Abstract
Objective
To investigate the pharmacokinetic properties of piperaquine after repeated oral administration of the antimalarial combination CV8 in healthy subjects.
Methods
Twelve healthy fasted Vietnamese males were administered four tablets CV8 (320 mg piperaquine phosphate, 32 mg dihydroartemisinin, 5 mg primaquine phosphate, 90 mg trimethoprim) on day 1, followed by two tablets every 24th hour, for a total of 3 days. Blood samples were frequently drawn on days 1 and 3 and sparsely drawn until day 29. Samples were analyzed for piperaquine using solid phase extraction followed by high-performance liquid chromatography. Population pharmacokinetic parameter estimates were obtained by nonlinear mixed effects modeling of the observed data using NONMEM.
Results
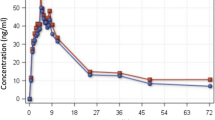
A two-compartment disposition model with an absorption lag time described the observed piperaquine concentrations. Absorption profiles were found to be irregular with double or multiple peaks. A dual pathway first-order absorption model improved the goodness of fit. Piperaquine pharmacokinetics were characterized by a large volume of distribution and a terminal half-life of several days. Estimates [95% confidence interval (CI)] of CL/F, Vss/F and t½z were found to be 56.4 (29–84) l/h, 6,000 (3,500–8,500) l and 11.7 (8.3–15.7) days, respectively.
Conclusion
Piperaquine pharmacokinetics after repeated oral doses were characterized by multiple concentration peaks and multiphasic disposition, resulting in a long terminal half-life. Sustained exposure to the drug after treatment should be taken into account when designing future clinical studies, e.g. duration of follow-up, and may also drive resistance development in areas of high malaria transmission.




Similar content being viewed by others
References
Chen L, Qu FY, Zhou YC (1982) Field observations on the antimalarial piperaquine. Chin Med J (Engl) 95:281–286
Davis TME, Hung TY, Sim IK, Karunajeewa HA, Ilett KF (2005) Piperaquine: a resurgent antimalarial drug. Drugs 65(1):75–87
White NJ, Olliaro PL (1996) Strategies for the prevention of antimalarial drug resistance: rationale for combination chemotherapy for malaria. Parasitol Today 12(10): 399–401 DOI 10.1016/0169–4758(96)10055–7
White NJ (1999) Delaying antimalarial drug resistance with combination chemotherapy. Parassitologia 41(1–3):310–308
World Health Organization (2001) Antimalarial Drug Combination Therapy. Report of a WHO Technical Consultation (35)
Yeung S, Pongtavornpinyo W, Hastings IM, Mills AJ, White NJ (2004) Antimalarial drug resistance, artemisinin-based combination therapy, and the contribution of modeling to elucidating policy choices. Am J Trop Med Hyg 71(2):179–186
Hien TT, Dolecek C, Mai PP, Dung NT, Truong NT, Thai LH, An DTH, Thanh TT, Stepniewska K, White NJ, Farrar J (2003) Dihydroartemisinin-piperaquine against multidrug-resistant plasmodium falciparum malaria in Vietnam: randomized clinical trial. Lancet 363:18–22. DOI 10.1016/S0140–6736(03)15163–X
Denis BM, Davis MET, Hewitt S, Incardona S, Nimol K, Fandeur T, Poravuth Yi, Lim C, Socheat D (2002) Efficacy and safety of dihydroartemisinine-piperaquine (Artekin) in Cambodian children and adults with uncomplicated falciparum malaria. Clin Infect Dis 35:1469–1476. DOI 1058–4838/2002/3512–0003
Karunajeewa H, Lim C, Hung TY, Ilett KF, Denis MB, Socheat D, Davis TME (2003) Safety evaluation of fixed combination piperaquine plus dihydroartemisinin (Artekin) in Cambodian children and adults with malaria. Br J Clin Pharmacol 57(1):93–99. DOI 10.1046/j.1365–2125.2003.01962.x
Ashley EA, Krudsood S, Phaiphun L, Srivilairit S, McGready R, Leowattana W, Hutagalung R, Wilairatana P, Brockman A, Looareesuwan S, Nosten F, White NJ (2004) Randomized, controlled dose-optimization studies of dihydroartemisinin-piperaquine for the treatment of uncomplicated multidrug-resistant falciparum malaria in Thailand. J Infect Dis 190:1773–1782. DOI 0022–1899/2004/19010–0009
Wilairatana P, Krudsood S, Chalermrut K, Pengruksa C, Srivilairit S, Silachamroon U, Treeprasertuk S, Looareesuwan S (2002) An open randomized clinical trial of Artecom vs artesunate-mefloquine in the treatment of acute uncomplicated falciparum malaria in Thailand. Southeast Asian J Trop Med Public Health 33(3):519–524
Giao PT, de Vries PJ, Hung LQ, Binh TQ, Nam NV, Kager PA (2004) CV8, a new combination of dihydroartemisinin, piperaquine, trimethoprim and primaquine, compared with atovaquone-proguanil against falciparum malaria in Vietnam. Trop Med Int Health 9(2):209–216. DOI 10.1046/j.1365–3156.2003.01180.x
Chen Q, Deng J, Wu D (1979) Study on absorption, distribution and excretion of 14C-piperaquine phosphate and 14C-piperaquine in mice. Pharm Ind 8:19–23
Sim IK, Davis TME, Ilett KF (2005) Effects of a high-fat meal on the relative oral bioavailability of piperaquine. Antimicrob Agents Chemother 49: 2407–2411
Hung T, Davis T, Ilett K, Karunajeewa H, Hewitt S, Denis MB, Lim C, Socheat D (2003) Population pharmacokinetics of piperaquine in adults and children with uncomplicated falciparum or vivax malaria. Br J Clin Pharmacol 57(3):253–262. DOI 10.1046/j.1365–2125.2003.02004.x
Lindegårdh N, Ashton M, Bergqvist Y (2003) Automated solid-phase extraction method for the determination of piperaquine in plasma by peak compression liquid chromatography. J Chromatogr Sci 40:1–6
Beal SL, Boeckman AJ, Sheiner LB (1992) NONMEM Users Guides. NONMEM Project Group, University of California at San Fransisco, San Fransisco
Jonsson EN, Karlsson MO (1999) Xpose-an S-PLUS based population pharmacokinetic/pharmacodynamic model building aid for NONMEM. Comput Methods Programs Biomed 58:51–64. DOI 10.1016/S0169–2607(98)00067–4
Karlsson MO, Sheiner LB (1993) The importance of modeling interoccasion variability in population pharmacokinetic analyses. J Pharmacokinet Biopharm 21(6):735–750
Beal SL, Sheiner LB (1982) Estimating population kinetics. Crit Rev Biomed Eng 8:195–222
Zhou H (2003) Pharmacokinetic strategies in deciphering atypical drug absorption profiles. J Clin Pharmacol 43:211–227. DOI 10.1177/0091270002250613
Oberle RL, Amidon GL (1987) The influence of variable gastric emptying and intestinal transit rates on the plasma level curve of cimetidine: an explanation for the double peak phenomenon. J Pharmacokinet Biopharm 15(5):529–544
Schoemaker RC, Cohen AF (1996) Estimating impossible curves using NONMEM. Br J Clin Pharmacol 42:283–290
Wahlby U, Jonsson EN, Karlsson MO (2001) Assessment of actual significance levels for covariate effects in NONMEM. J Pharmacokinet Pharmacodyn 28(3):231–252. DOI 10.1023/A:1011527125570
Wahlby U, Bouw MR, Jonsson EN, Karlsson MO (2002) Assessment of type I error rates for the statistical sub-model in NONMEM. J Pharmacokinet Pharmacodyn 29(3):251–269. DOI 10.1023/A:1020254823597
Acknowledgements
This study was supported by the UNICEF/UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases (TDR). The authors would gratefully like to thank Dr. Ulrika Wählby for her helpful comments.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
The differential equations describing the pharmacokinetic model in Fig. 2 are:
where
and A1 is the amount of piperaquine base administered, A2 is the amount in the central compartment and A3 and A4 are the amounts in the peripheral compartments.
Rights and permissions
About this article
Cite this article
Röshammar, D., Hai, T.N., Friberg Hietala, S. et al. Pharmacokinetics of piperaquine after repeated oral administration of the antimalarial combination CV8 in 12 healthy male subjects. Eur J Clin Pharmacol 62, 335–341 (2006). https://doi.org/10.1007/s00228-005-0084-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-005-0084-9