. Pharm Res 8(8):1039–1043
Suverkrup R, Weichselbaum A, Diestelhorst M (1999) Pilocarpine dry drops: miotic effect and discomfort upon administration compared to conventional eye drops. IOVS Supl 40(4):85
Diestelhorst M, Grunthal S, Suverkrup R (1999) Dry drops: a new preservative-free drug delivery system. Graefes Arch Clin Exp Ophthalmol 237(5):394–398
Mitra AK (2003) Ophthalmic drug delivery system; drugs and the pharmaceutical sciences, 2nd edn. Marcel Dekker, New York
Dinslage S, Diestelhorst M, Weichselbaum A, Süverkrüp R (2002) Lyophilisates for drug delivery in ophthalmology: pharmacokinetics of fluorescein in the human anterior segment. Br J Ophthalmol 86(10):1114–1117
Lux A, Maier S, Dinslage S, Süverkrüp R, Diestelhorst M (2003) A comparative bioavailability study of three conventional eye drops versus a single lyophilisate. Br J Ophthalmol 87(4):436–440
Steinfeld A, Lux A, Maier S, Süverkrüp R, Diestelhorst M (2004) Bioavailability of fluorescein from a new drug delivery system in human eyes. Br J Ophthalmol 88(1):48–53
Süverkrüp R, Grunthal S, Krasichkova O, Maier S, Weichselbaum A, Neff B, Diestelhorst M, Dinslage S, Lux A (2004) The ophthalmic lyophilisate carrier system (OLCS): development of a novel dosage form, freeze-drying technique, and in vitro quality control tests. Eur J Pharm Biopharm 57(2):269–277
Schraermeyer U, Diestelhorst M, Bieker A, Theisohn M, Mietz H, Ustundag C, Joseph G, Krieglstein GK (1999) Morphologic proof of the toxicity of mitomycin C on the ciliary body in relation to different application methods. Graefes Arch Clin Exp Ophthalmol 237(7):593–600
Coakes RL, Brubaker RF (1979) Method of measuring aqueous humor flow and corneal endothelial permeability using a fluorophotometry nomogram. Invest Ophthalmol Vis Sci 18(3):288–302
Mishima S (1981) Clinical pharmacokinetics of the eye:Proctor Lecture. Invest Ophthalmol Vis Sci 21(4):504–541
Maurice DM (1967) The use of fluorescein in ophthalmological research. Invest Ophthalmol 6(5):464–477
Adler CA, Maurice DM, Paterson ME (1971) The effect of the viscosity of the vehicle on the penetration of fluorescein into the human eye. Exp Eye Res 11(1):34–42
Linden C, Alm A (1997) Effect of consecutively applied fluorescein eye drops on corneal and aqueous concentrations of fluorescein. Ophthalmic Res 29(2):57–60
Ludwig A, van Haeringen NJ, Bodelier VM, Van Ooteghem M (1992) Relationship between precorneal retention of viscous eye drops and tear fluid composition. Int Ophthalmol 16(1):23–26
Benedetto DA, Shah DO, Kaufman HE (1975) The instilled fluid dynamics and surface chemistry of polymers in the preocular tear film. Invest Ophthalmol 14(12):887–902
McLaren JW, Ziai N, Brubaker RF (1993) A simple three-compartment model of anterior segment kinetics. Exp Eye Res 56(3):355–366
Acknowledgements
The authors are grateful to Olena Krasichkova and Stefan Mayer for the preparation of lyophilisates.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abduljalil, K., Diestelhorst, M., Doroshyenko, O. et al. Modelling ocular pharmacokinetics of fluorescein administered as lyophilisate or conventional eye drops. Eur J Clin Pharmacol 64, 521–529 (2008). https://doi.org/10.1007/s00228-007-0457-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-007-0457-3