Abstract
Purpose
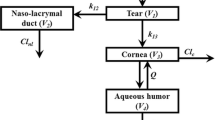
Treating posterior segment diseases presents significant challenges because of the intricate anatomical and physiological barriers within the eye that limit drug penetration. Enavogliflozin eye drops (DWRX2008) are a novel candidate for diabetic retinopathy; however, their ocular distribution characteristics have not yet been identified. In this study, the ocular and systemic pharmacokinetics (PKs) of enavogliflozin were investigated using a multicompartmental PK model for DWRX2008.
Methods
Following topical instillation of a single dose of DWRX2008 (0.025 mg/eye) in both eyes of the rats, the concentration of enavogliflozin was quantified in five biological matrices (cornea, vitreous humor, retina, choroid, and plasma) using bioanalytical methods. A six-compartment model considering protein binding in the vitreous humor and systemic circulation was developed based on the disposition trends of eye drop formulations. The performance of the model was verified by visual inspection, fold-error, and sensitivity analyses.
Results
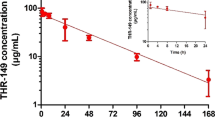
Through this model, the peak concentration (Cmax) and area under the curve from zero to infinity (AUC0-∞) of enavogliflozin in retina were estimated at 39.51 ng/g and 151.12 ng∙h/g, respectively, indicating its reach to the target tissue. It was confirmed that enavogliflozin primarily follows the conjunctival-scleral pathway after topical instillation of eye drop formulations. The prolonged half-life of the drug in vitreous humor may be attributed to its interaction with the albumin present in the vitreous humor.
Conclusion
These findings established a scientific basis for the further development of enavogliflozin eye drops as a treatment for diabetic retinopathy.






Similar content being viewed by others
References
Acheampong AA, Shackleton M, John B et al (2002) Distribution of brimonidine into anterior and posterior tissues of monkey, rabbit, and rat eyes. Drug Metab Dispos 30:421–429
Angi M, Kalirai H, Coupland SE et al (2012) Proteomic analyses of the vitreous humour. Mediators Inflamm 2012:148039
Bourne RRA, Stevens GA, White RA et al (2013) Causes of vision loss worldwide, 1990–2010: a systematic analysis. Lancet Glob Health 1:e339–e349. https://doi.org/10.1016/S2214-109X(13)70113-X
Brown E, Wilding JPH, Barber TM et al (2019) Weight loss variability with SGLT2 inhibitors and GLP-1 receptor agonists in type 2 diabetes mellitus and obesity: Mechanistic possibilities. Obes Rev 20:816–828
Chastain JE, Sanders ME, Curtis MA et al (2016) Distribution of topical ocular nepafenac and its active metabolite amfenac to the posterior segment of the eye. Exp Eye Res 145:58–67
Choi EJ, Choi GW, Kim JH et al (2020a) A novel eye drop candidate for age-related macular degeneration treatment: studies on its pharmacokinetics and distribution in rats and rabbits. Molecules 25(3):663
Choi MK, Nam SJ, Ji HY et al (2020b) Comparative pharmacokinetics and pharmacodynamics of a novel sodium-glucose cotransporter 2 inhibitor, DWP16001, with dapagliflozin and ipragliflozin. Pharmaceutics 12(3):268
Daewoong Pharmaceutical Co., Ltd. (2023a) Daewoong Pharmaceutical's Envlo to enter the global market in full swing with filing for product license in three ASEAN countries. https://www.prnewswire.com/apac/news-releases/daewoong-pharmaceuticals-envlo-to-enter-the-global-market-in-full-swing-with-filing-for-product-license-in-three-asean-countries-301778615.html. Accessed 24 November 2023
Daewoong Pharmaceutical Co., Ltd. (2023b) Daewoong Pharmaceutical has obtained approval for SGLT2i + metformin combination drug Envlomet. Cision PR Newswire. https://www.prnewswire.com/in/news-releases/daewoong-pharmaceutical-has-obtained-approval-for-sglt2i--metformin-combination-drug-envlomet-301880411.html. Accessed 24 November 2023
del Amo EM, Rimpelä A-K, Heikkinen E et al (2017) Pharmacokinetic aspects of retinal drug delivery. Prog Retin Eye Res 57:134–185
Dugel PU, Koh A, Ogura Y et al (2020) HAWK and HARRIER: phase 3, multicenter, randomized, double-masked trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology 127:72–84
Farkouh A, Frigo P, Czejka M (2016) Systemic side effects of eye drops: a pharmacokinetic perspective. Clin Ophthalmol 10:2433–2441
Fayyaz A, Vellonen KS, Ranta VP et al (2021) Ocular pharmacokinetics of atenolol, timolol and betaxolol cocktail: Tissue exposures in the rabbit eye. Eur J Pharm Biopharm 166:155–162
Fraunfelder FT, Meyer SM (1987) Systemic reactions to ophthalmic drug preparations. Med Toxicol Adverse Drug Exp 2:287–293
Fuchs H, Igney F (2017) Binding to ocular albumin as a half-life extension principle for intravitreally injected drugs: evidence from mechanistic rat and rabbit studies. J Ocul Pharmacol Ther 33:115–122
Fuchs H, Chen LZ, Low S, Yu H (2021) Ocular and systemic pharmacokinetics of BI-X, a nanobody targeting VEGF and Ang-2, after intravitreal dosing in cynomolgus monkeys - Evidence for half-life extension by albumin. Exp Eye Res 205:108486
Gaudana R, Ananthula HK, Parenky A, Mitra AK (2010) Ocular drug delivery. AAPS J 12:348–360
Ghanchi F, Bourne R, Downes SM et al (2022) An update on long-acting therapies in chronic sight-threatening eye diseases of the posterior segment: AMD, DMO, RVO, uveitis and glaucoma. Eye 36:1154–1167
Goel M, Picciani RG, Lee R, Bhattacharya S (2010) Aqueous humor dynamics: a review. Open Ophthalmol J 4:52–59
Gote V, Sikder S, Sicotte J, Pal D (2019) Ocular drug delivery: present innovations and future challenges. J Pharmacol Exp Ther 370:602–624
Hanaguri J, Yokota H, Kushiyama A et al (2022) The effect of sodium-dependent glucose cotransporter 2 inhibitor tofogliflozin on neurovascular coupling in the retina in type 2 diabetic mice. Int J Mol Sci 23(3):1362
Horita S, Watanabe M, Katagiri M et al (2019) Species differences in ocular pharmacokinetics and pharmacological activities of regorafenib and pazopanib eye-drops among rats, rabbits and monkeys. Pharmacol Res Perspect 7(6):e00545
Iacobellis G, Baroni MG (2022) Cardiovascular risk reduction throughout GLP-1 receptor agonist and SGLT2 inhibitor modulation of epicardial fat. J Endocrinol Invest 45:489–495
ICH (2022) Guideline M10 on bioanalytical method validation and study sample analysis
Kang DW, Kim KM, Kim JH, Cho HY (2023) Application of minimal physiologically-based pharmacokinetic model to simulate lung and trachea exposure of pyronaridine and artesunate in hamsters. Pharmaceutics 15(3):838
Kim SJ, Choi EJ, Choi GW et al (2019) Exploring sex differences in human health risk assessment for PFNA and PFDA using a PBPK model. Arch Toxicol 93:311–330
Kim KS, Han KA, Kim TN, Park CY, Park JH et al (2023a) Efficacy and safety of enavogliflozin versus dapagliflozin added to metformin plus gemigliptin treatment in patients with type 2 diabetes: A double-blind, randomized, comparator-active study: ENHANCE-D study. Diabetes Metab 49(4):101440
Kim MS, Song YK, Choi JS et al (2023b) Physiologically based pharmacokinetic modelling to predict pharmacokinetics of enavogliflozin, a sodium-dependent glucose transporter 2 inhibitor. Humans Pharmaceutics 15(3):942
King G, Hirst L, Holmes R (1999) Human corneal and lens aldehyde dehydrogenases: Localization and function(s) of ocular ALDH1 and ALDH3 isozymes. Adv Exp Med Biol 463:189–198
Kwak SH, Han KA, Kim KS et al (2023) Efficacy and safety of enavogliflozin, a novel SGLT2 inhibitor, in Korean people with type 2 diabetes: A 24-week, multicentre, randomized, double-blind, placebo-controlled, phase III trial. Diabetes Obes Metab 25:1865–1873
Lambiase A, Tirassa P, Micera A et al (2005) Pharmacokinetics of conjunctivally applied nerve growth factor in the retina and optic nerve of adult rats. Invest Ophthalmol vis Sci 46:3800–3806
Le Merdy M, Fan J, Bolger MB et al (2019) Application of mechanistic ocular absorption modeling and simulation to understand the impact of formulation properties on ophthalmic bioavailability in rabbits: a case study using dexamethasone suspension. AAPS J 21(4):65
Lin J, Sun J, Wang Y et al (2015) Ocular pharmacokinetics of naringenin eye drops following topical administration to rabbits. J Ocul Pharmacol Ther 31:51–56
Löscher M, Seiz C, Hurst J, Schnichels S (2022) Topical drug delivery to the posterior segment of the eye. Pharmaceutics 14(1):134
Matthews J, Herat L, Rooney J et al (2022) Determining the role of SGLT2 inhibition with Empagliflozin in the development of diabetic retinopathy. Biosci Rep. https://doi.org/10.1042/BSR20212209
Mayumi K, Ohnishi S, Hasegawa H (2019) Successful prediction of human pharmacokinetics by improving calculation processes of physiologically based pharmacokinetic approach. J Pharm Sci 108:2718–2727
McLaughlin T, Medina A, Perkins J et al (2022) Cellular stress signaling and the unfolded protein response in retinal degeneration: mechanisms and therapeutic implications. Mol Neurodegener 17(1):25
Nees DW, Fariss RN, Piatigorsky J (2003) Serum albumin in mammalian cornea: implications for clinical application. Invest Ophthalmol vis Sci 44:3339–3345
Nickla DL, Wallman J (2010) The multifunctional choroid. Prog Retin Eye Res 29:144–168
Pang M, Jeon SY, Choi MK et al (2022) Pharmacokinetics and tissue distribution of enavogliflozin in mice and rats. Pharmaceutics 14(6):1210
Panova IG, Tatikolov AS, Smirnova YA et al (2017) Albumin in the vitreous body, retina and lens of human fetal eye. Bull Exp Biol Med 162:629–631
Patel A, Cholkar K, Agrahari V, Mitra AK (2013) Ocular drug delivery systems: An overview. World J Pharmacol 2:47
Rasool MF, Ali S, Khalid S et al (2021) Development and evaluation of physiologically based pharmacokinetic drug-disease models for predicting captopril pharmacokinetics in chronic diseases. Sci Rep 11:8589
Rhee B, Mahbubur RM, Jin C et al (2022) Evaluation of safety and anti-obesity effects of DWP16001 in naturally obese dogs. BMC Vet Res 18(1):237
Sabah J, McConkey E, Welti R et al (2005) Role of albumin as a fatty acid carrier for biosynthesis of lens lipids. Exp Eye Res 80:31–36
Sakanaka K, Kawazu K, Tomonari M et al (2008) Ocular pharmacokinetic/pharmacodynamic modeling for multiple anti-glaucoma drugs. Biol Pharm Bull 31:1590–1595
Schappacher-Tilp G, Fuertinger DH, Kotanko P (2019) A multi-compartment model capturing the pharmacokinetics of the calcimimetic cinacalcet. Cell Physiol Biochem 53:429–438
Skeie JM, Mahajan VB (2014) Proteomic landscape of the human choroid-retinal pigment epithelial complex. JAMA Ophthalmol 132:1271–1281
Daewoong Therapeutics (2023) Daewoong Therapeutics Gets MFDS Nod for Phase 1 IND for the World's First Eyedrop Treatment for Diabetic Retinopathy and Macular Edema. https://www.prnewswire.com/news-releases/daewoong-therapeutics-gets-mfds-nod-for-phase-1-ind-for-the-worlds-first-eyedrop-treatment-for-diabetic-retinopathy-and-macular-edema-301937366.html. Accessed 28 November 2023
Toffoletto N, Chauhan A, Alvarez-Lorenzo C et al (2021) Asymmetry in drug permeability through the cornea. Pharmaceutics 13:694
Wołos-Kłosowicz K, Matuszewski W, Rutkowska J et al (2022) Will GLP-1 analogues and SGLT-2 inhibitors become new game changers for diabetic retinopathy? J Clin Med 11(20):6183
Yang YS, Min KW, Park SO et al (2023) Efficacy and safety of monotherapy with enavogliflozin in Korean patients with type 2 diabetes mellitus: Results of a 12-week, multicentre, randomized, double-blind, placebo-controlled, phase 2 trial. Diabetes Obes Metab 25(8):2096–2104
Acknowledgements
This research was supported by Korea Drug Development Fund funded by Ministry of Science and ICT, Ministry of Trade, Industry, and Energy, and Ministry of Health and Welfare (HN22C0450, Republic of Korea).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors (S. Cho, D.W. Kang, J.H. Kim, G.-W. Choi, M. Kang, and H.-Y. Cho) declare that they have no conflict of interest.
Research involving human and animal rights
All experimental procedures and protocols were reviewed and approved by the Institutional Animal Care and Use Committee of the Laboratory Animal Research Center in CHA University (Approval number: IACUC 220109) (Seongnam, Republic of Korea).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cho, Sj., Kang, D.W., Kim, J.H. et al. Multicompartmental pharmacokinetic evaluation of enavogliflozin eye drop formulation: Understanding its distribution to posterior segments. J. Pharm. Investig. 54, 329–343 (2024). https://doi.org/10.1007/s40005-023-00653-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40005-023-00653-8