Abstract
Coral reef fishes are usually assumed to be most strongly associated with reef-building corals. However, sponges can be a significant structural component of coral reef ecosystems and their framework can enhance the local abundance and biodiversity of fish assemblages. Little is known regarding the range of fish species using complex sponges as either shelter or feeding substrata. Here we use a combination of stationary video cameras and focal animal sampling to document fish species positively associated with complex sponges in Kimbe Bay, Papua New Guinea. Stationary cameras identified 45 fish species using the sponges for either shelter, feeding substrata or as sites for ambush predation. A guild of 10 individual fish species from five families (Blenniidae, Chaetodontidae, Gobiidae, Labridae and Pomacentridae) were observed to quantify sponge and other habitat use and compared with habitat availability to determine the level of sponge selectivity. One species, Pleurosicya elongata (the Slender Spongegoby), lived in obligate association with Ianthella basta (Elephant Ear sponge), and there was a positive relationship between sponge size and number of resident fish, however this was not significant for all life stages. Five other fish species appeared to preferentially select sponges as habitat (Amblyglyphidodon aureus, Chaetodon kleinii, Coradion chrysozonus, Escenius prooculis and Pomacentrus nigromanus), while for others, sponge use appeared incidental. When selectivity indices were calculated for specific sponge species it was apparent that some fishes exhibited preferences for particular sponge species or growth forms. These results suggest more fish species may be reliant on sponges than is widely appreciated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Habitat complexity, often defined as the physical three-dimensional structure of an ecosystem, has long been known to influence associated animal communities (Pianka 1966; Painka 1973; Gratwicke and Speight 2005). Many ecosystems are known to be structurally complex (e.g., rainforests, grass plains, kelp forests, seagrass beds and coral reefs) with the majority of this physical structure being provided by living, space-holding organisms (e.g., trees, grasses, seagrasses, kelp, mangroves, hard corals) (Hyndes et al. 2018; Christie et al. 2009; Nagelkerken et al. 2008; Coker et al 2014). Collectively, these sedentary, structurally complex organisms are often referred to as ecosystem engineers (Bruno and Bertness 2000). Ecosystem engineers provide a range of physical and biological resources, including food (Chong-Seng et al. 2011, 2014), shelter (Christie et al. 2009; Graham and Nash 2013) and camouflage that promotes the hunting efficiency of predators (Menge 1976; Menge and Lubchenco 1981; Norbury and Overmeire 2019). The microhabitats they provide promote the evolution of distinct ecological niches, which in turn, support greater biodiversity, leading to the well-known positive habitat complexity – species diversity relationship (MacArthur and MacArthur 1961).
Coral reef ecosystems are among the largest and most complex biological structures on earth (Veron 2000). Here the structural framework is dominated by hard corals (Alvarez-Filip et al. 2009; Coker et al. 2014; Darling et al. 2017). Clearly many coral reef fishes are dependent on corals and coral cover and diversity can have a major influence on reef fish assemblages (Jones et al. 2004; Darling et al. 2017; Pratchett et al. 2008). Losses of hard coral cover and a flattening of reef architecture, have led to marked changes in coral reef benthic communities and their associated fishes (Hoegh-Guldberg et al. 2007; Veron et al. 2009; Darling et al. 2017; Hughes et al., 2016; 2017; 2018). Despite the importance of corals, other benthic habitat-forming organisms (e.g., soft corals, ascidians, macroalgae and sponges) are frequently highly abundant over a wide depth range (e.g., Wilkinson and Cheshire 1989; Bridge et al. 2019; Pomponi et al. 2019; Spalding et al. 2019) and can also play significant roles in the benthic ecology in coral reef ecosystems (Emmett et al. 2022). Yet, surprisingly little is known about the range of fish species using these alternative habitats, either as shelter or as feeding substrata, or the extent to which fishes may prefer these habitats over hard corals (Fabricius 1997; Epstein and Kingsford 2019; Moynihan et al. 2022). The degree to which other substrate-forming organisms provide a viable alternative to corals in the face of declining coral cover is an important question. This paper addresses the concern that, to date, our knowledge of other coral reef structure forming invertebrates, and their interactions with fishes is limited (Carballo et al. 2019).
Sponges (Porifera, Class: Demospongiae) are increasingly being recognised as important organisms in coral reef ecosystems (Maldonado et al. 2017). They exhibit a diverse range of morphologies, contribute substantially to the biological and physical architecture of reefs, and perform several key functional roles (e.g., substrate stabilisation and nutrient cycling, Diaz and Rützler 2001; Wulff 2006; Rix et al. 2018). Their three-dimensional structure enhances local levels of biodiversity by providing microhabitats for numerous invertebrate species and some fishes (Heyward et al. 2010; Schönberg et al. 2016; Maldonado et al. 2017; Chin et al. 2020) and their faster growth and reproductive rates (than their hard coral counterparts), make them successful competitors for benthic space (Dunlap and Pawlik 1996; Wulff 2005, 2006, 2012; Pawlik et al. 2018). Furthermore, sponges are comparatively tolerant to changing climatic conditions, with available evidence suggesting that they may more successfully withstand the environmental pressures (e.g., ocean acidification, increased sea surface temperatures) currently considered deleterious to hard coral populations (Bell and Smith 2004; Emmett et al. 2022). The potential importance of sponges in changing reef environments, therefore, should not be underestimated. While some studies consider the prospect of sponge dominated reefs to be true (e.g., Bell and Smith 2004), others suggest that these relationships may be far more nuanced, and that sponge distribution will likely be constrained by food availability (e.g., Lesser et al. 2020). However, both the role that sponges might fulfil in these scenarios, or whether they (and their associated fish species) can endure continued perturbations, remains unclear (Bell and Smith 2004; Coppock et al. 2022).
Past studies documenting fish-sponge interrelationships have commonly focussed upon describing obligate sponge-users (i.e., obligate spongivores or obligate sponge-dwelling species, Karplus 2014; Coppock et al. 2022). Many obligate sponge-dwellers are highly specialised, small species (≤ 5 cm), adapted to inhabiting the sponges’ internal cavities (Tyler and Böhlke 1972; Henkel and Pawlik 2005). Their abundance is commonly related to the size or availability of their host sponge (Karplus 2014; Majoris et al. 2018a, b). Potential facultative relationships, where fish species use sponges along with other substrata, but show a preference for sponges, have been largely neglected. In addition, the degree to which fish species select for particular sponge species or growth forms has received limited attention. Facultative sponge-dwelling fishes are likely to be morphologically generalised and possess no known adaptations for inhabiting sponges, they may associate with sponges for at least part of their lives but are also commonly linked to other microhabitats (Tyler and Böhlke 1972). It is presumed that the sponges are primarily used either as a temporary shelter or a source of prey organisms (Freese and Wing 2003; Stoner and Titgen 2003; Ryer et al. 2004; Karplus 2014), but this has yet to be corroborated for many tropical coral reef fish species. Furthermore, studies have consistently centred upon their associations with their sponge hosts, seldom providing comparisons of their associations with alternate available habitat types (Chin et al. 2020). Although some understanding has been garnered concerning habitat preferences and selectivity by obligate sponge-dwelling fishes (e.g., Majoris et al. 2018a, b), species- or morphology-specific selectivity has not been considered for facultative sponge-dwelling fishes.
The fish-sponge interrelationships that occur within the tropical Atlantic (a region encompassing the Caribbean, but also including the central America and the Gulf of Mexico) have been the best documented in the literature (e.g., Tyler and Böhlke 1972; Wulff 1994, 2006, 2012; Pawlik et al. 2018; Lesneski et al. 2019). Here, sponges rival hard corals in abundance and in providing three-dimensional structure to the reef (Rohde and Schupp 2012). Far less is known regarding fish-sponge associations within the Indo-Pacific region, an area noted for its high levels of sponge abundance and biomass (Bell and Smith 2004; Van Soest et al. 2012; de Goeij et al. 2017; Rovellini et al. 2019). In comparison to the Indo-Pacific, the Caribbean is considerably more homogeneous in terms of both fish assemblages and sponge species composition, and does not support certain fish (e.g., Siganus spp.) and complex (i.e., free-standing sponges with the capacity to shelter small fishes and invertebrates) sponge genera (Ianthella spp., Lamellodysidia spp., Phyllospongia spp.) that are common throughout the Indo-Pacific (Hoey and Bellwood 2009; Fox et al. 2009; Hoey et al. 2013; Maldonado et al. 2017). Increased research effort in recent years has now begun to clarify fish-sponge relationships for some areas of the Indo-Pacific (e.g., Powell et al. 2014, 2015, 2018; Mortimer et al. 2021). However, if the role of sponges becomes more critical as a result of increasing coral reef degradation (González-Rivero et al. 2012), a baseline understanding of fish-sponge relationships at a wider geographic scale, is urgently required. Only then can we anticipate the role that sponges might fulfil in a future scenario.
The nearshore and island reefs of Kimbe Bay are noted for their high sponge abundance and diversity (González-Murcia et al. 2022). However, fish-sponge interrelationships for this region have yet to be examined. The objective of this study was to explore the importance of structurally complex and/or massive sponges as a source of habitat and/or feeding substrata for local coral reef fish species. Our specific objectives were as follows: (1) determine which fish species associate with four large complex sponge species, describe the nature of each relationship (shelter, feeding, etc.) and determine the degree to which fishes are species-specific in terms of use of sponges; (2) quantify habitat use, including sponges and other substrata, for 10 focal fish species (Families: Blenniidae, Chaetodontidae, Gobiidae, Labridae and Pomacentridae) known to associate with sponges to varying degrees; (3) compare habitat use with habitat availability to assess which fish species have an obligate association with or are preferentially selecting sponges, and if so, which sponge species; and finally) to elucidate the relationship between obligate sponge dwelling fish species Pleurosicya elongata and its sponge host Ianthella basta.
Materials and Methods
Study location
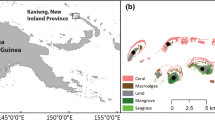
The study area was located in Kimbe Bay (Fig. 1B), West New Britain Province, Papua New Guinea (Fig. 1A) (05°12′ 0.530 S, 150°22′ 0.801 E), and is recognised as a region of high biodiversity and species richness (Roberts et al. 2002). The study was conducted on six nearshore reefs (< 1 km from land; Fig. 1C) and one island reef (≤ 5 km from land; Fig. 1D). Data collection took place at Mahonia Na Dari Research and Conservation Centre from 3 June through to 4 July 2019.
Fish species associated with four focal sponges: stationary video observations
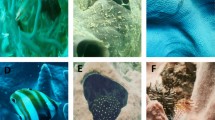
To ascertain what behavioural associations were occurring between fishes and sponges within the region, video footage was collected for four widely distributed local sponge species with distinct structurally complex and/or massive morphologies (Fig. 2). Focal species were (1) hanging sponge Cribrochalina sp. (Fig. 2A); a ramose tangled mass of irregularly anastomising and dividing branches (Busutil et al. 2018), while dominated by Cribrochalina sp. the mass often encompasses multiple species (e.g., Gelliodes spp., Cribrochalina spp., and Callyspongia spp.), which are predominantly spikey, and occasionally smooth; (2) Coelocarteria singaporensis (the daisy sponge; Fig. 2B), a common endopsammic sponge that demonstrates a fistular morphology with a globular, irregular body form (Schönberg and Lim 2019); (3) Xestospongia testudinaria (the giant barrel sponge; Fig. 2C) barrel-like morphology, which commonly occurs in two distinct morphotypes, the first of which is represented by individuals with a digitate surface area, the second morphotype includes those individuals with a predominantly smooth surface area (Swierts et al. 2013); and (4) Ianthella basta (the elephant ear sponge; Fig. 2D) which demonstrates conspicuous fan- or funnel-like growth forms (Rohde and Schupp 2012).
Structurally complex and/or massive sponge species sampled from Kimbe Bay. Focal species were comprised of (A) Cribrochalina sp., arborescent/ branching morphology; (B) Coelocarteria singaporensis, fistular morphology, has a globular irregular body from which fistules arise (Schönberg and Lim 2019); (C) Xestospongia testudinaria a barrel morphology: and (D) Ianthella basta, foliaceous morphology. Photo credits A. Coppock (B); G. Jones (A, C); C. Battershill (D)
To observe fish associated with sponges, two Go Pros (Hero 4) were positioned on weighted platforms and deployed (on SCUBA) on either side of the focal sponges. Cameras were then allowed to film continuously for a period of 30 min. Divers vacated the location for the duration of filming to minimise the disruption to fish behaviours (Emslie et al. 2018). Video footage was collected on windward areas of reef slope and reef wall/overhangs (5–15 m depth) during daylight hours (08:00–15:00). Approximately 18 h of video footage was recorded across the seven reef sites (Supplementary Material Table 2).
The first and last 5 min of video footage were disregarded to account for any disturbance due to divers leaving or approaching the site (sensu Emslie et al. 2018), the remaining footage was quantified. Fish were identified to species level and the manner of behavioural association designated one of four distinct categories (Table 1). Care was taken to avoid recounting fish that exited the video-frame, and analysis was conducted for both sides of the sponge simultaneously to account for any movement of individual fishes. Hereafter, the term ‘sponge-grazer’ was applied to fishes adopting ‘apparent feeding’ behaviours, where fish were seen to bite at the surface of the sponge, but biomass removal could not be verified (sensu Mortimer et al. 2021). It should also be noted that technological limitations (e.g., camera resolution) may have prevented some cryptobenthic fish species from being identified. Furthermore, while some of the cryptobenthic fish species observed inhabiting the surfaces of the sponges were collected using clove oil and identified to species level as part of a pilot study, those that occupied the internal cavities of the sponges were not accounted for. This likely resulted in an underestimation of localised fish diversity on sponges.
Habitat use by 10 focal fish species
Following analysis of the literature (e.g., Noonan et al. 2012, McDonald et al. 2018; Coppock et al 2022) and video footage, 10 focal fish species were selected for further fish-habitat observations. Focal fish species were the Blackbelly Dwarfgoby (Eviota atriventus), the Redspeckled Dwarfgoby (E. rubrisprasa), the Slender spongegoby (Pleurosicya elongata), the Striped Coralblenny (Ecsenius prooculis), the Golden Damsel (Amblyglyphidodon aureus), the Black-bar Chromis (Chromis retrofasciata), the Goldback Damsel (Pomacentrus nigromanus), the Pinstriped Wrasse (Halichoeres melanurus), Klein’s Butterflyfish (Chaetodon kleinii), and the Orange-banded Coralfish (Coradion chrysozonus) (Fig. 3). All focal fish species are known (e.g., Nagelkerken et al. 2008; Noonan et al. 2012; Macdonald et al. 2016; Coppock et al. 2022) or observed (pers.obs., Coppock A., Jones G.) to exhibit some form of association with sponges and are locally abundant. While most were assumed to have facultative sponge associations, documented within this study, Pl. elongata are often considered an obligate sponge-dwelling species (Kuiter and Tonozuka 2001).
Focal fish species. (A) the Striped Coralblenny (Ecsenius prooculis) (Standard Length: 4cm); (B) the Goldback Damsel (Pomacentrus nigromanus) (SL: 9cm); (C) the Blackbelly Dwarfgoby (Eviota atriventus) (SL: 2 cm); (D) the Orange-banded Coralfish (Coradion chrysozonus) (SL: 15 cm); (E) the Redspeckled Dwarfgoby (E. rubrisprasa) (SL:2cm); (F) the Black-bar Chromis (Chromis retrofasciata) (SL: 4cm); (G) the Golden Damsel (Amblyglyphidodon aureus) (SL: 13cm); H) the Pinstriped Wrasse (Halichoeres melanurus) (SL:12cm); (I) Klein’s Butterflyfish (Chaetodon kleinii) (SL: 15cm); (J) the Slender spongegoby (Pleurosicya elongata) (SL: 4cm). Photo credits A. G. Coppock, G. P. Jones
The habitat use was categorised for the ten focal species. Here, divers on SCUBA surveyed the windward areas of reef slope and reef wall/ overhangs (5–15 m depth) during daylight hours (08:00–15:00) of the seven reef sites. Starting at depth (15 m), divers adopted an ascending grid (200 × 15 m) search pattern. Focal species were observed haphazardly, as encountered, and the microhabitat situated immediately underneath the individual at the time of observation was recorded. Substrata were classified to the level of species, where known. Where possible a minimum of 100 individuals were recorded for each species. Where it was not possible to collect 100 observations for a particular fish species (i.e., due to low local species abundance) recorded numbers were lower. Where a group of individuals occupied the same microhabitat, up to a maximum of five individuals were recorded, eliminating the potential for any microhabitat bias.
Habitat availability and selectivity indices
To calculate the degree of habitat selectivity in the 10 focal species, habitat use data was compared to habitat availability data. Benthic surveys were conducted to collect habitat availability data to estimate the percent cover of different substratum types within each of the six nearshore sites in Kimbe Bay: Rakaru Diri, Matane Huva, Gava Gava, Matane Walindi, Hanging Gardens, and Limuka (Fig. 1C) and the one island reef site (Restorff Island, Fig. 1D), where habitat usage had previously been determined. At each site 12 (4 × 50 m) transects were laid parallel to the shore on the windward exposed side of the site, within three depth strata (5, 10, 15 m). Video-recordings were then conducted along each transect using the line-point intercept (LPI) technique to quantify substratum cover. Habitat availability was then quantified retrospectively, here, images were extracted from the video footage and the substratum directly underlying the transect at random intervals (n = 100) was identified. Five overarching substratum types were recognised: live hard coral, live soft coral, macroalgae, sponges and non-living (e.g., coral rubble, rock, etc.). Within each of these categories the major genera/ species were identified to the lowest taxonomic level where possible. Sponge species were classified as per González-Murcia et al. (2022) and Coppock et al. (In prep); and due to similarities across reef sites (Supplementary Material Tables 1 and 3A–J; one-way ANOVA: F(6,42) = 0.013, p = 0.999) percent cover was pooled across sampling sites (nsites = 7). Results were then graphed to compare the relative availability of each substratum type within these nearshore reefs. Only windward exposures were investigated as it was previously determined that no significant differences occurred in sponge distributions with exposure (González-Murcia et al. 2022).
To compare habitat use and availability, data was then pooled across sites (nsites = 7) and divided into five major substrate categories: hard coral, soft coral, sponges, macroalgae and non-living. Electivity indices were then calculated. Ivlev’s electivity index (IV) was used to determine observational electivity on sponge habitats in the focal fish species. Here, the relationship between percentage habitat use (U) was compared to percentage habitat availability (A) using the following formula (Ivlev 1961; as per Moynihan et al. 2022). Positive values were indicative of associations with particular habitat types, whereas negative values were indicative of habitat avoidance.
Upon the identification of focal fish species that demonstrated an association with complex sponge habitats, Ivlev’s electivity index was then reapplied to assess the degree of selectivity for specific sponge species (Supplementary Material Table 4).
The obligate relationship between the slender spongegoby, Pleurosicya elongata, and the elephant ear sponge, Ianthella basta
To further understand fish-sponge associations for a local obligate sponge-dwelling fish species, the relationship between Ianthella basta (the elephant ear sponge) and Pleurosicya elongata (the slender spongegoby) was investigated. Resident I. basta populations (Restorff Island – Fig. 1d) were mapped. A total of 39 I. basta, occupying the N-NE fringing reef slope were surveyed (5–25 m depth) on SCUBA. Sponges were photographed against a scale. A minimum of two images were recorded for each sponge, one representing the planar view (i.e., from above) and one representing the non-planar view (i.e., from the side). Imaging software (ImageJ) was then used retrospectively to calculate estimates of height, width and depth, thus volume (m3) estimates for each sponge could be calculated and size could be assessed (sensu McMurray et al. 2018). Where present, divers recorded the total number and ontogenetic life stage of Pl. elongata occupying the surface of the sponge (Adults (≤ 4 cmSL), juveniles (≤ 3 cmSL) and new recruits (≤ 2 cmSL)) in situ. An ANOVA was used to determine if the slopes of the relationships were significantly different from zero. The relationship between sponge size and the number of resident Pl. elongata was then tested with least-squared regression. The relationship between volume of sponge and number of fish was tested separately for new recruits, juveniles and adults.
Results
Fish species associated with 4 focal sponges: stationary video observations
Approximately 18 h of video footage was recorded across the seven reef sites (Supplementary Material Table 2). A total of 278 individual fishes, comprised of 45 different species from 13 different families were identified as interacting with sponges (Tables 2 and 3). Of the four different behavioural associations defined, apparent feeding, shelter, swimming, and ambush, 63 percent of fish species (nspecies = 27) used the structurally complex sponges as a source of shelter. This was evident regardless of ontogenetic life stage, and although more common amongst those species with small body sizes (≤ 15 cm SL; e.g., Gobiidae, Pomacentridae, Labridae), did not solely apply to small fishes. Apparent feeding was also prevalent (nspecies = 14). This behavioural category was dominated by species from Families: Acanthuridae, Pomacanthidae and Chaetodontidae, where grazing on the surface of sponges was conspicuous and constant. Swimming was the third most common behavioural association (nspecies = 10). Lastly, ambush was the least common behavioural association noted (nspecies = 3), with only three species of piscivores/mesopredators (Cephlopholis cyanostigma, C. mircroprion and Pseudochromis fuscus) interacting with sponges in this fashion. It must be noted that behavioural associations rarely appeared in isolation, rather they occurred in conjunction with one another (e.g., sheltering as an ambush predator).
Fish were usually restricted to a single host species of sponge (Fig. 4). While some fish species (e.g., Pomacentrus nigromanus and Cephalopholis microprion) were present on all four focal sponge species (Fig. 4, Table 1) other more specialised sponge-dwelling species, for example, Pleurosicya elongata (nfish = 5) only occupied their preferred sponge shelter (Ianthella basta). Several fishes, however, associated with two or three of the focal sponge species. Sponges that exhibited higher levels of structural complexity (e.g., Cribrochalina sp. and Coelocarteria singaporensis) had noticeably more fish species and individuals associating with them (nspecies = 23, 16; nindividuals140, 72, respectively) than either X. testudinaria or I. basta (nspecies = 16, 5,; nindividuals 21, 42, respectively). Cribrochalina sp., for example, had more than four times as many sponge-associated fish species than Ianthella basta (nspecies = 23:5, respectively) and approximately seven times as many individuals (nindividuals= 140; 21, respectively). Aggregations of small-bodied fishes were common in these sponges.
Venn diagram showing the fish-sponge associations identified from video footage. The four focal sponge species are represented by ellipses. n denotes the number of fish species that are associated with each sponge species or combination of species. Regions of no overlap show fish species that are specific to one of the four sponge species. Regions of overlap show numbers of fish species associated with 2 or more sponge species. Fish species represented in each ellipse are examples of some, but not all, of the fishes found associating with focal sponge species. Further detail of all fish species observed in video footage and their behavioral associations with focal sponge species is provided in Table 2
Habitat use by 10 focal fish species
Across our 10 focal fish species, a total of 1362 fish-habitat associations were recorded. The degree of association with habitats, however, differed among fish species (Fig. 5). It was evident that while some fish species associated with habitats more generally (e.g., E. atriventus, H. melanurus, Fig. 5A, H), other fish species demonstrated strong associations with specific habitat types (e.g., E. rubrisprasa, Pl. elongata, Co. chrysozonus, Ch. kleinii) (Fig. 5B, C, I, J). Halichoeres melanurus (Fig. 5H) were abundant and ubiquitous across nearly all substrata (except for macroalgae). Similarly, Escenius prooculis (Fig. 5D) were widely distributed across multiple substrata. However, they were particularly abundant upon macroalgae, Porites spp. and sponges.
Habitat use (± SEM) of focal fish species. Data was pooled across reef sites (n = 7). Habitat use refers to the percentage of occurrences focal fish species were observed directly above each substrate type. Bar colour is indicative of overarching substrate type: grey = non-living, green = macroalgae, orange = hard coral, pale orange = soft coral, blue = sponges, dark blue = other invertebrate, white = unidentified. ‘Unidentified’ encompasses all substrata observed directly underneath the focal fish species that could not be identified. Further description of species/ genera and morphology denoted on the x-axis. All observations were recorded on the windward exposures of reef sites. Y-axes scales differ among charts
Eviota atriventus and E. rubrisprasa (Fig. 5A, B) were prominent on massive and encrusting Porites spp. and non-living substrata (E. atriventus, rubble: 24% ± 9 SEM). Likewise, Chromis retrofasciata (Fig. 5F) commonly occurred in association with hard coral species, the majority being recorded within branching coral species. The two remaining damselfish species displayed markedly different habitat associations. Amblyglyphidodon aureus were primarily noted in association with structurally complex sponges and soft corals, but also recorded elsewhere, whereas Pomacentrus nigromanus, was primarily documented on Porites spp. (massive and encrusting: 30% ± 3 SEM) or hiding amongst the ramose branches of Cribrochalina sp. and Coelocarteria singaporensis. While the proportion of time Ch. kleinii and Co. chrysozonus spent in proximity to sponges was relatively high (Figs. 5I, J), sample sizes for these species were low (nfish = 19: nfish = 6, respectively).
Habitat availability and selectivity indices
Hard coral was the most abundant benthic space-holder (Fig. 6). Here, massive, and encrusting forms of Porites spp. were prolific (12% ± 3 SEM), while branching morphologies (Acropora spp., Porites spp., Pocillopora spp., Seriatopora spp.) and foliose morphologies (Pachyseris spp. and Echinopora spp.) accounted for the remainder. Non-living substrata was also prevalent, of which, sand and rubble were substantial contributors. Here, dead coral and rock made up the smaller components. Sponges and macroalgae were the next most abundant. Soft coral cover (inclusive of gorgonians and whip corals), in this instance, was comparatively low contributing minimally (relatively speaking) to local three-dimensional structure.
Of the 13.8% substrate cover that was made up of sponges (Fig. 6), 3.8% (± 1.5EM) were encrusting sponge species. Cribrochalina sp. was the most abundant structurally complex sponge species recorded, but Coelocarteria singaporensis and Xestospongia testudinaria cover was also substantial. Melophlus sarasinorum, Hyrtios spp., and Ianthella basta made up a smaller component. All other structurally complex sponge species contributed to the remaining sponge cover.
Patterns of substrate selectivity by focal fish species were evident. A. aureus, Co. chrysozonus, Esc. prooculis, P. nigromanus, and Pl. elongata all selected sponges over other available habitat (Fig. 7C–E, G, J). Except for the damselfish species (A. aureus and P. nigromanus), which exhibited neutral- to weak avoidance responses toward soft coral substrata, no evidence of any other associations was apparent. Selectivity calculations revealed negative responses toward macroalgal, hard coral and non-living substrata. Preferences for sponges was strongest for Co. chrysozonus and Pl. elongata. Associations with hard coral were detected for C. retrofasciata, E. atriventus and E. rubrisprasa, again, all other substrata were largely avoided (Fig. 7A, B, F). Chaetodon kleinii and H. melanurus associated with multiple substrata. Ch. kleinii demonstrated strong positive responses toward hard coral (Fig. 7H, I). While H. melanurus was pervasive across most substrata (Fig. 5H), slight positive relationships were detected toward hard coral and sponges. However, this habitat usage was scarcely more than what was available.
Slectivity indices of (A) Eviota atriventus, (B) E. rubrisparsa, (C) Pleurosicya elongata, (D) Escenius prooculis, (E) Amblyglyphidodon aureus, (F) Chromis retrofasciata, (G) Pomacentrus nigromanus, (H) Halichoeres melanurus, (I) Chaetodon kleinii and (J) Coradion chrysozonus for substrate, using Ivlev’s selectivity index (± SEM). The index relates the relationship between the percentage availability of habitat compared to the proportion of habitat use. Positive values indicate association, negative values indicate avoidance. Data pooled across seven sample sites
For those fish species that selected for sponges, it was clear that some fish species were selectively associating with certain species of sponge (Fig. 8). Pleurosicya elongata, for example, was only found on Ianthella basta (Fig. 8A), 100% of their occurrences were recorded here. Evidence of them occurring on any other species of sponge was not apparent. As such, they have been defined as an.
Selectivity indices of (A) Pl. elongata, (B) Esc. prooculis, (C) Amblyglyphidodon aureus (D) Pomacentrus nigromanus, (E) Halichoeres melanurus (F) Chaetodon kleinii, (G) Coradion chrysozonus, for sponge taxa, using Ivlev’s selectivity index (± SEM). Data pooled across seven sample sites. The index relates the relationship between the percentage availability of sponge taxa compared to the proportion of sponge use. Positive values indicate association, negative values indicate avoidance
Obligate sponge-dwelling species within this study. All other fish-sponge associations documented within this study, however, were only facultative associations. Escenius prooculis was observed associating with Melophlus sarasinorum (Fig. 8B) but did not select for any other sponge species despite also being recorded upon other sponges, e.g., Hyrtios spp., non-living substrata and hard coral species. Amblyglyphidodon aureus and Pomacentrus nigromanus (Fig. 8C, D) were noted to primarily associate with Cribrochalina sp., however, an association with Coelocarteria singaporensis was also evident for P. nigromanus. Despite only exhibiting a weak association with sponges, when individual sponge species were investigated, H. melanurus (Fig. 8E) was noted to demonstrate slight associations with M. sarasinorum and C. singaporensis. Chaetodon kleinii and Coradion chrysozonus (Fig. 8F, G) both exhibited positive associations toward Cribrochalina sp. Co. chrysozonus also exhibited an association with Haliclona spp., another ramose, hanging sponge species, but interestingly, demonstrated a neutral response toward Xestospongia testudinaria.
The obligate relationship between the slender spongegoby Pleurosicya elongata and the elephant ear sponge Ianthella basta
A total of 349 individuals were noted across 39 sponges, of which 105 individuals were classified as adults (≤ 4 cmSL), 171 as juveniles (≤ 3 cmSL), and 72 as new recruits (≤ 2 cmSL). I. basta ranged in size from 0.06m2 to 2.7m2 and while the majority exhibited fan-type morphologies, several resembled funnels. The ANOVA indicated that slopes significantly differed from zero (F = 7.39, p ≤ 0.01). The least-squared regression revealed a positive relationship, whereby the larger the sponge (area, m2) the greater the number of resident Pl. elongata (Fig. 9). This relationship was most pronounced for the juvenile life-stage.
Discussion
Sponges are conspicuous and abundant contributors to coral reef structural complexity in Kimbe Bay, Papua New Guinea (González-Murcia et al. 2022; Coppock et al. In prep). They are functionally important, with numerous local fish species associated with them for food, shelter, and other resources. In this study we identified 45 fish species that interact with complex and massive sponge morphologies. Further, we documented several facultative relationships between fish and sponges, where sponges appear to be used both to ameliorate predation or as a source of concealment (for predators), from which to ambush prey (e.g., Cp. cyanostigma). Additionally, sponges appeared to be an important feeding substratum for several key coral reef fish families (Pomacanthidae, Chaetodontidae, Acanthuridae), although most observations suggested no damage was done to the sponge. The degree to which the sponge itself, or its resident inhabitants are, a source of nutrition requires further investigation. We also documented a clear obligate relationship between the goby Pleurosicya elongata and the elephant ear sponge Ianthella basta, with the goby abundance closely linked to the availability and size of its host.
Fish species associated with four focal sponges
Sponges are important habitats, providing living space for a wide variety of organisms (e.g., polychaetes, Lopez et al. 2001; echinoderms, Henkel and Pawlik 2005; crustaceans, Rios and Duffy 2007; Butler et al. 2017). It has been hypothesised that the physical characteristics of sponges (e.g., volume, structural complexity) may control associated biodiversity (Koukouras et al. 1992, 1996; Abdo 2007), as they provide spatial refuge for potential prey organisms resulting in increased survivorship (Scharf et al. 2006). While most associated fauna inhabit the sponges’ internal canals (Koukouras et al. 1996; Karplus 2014, George et al. 2023) others occupy the surface of the sponge and/or the negative space created by complex morphological structures (Karplus 2014). Although epiphytic, inquilinist and cryptobenthic mesofauna, including obligate sponge-dwelling fishes, have been repeatedly quantified (e.g., Tyler and Böhlke 1972; Bell and Smith 2004; Henkel and Pawlik 2005; Majoris et al 2018a, b), associations with facultative sponge-dwelling fishes have not (Tyler and Böhlke 1972; Karplus 2014). Complex and massive type sponge morphologies contribute substantially to the local reef framework of Kimbe Bay, where some species reach considerable sizes (Coppock et al. In prep). Here we demonstrate that their influence also extends to facultative fishes and fish assemblages, and that through enhancing local habitat complexity, biodiversity was also enriched (Abdo 2007). Furthermore, larger, more complex sponge morphologies are presumed to host higher diversity and abundance of associated communities (Chin et al. 2020). While this relationship is not overarching and is not apparent for several temperate sponge species (e.g., Koukouras et al. 1996), it was evident here. As the complexity of the host sponge increased, so did the associated fish biodiversity (I. basta, nfish = 5; X. testudinaria, nfish = 42; C. singaporensis, nfish = 72 and Cribrochalina sp., nfish = 140). However, the degree of complexity required by fishes may be species-specific. Additionally, further replicates of each morphotype would be needed to confirm this relationship. Whether these relationships differ with increased depth, is also unclear. Sponges generally exhibit faster growth rates and increased size with depth (≥ 30 m; Lesser and Slattery 2013, 2018) where the availability and influence of other structure-forming organisms may be reduced, thus providing an important source of vertical relief (Wilkinson and Cheshire 1989; Hooper 2019; Pomponi et al. 2019). Understanding sponge interactions with local fauna, therefore, is essential. The number of species we identified in facultative association with sponges (n = 26) was substantially higher than any other records we could find. Only one additional fish species, Pleurosicya elongata, was documented using sponges as shelter, here an obligate relationship with its’ sponge host (I. basta) was noted.
Sponges were widely used in a facultative manner as shelter by fishes. Here we identified 26 fish species utilising sponges in this manner, accounting for 96% of all shelter-related observations observed. Fishes from the families Apogonidae, Gobiidae, Labridae and Pomacentridae were particularly prevalent. Here, juvenile fishes and /or aggregations of small-bodied individuals (≤ 15 cm SL) commonly sheltered within the three-dimensional structure created by the sponge. Some of the associations noted here, specifically damselfish species, have previously been documented as part of broader scale habitat investigations (e.g., C. retrofasciata, Noonan et al. 2012; P. nigromanus, MacDonald et al. 2018), however, these were mostly anecdotal and observations were not quantified. The majority of fish-sponge relationships identified here had not been previously recorded. These relationships with digitate, fistulate and branching sponge morphologies should not be surprising. Branching hard coral species have long been considered refuges for small-bodied and juvenile fishes (Coker et al. 2014); likewise, digitate hard coral morphologies (e.g., some Acropora species) often provide important nursery areas for juvenile fishes. Several recent studies have indicated that sponges with complex morphologies may provide suitable shelter and/or nursery areas in the absence of hard corals (Cabaitan et al. 2016; Seemann et al. 2018). Perhaps one explanation for these observed associations is that the sponges’ physical and chemical defences provide refuge from predators, protection from local environmental stressors and distance from competitors (Safriel and Ben-Eliahu 1991; Abdo 2007; Huang et al. 2008). However, despite this progress, relatively little is known regarding most of these partnerships (Karplus 2014). Whether these relationships are mutualistic, commensal, or otherwise has not yet been determined. Furthermore, whether fishes are actively choosing to inhabit sponge structures, or whether it is the result of competition between local fish species or other biotic interaction is not yet clear.
Three fish species, adopted the differing behavioural strategy of potential ambush. Cephlopholis cyanostigma, Cp. microprion, Pseudochromis fuscus are known to prey upon small fishes and crustaceans (Leiske and Myers 2002; Holmes and McCormick 2010). Sheltering in sponges not only conceals them from predators, but the camouflage gained reduces their visibility to potential prey (James and Heck 1994). Sponges, therefore, have the capacity to assist with predation efforts. Additionally, Ps. fuscus are known to aggressively mimic the colouration of their damselfish prey (Munday et al. 2003; Cortesi et al. 2015). Unsurprisingly they were only present on sponges (Coelocarteria singaporensis) inhabited by their preferred damselfish prey (e.g., Pomacentrus. amboinensis). Although no predation events were recorded within the current study, this behavioural association should not be discounted and warrants further investigation.
Sponge-grazing and/or predation by fishes may have important implications in structuring local benthic communities (Batista et al. 2012; Boaden and Kingsford 2015; Pawlik et al. 2018). Apparent feeding by sponge-grazers was both conspicuous and consistent. Thus, sponges and associated fauna appear to be an important source of nutrition for local acanthurid, chaetodontid, siganid and pomacanthid species. Our findings are consistent with Mortimer et al. (2021) and Powell et al (2015), who noted Acanthurus pyroferus, Ctenochaetus binotatus, Ct. striatus, Chaetodon kleinii and Pygoplites diacanthus to be prolific sponge-grazers in the Sulawesi region of Indonesia. However, while apparent feeding was evident, we cannot determine the exact dietary source consumed. Sponges are an important food source for obligate spongivorous fishes such as angelfish, however, fish may bite sponges and have minimal predatory impact (Bell and Smith 2004; Pawlik et al. 2018; Mortimer et al. 2021). Moreover, fish may consume small quantities of sponge, remove their target prey (e.g., commensal crustaceans, polychaetes, or cyanobacteria), and then regurgitate unwanted sponge material. The implications of consuming sponge material, irrespective of target prey, are likely the same from the perspective of the sponge (Coppock et al. 2022). Further quantification of dietary preferences is therefore still required to refine our knowledge of local sponge-grazing behaviours.
Swimming was the least common behavioral association noted. It was likely a temporary behaviour either used in conjunction with, or as a precursor to other behavioral associations. For planktivorous fishes such as damselfishes it is likely that they left their sponge shelters, briefly, to feed before returning to safety (Moynihan et al. 2022). Similarly, sponge-grazers were presumably swimming between appropriate foraging sites. It must be noted that behavioural associations rarely appeared in isolation, rather they occurred in conjunction with one another.
Habitat availability, habitat use and sponge selectivity by fishes
The nearshore reefs of Kimbe Bay are sheltered with limited exposure to wave action, weak wind and tidal generated current regimes and minimal seasonal variations (Brodie and Turak 2004). As a result, although hard corals dominate the benthic substrata (35.54%), a common trait amongst of many coral reefs in the Indo-Pacific biogeographic region, sponges are prevalent (≥ 13%). Their abundance, while comparable to the wider biogeographic region, exceeds that found upon tropical Atlantic, Central Pacific and Great Barrier Reef (Rovellini et al. 2019; Bell and Smith 2004; González-Murcia et al. 2022). Macroalgal abundance was nearly equal to sponges (16.26%) and recent localised increases in macroalgal abundance (in line with coral degradation; Jones et al. 2004; pers obs Coppock AG) are likely the result of nutrient input from nearby palm oil plantations and logging developments (Brodie and Turak 2004). In contrast soft corals (Alcyonea, Gorgonacea), while present, are noticeably lower in abundance than other similar biogeographic regions (e.g., the Great Barrier Reef, Australia) where soft corals are evidently more abundant (Wilkinson and Cheshire 1989; Fabricius and De’ath 2009; Moynihan et al. 2022). This study largely corroborates the findings of González-Murcia et al (2022), further suggesting that sponges are important space-competitors in the region.
Several of our focal fish species exhibited associations with sponges; Amblyglyphidodon aureus, Pomacentrus nigromanus, Escenius prooculis, Pleurosicya elongata, Halichoeres melanurus Chaetodon kleinii and Coradion chrysozonus. All species were regularly encountered amongst/or within close proximity to sponges; Pl. elongata (100%), Co. chrysozonus (100%), Ch. Kleinii (45%), P. nigromanus (26%), Esc. Prooculis (18%). H. melanurus (20%). Pomacentrus nigromanus selected for the branching digitate sponge morphologies Cribrochalina sp. And C. singaporensis. Perhaps surprisingly some of the least abundant sponges seemingly attracted certain fish species (Ianthella basta, Melophlus sarasinorum; % availability of each species: 0.23%:0.13%, respectively). This was in part due to the obligate relationship revealed between Ianthella basta and Pleurosicya elongata. While the associations noted with M. sarasinorum were not obligate, they were substantial, with selectivity indices indicating that Esc. prooculis and H. melanurus preferentially select M. sarasinorum over other available sponge species and substrata. While the proportion of time Ch. kleinii and Co. chrysozonus spent in proximity to sponges was relatively high (45:100%, respectively), sample sizes were low (nindividuals = 19: 6, respectively) and although selectivity indices indicated preferences for certain habitat types and sponge species, caution is required before inferring habitat use and/ or prey preferences without further investigation. Observations of these species also occurred across a limited number of sites (Restorff Island, Limuka and Hanging Gardens), in different habitats, often where locations were more exposed and soft coral species were more abundant. This suggested that other variables may be responsible, in part, for their distribution and use of available habitat. Contrary to video observations where Co. chrysozonus consistently fed upon sponges (specifically X. testudinaria) selectivity indices indicated a neutral response to barrel sponges.
The obligate relationship between the goby Pleurosicya elongata and the elephant ear sponge Ianthella basta
Despite the low availability of Ianthella basta, the strongest association was demonstrated by the obligate sponge-dwelling goby Pleurosicya elongata. Although absent from the tropical Atlantic I. basta is widely distributed across the Indo-Pacific (Cheshire et al. 1997; Wahab et al. 2017). It is predominantly found along the edge of coral slopes where hard substrates change to soft bottomed sediments, with occasional rocky outcrops to which it is attached. Here, I. basta adds to the local rugosity, providing shelter for other invertebrates and fishes (Rohde and Schupp 2012). While the number of associated Pl. elongata recorded in the video-footage was minimal (n = 5) this was likely due to technological limitations, where the cameras were unable to effectively distinguish the presence of the cryptobenthic fishes. Pleurosicya elongata are small in size (≤ 4cmSL), typically translucent in colour, and often mimic the colouration of their host sponge (Larson 1990). In situ observations, however, revealed them to be prolific (nindividuals = 395) and Pl. elongata consistently inhabited I. basta in conspecific groups. Fish numbers, however, were highly variable, often dependent on sponge size and / or morphology (foliaceous vs. funnel) and location, however a preference for this sponge was evident. Although it has been described on several occasions (e.g., Larson 1990; Colin and Arneson 1995) we currently know very little regarding this relationship. However, it was reflected in the selectivity indices, and a positive relationship between sponge size and the number of resident Pl. elongata individuals was noted. Numerous small coral reef fish species co-exist in conspecific groups (Allen 1991; De Brauwer et al 2015). The benefits of group-living are three-fold: (1) resident conspecific adults are indicative of a suitable living environment, (2) individual predation risks may be reduced resulting in enhanced survivorship, and (3) heightened predator awareness (Beukers and Jones 1998; Booth 2002). Thus, subsequent fitness, growth and reproduction may all be improved (Coppock et al. 2013, 2016). Within the current study the number of juveniles increased notably, relative to adults with increased sponge size. While this may be indicative of a species-specific social structure, it may also be due to high subsequent mortality rates. As such group-living in this instance may enhance survival rates.
Future directions and conclusions
In contrast to the mass bleaching events, habitat degradation and reef flattening that hard corals are exposed to (Hoegh-Guldberg et al. 2007; Veron et al. 2009; Hughes et al. 2018), sponges appear better suited to changing environmental conditions. Their siliceous skeletons are less susceptible to erosion due to ocean acidification (Pawlik 2011) and they are more tolerant to changes in sea surface temperature (Przeslawski et al. 2008; Bell and Smith 2004; Ramsby et al. 2018). They are clearly used by resident fishes /fish assemblages, but the manner of these relationships still needs to be further refined. As such, this further enforces the theory that sponges may have the capacity to act as an alternative for hard corals, providing shelter for fishes that prefer morphologically complex habitat structures, where other structures are lacking (Ryer et al. 2004; Cabaitan et al. 2016; Seemann et al. 2018). It was demonstrated in the current study that the more complex sponge species, Coelocarteria singaporensis and Cribrochalina sp., were important sources of structure for a number of coral reef fishes (e.g., Families: Pomacentridae. Labridae, Gobiidae). Such patterns may also be true for regions where the prospect of sponge dominated reef assemblages are becoming increasingly likely (Fox et al. 2009), and with increased depth where they are more dominant members of the benthic substratum. Here, the importance of sponges, particularly as a source of shelter, may well increase with depth (Wilkinson and Cheshire 1989; Hooper 2019; Pomponi et al. 2019) The impact of changing environmental conditions on coral reef fishes, however, will likely vary among species and ontogenetic life-stage (Munday et al. 2008). A shift from a coral-dominated to sponge-dominated ecosystem will substantially alter local productivity and nutrient fluxes and have important implications for larval fish recruitment (Coppock et al. 2022). Such changes are likely to be problematic for obligate corallivores and coral-dwelling fishes (Jones et al. 2004). An understanding of how sponges might respond to both localised and global environmental pressures, and their current interrelationships with fishes is needed if we are to truly understand how sponges might assist/ the role sponges might play for more generalist fishes in the future (Coppock et al. 2022). Baselines detailing local sponge species and their interrelationships with fishes are currently lacking for many biogeographic regions, need to be obtained.
It was demonstrated that 40 + species of fish associate with sponges as a source of shelter. The utilisation of sponges (by fishes) within coral reef ecosystems is evidently more common than previously considered (Chin et al. 2020; Stella et al. 2011) and requires further investigation. The relationships between fish and sponges are currently less predictable than similar relationships with hard coral species. Structurally complex and massive sponge morphologies may affect fish that require benthic space-holders in multiple ways, yet historically this relationship has been overlooked in many localities. We have shown that sponges of Kimbe Bay are used extensively as shelter by local fishes in a primarily facultative, but also an obligate manner. Here sponges may provide shelter in place of other three-dimensional structures. Furthermore, sponges appear to be a valuable source of nutrition for some of the fish species observed in this study. Whether these relationships are reciprocated elsewhere is currently unknown. As such, managers may need to consider how sponge habitats add to the mosaic of habitats on reefs and how they affect species diversity and other metrics of reef health.
Data availability
Data available upon request.
References
Abdo DA (2007) Endofauna differences between two temperate marine sponges (Demospongiae; Haplosclerida; Chalinidae) from Southwest Australia. Mar Biol 152:845–854
Allen GR (1991) Damselfishes of the world. Mergus Publishers, Melle, Germany, pp 9–12
Alvarez-Filip L, Dulvy NK, Gill JA, Côté IM, Watkinson AR (2009) Flattening of Caribbean coral reefs: region-wide declines in architectural complexity. Proc Royal Soc B 276:3019–3025. https://doi.org/10.1098/rspb.2009.0339
Batista D, da Silva Muricy GR, Rustum Andréa B, Campos Villaça R (2012) High intraspecific variation in the diet of the french angelfish Pomacanthus paru in the south-western Atlantic. Braz J Oceanogr 60:449–454
Bell JJ, Smith D (2004) Ecology of sponge assemblages (Porifera) in the Wakatobi region, south-east Sulawesi, Indonesia: richness and abundance. J Mar Biol Assoc UK 84:581–591. https://doi.org/10.1017/S0025315404009580h
Beukers JS, Jones GP (1998) Habitat complexity modifies the impact of piscivores on a coral reef fish population. Oecologia 114:50–59
Boaden AE, Kingsford MJ (2015) Predators drive community structure in coral reef fish assemblages. Ecosphere 6:46
Booth DJ (2002) Distribution changes after settlement in six species of damselfish (Pomacentridae) in One Tree Island Lagoon, Great Barrier Reef. Mar Ecol Prog Ser 226:157–164
Bridge TCL, Webster JM, Sih TL, Bongaerts P (2019) The Great Barrier Reef outer shelf. In: Hutchings P, Kingsford MJ, Hoegh-Guldberg O (eds) The great barrier reef: biology, environment and management. CSIRO Publishing and CRC Press, Australia, pp 229–246
Brodie J, Turak E (2004) Land use practices in the Stettin Bay catchment area and their relation to the status of the coral reefs in Kimbe Bay. Australian Centre for Tropical Freshwater Research Report No. 04/01, Townsville
Bruno JF, Bertness MD (2000) Habitat modification and facilitation in benthic marine communities. Mar Community Ecol 413:201–218
Busutil L, Garcia-Hernandez MR, Diaz MC, Pomponi SA (2018) Mesophotic sponges of the genus Callyspongia (Demospongiae, Haplosclerida) from Cuba, with the description of two new species. Zootaxa 4466(1):78–94
Butler MJ, Dolan TW, Hunt JH, Rose KA, Herrnkind WF (2017) Recruitment in degraded marine habitats: a spatially explicit, individual-based model for spiny lobster. Ecol Appl 15:902–918
Cabaitan PC, Gomez ED, Yap HT (2016) The spaghetti sponge Callyspongia samarensis (Wilson, 1925) provides temporary habitat for reef fish recruits. Mar Biodivers 46:541–542. https://doi.org/10.1007/s12526-015-0410-0
Carballo JL, Cruz-Barraza JA, Vega C, Nava H, del Chávez-Fuentes MC (2019) Sponge diversity in Eastern Tropical Pacific coral reefs: an interoceanic comparison. Sci Rep 9:9409. https://doi.org/10.1038/s41598-019-45834-4
Cheshire AC, Wilkinson CR, Seddon S, Westphalen G (1997) Bathymetric and seasonal changes in photosynthesis and respiration of the phototrophic sponge Phyllospongia lamellosa in comparison with respiration by the heterotrophic sponge Ianthella basta on Davies Reef, Great Barrier Reef. Mar Freshwat Res 48(7):589–599
Chin YY, Prince J, Kendrick G, Wahab MAA (2020) Sponges in shallow tropical and temperate reefs are important habitatsfor marine invertebrate biodiversity. Mar Biol 167(11):164
Chong-Seng KM, Cole AJ, Pratchett MS, Willis BL (2011) Selective feeding by coral reef fishes on coral lesions associated with brown band and black band disease. Coral Reefs 30:473–481. https://doi.org/10.1007/s00338-010-0707-1
Chong-Seng KM, Graham NAJ, Pratchett MS (2014) Bottlenecks to coral recovery in the Seychelles. Coral Reefs 33:449–461
Christie H, Norderhaug KM, Fredriksen S (2009) Macrophytes as habitat for fauna. Mar Ecol Prog Ser 396(December):221–233. https://doi.org/10.3354/meps
Coker DJ, Wilson SK, Pratchett MS (2014) Importance of live coral habitat for reef fishes. Rev Fish Biol Fisher 24:89–126. https://doi.org/10.1007/s11160-013-9319-5
Colin PL, Arneson C (1995) Tropical pacific invertebrates. Coral Reef Press, Beverly Hills, CA
Coppock AG, Gardiner NM, Jones GP (2013) Olfactory discrimination in juvenile coral reef fishes: response to conspecifics and coral. J Exp Mar Biol Ecol 443:21–26. https://doi.org/10.1016/j.jembe.2013.02.026
Coppock AG, Gardiner NM, Jones GP (2016) Sniffing out the competition? Juvenile coral reef damselfishes use chemical cues to distinguish the presence of conspecific and heterospecific aggregations. Behav Proc 125:43–50. https://doi.org/10.1016/j.beproc.2016.02.001
Coppock AG, Kingsford MJ, Battershill CN, Jones GP (2022) Significance of fish–sponge interactions in coral reef ecosystems. Coral Reefs. https://doi.org/10.1007/s00338-022-02253-8
Cortesi F, Feeny WE, Marshall NJ, Cheney KL (2015) Phenotypic plasticity confers multiple fitness benefits to a mimic. Curr Biol 25:949–954
Darling ES, Graham NAJ, Januchowski-Hartley FA, Nash KL, Pratchett MS, Wilson SK (2017) Relationships between structural complexity, coral traits, and reef fish assemblages. Coral Reefs 36(2):561–575. https://doi.org/10.1007/s00338-017-1539-z
De Brauwer M, Camp E, Jompa J, Smith DJ (2015) High levels of heterospecific cohabitation among anemonefishes in Hoga Island. Indones Mar Biodivers. https://doi.org/10.1007/s12526-015-0343-7
de Goeij JM, Lesser MP, Pawlik JR (2017) Nutrient fluxes and ecological functions of coral reef sponges in a changing ocean. In: Carballo JL, Bell JJ (eds) Climate change, ocean acidification and sponges. Springer, Cham, Switzerland, pp 373–410
Diaz CM, Rützler K (2001) Sponges: an essential component of Caribbean coral reefs. Bull Mar Sci 69:535–546
Dunlap M, Pawlik JR (1996) Video-monitored predation by caribbean reef fishes on an array of mangrove and reef sponges. Mar Biol 126:117–123
Emmett JS, Raj KD, Mathews G, Laju RL (2022) Opportunistic spongivore fishes in a reef of Gulf of Mannar. India Environ Biol Fishes 104:1251–1262. https://doi.org/10.1007/s10641-021-01150-3
Emslie MJ, Cheal AJ, MacNeil MA, Miller IR, Sweatman HPA (2018) Reef fish communities are spooked by SCUBA surveys and may take hours to recover. Peer J. https://doi.org/10.7717/peerj.4886]
Epstein HE, Kingsford MJ (2019) Are soft coral habitats unfavourable? A closer look at the association between reef fishes and their habitat. Environ Biol Fish 102:479–497. https://doi.org/10.1007/s10641-019-0845-4
Fabricius KE (1997) Soft coral abundanceon the central Great Barrier Reef: effects of Acanthaster planci, space availability and aspects of the physical environment. Coral Reefs 16:159–167
Fabricius K, De’ath G (2009) Biodiversity on the Great Barrier Reef: large-scale patterns and turbidity-related local loss of soft cora taxa. In: Wolinski E (ed) Oceanographic processes in coral reefs, chap 9. CRC Press, pp 127–144
Fox RI, Sunderland TI, Hoey AS, Bellwood DR (2009) Estimating ecosystem function: contrasting roles of closely related herbivorous rabbitfishes (Siganidae) on coral reefs. Mar Ecol Prog Ser 385:261–269. https://doi.org/10.3354/meps08059
Freese LJ, Wing BL (2003) Juvenile red rockfish, Sebastes sp., associations with sponges in the Gulf of Alaska. Mar Fish Rev 65(3):38–42
George AM, Abdo D, Wahab MAA, Ekins M, Hooper JNA, Whalan S (2023) Cryptic biodiversity inhabiting coral reef sponges. Mar Ecol 44(2):e12747. https://doi.org/10.1111/maec.12747
González-Rivero M, Ferrari R, Schönberg CHL, Mumby PJ (2012) Impacts of macroalgal competition and parrotfish predation on the growth of a common bioeroding sponge. Mar Ecol Prog Ser 444:133–142
González-Murcia S, Coppock AG, Ekins M, Battershill CN, Jones GP (2022) Effects of xxposure, depth and aspect on sponge communities on a coral reef. Mar Ecol Prog Ser. https://doi.org/10.3354/meps13981
Graham NAJ, Nash KL (2013) The importance of structural complexity in coral reef ecosystems. Coral Reefs 32:315–326. https://doi.org/10.1007/s00338-012-0984-y
Gratwicke B, Speight MR (2005) The relationship between fish species richness, abundance and habitat complexity in a range of shallow tropical marine habitats. J Fish Biol 66:650–667. https://doi.org/10.1111/j.0022-1112.2005.00629.x
Henkel TP, Pawlik JR (2005) Habitat use by sponge-dwelling brittlestars. Mar Biol 146:301–313
Heyward A, Fromont J, Schonberg CHL, Colquhoun J, Radford B, Gomez O (2010) The sponge gardens of Ningaloo Reef, Western Australia. Open Mar Biol J 4:3–11
Hoegh-Guldberg O, Mumby PJ, Hooten AJ, Steneck RS, Greenfield P, Gomez E, Harvell CD, Sale PF, Edwards AJ, Caldeira K, Knowlton N, Eakin CM, Iglesias-Prieto R, Muthiga N, Bradbury RH, Dubi A, Hatziolos ME (2007) Coral reefs under rapid climate change and ocean acidification. Science 318:1737–1742
Hoey AS, Bellwood DR (2009) Limited functional redundancy in a high diversity system: Single species dominates key ecological process on coral reefs. Ecosystems 12:1316–1328. https://doi.org/10.1007/s10021-009-9291-z
Hoey AS, Brandl SJ, Bellwood DR (2013) Diet and cross-shelf distribution of rabbitfishes (f. Siganidae) on the northern Great Barrier Reef: implications for ecosystem function. Coral Reefs 32:973–984
Holmes TH, McCormick MI (2010) Size-selectivity of predatory reef fish on juvenile prey. Mar Ecol Prog Ser 399:273–283
Hooper JNA (2019) Sponges. In: Hutchings P, Kingsford MJ, Hoegh-Guldberg O (eds) The Great Barrier Reef: biology, environment and management. CSIRO Publishing and CRC Press, Australia, pp 229–246
Huang JP, McClintock JB, Amsler CD, Huang YM (2008) Mesofauna associated with the marine sponge Amphimedon viridis. Do its physical or chemical attributes provide a prospective refuge from fish predation? J Exp Mar Biol Ecol 362:95–100
Hughes TP, Barnes ML, Bellwood DR, Cinner JE, Cumming GS, Jackson JBC, Kleypas J, van de Leemput IA, Lough J, Morrison TH, Palumbi SR, van Nes EH, Scheffer M (2017) Coral reefs in the anthropocene. Nature 546:82–90
Hughes TP, Kerry JT, Baird AH, Connolly SR, Dietzel A, Eakin CM, Heron SF, Hoey AS, Hoogenboom MO, Liu G, McWilliam MJ, Pears RJ, Pratchett MS, Skirving WJ, Stella JS, Torda G (2018) Global warming transforms coral reef assemblages. Nature 556:492–496
Hyndes GA, Francour P, Guidetti P, Heck KL, Jenkins G (2018) The roles of seagrasses in structuring associated fish assemblages and fisheries. In: Larkum A, Kendrick G, Ralph P (eds) Seagrasses of Australia. Springer, Cham. https://doi.org/10.1007/978-3-319-71354-0_18
Ivlev VS (1961) Experimental ecology of the feeding of fishes. Yale University Press, New Haven, CT
Jones GP, McCormick MI, Srinivasan M, Eagle JV (2004) Coral decline threatens fish biodiversity in marine reserves. Proc Natl Acad Sci B 101:8251–8253. https://doi.org/10.1073/pnas.0401277101
Karplus I (2014) The associations between fishes and sponges. Symbiosis in fishes: the biology of interspecific partnerships. Wiley, pp 371–430
Koukouras A, Russo A, Voultsiadou-Koukoura E, Dounas C, Chintiroglou C (1992) Relationship of sponge macrofauna with the morphology of their hosts in the North Aegean Sea. Internationale Revue der gesmten Hydrobiologie und Hydrographie 77:609–619
Koukouras A, Russo A, Voultsiadou-Koukoura E, Arvanitidis C, Stefanidou D (1996) Macrofauna associated with sponge species of different morphology. Mar Ecol 17(4):569–582
Kuiter RH, Tonozuka T (2001) Pictoral guide to Indonesian reef fishes. Part 3 Jawfishes – Sunfishes, Opistognathidae – Molidae. Zoonetics, Auustralia. p 623–893
Larson HK (1990) A revision of the commensal gobiid fish genera Pleurosicya and Luposicya (Gobiidae), with descriptions of eight new species of Pleurosicya and discussion of related genera. Beagle 7:1
Leiske E, Myers R (2002) Coral reef fishes: Indo-Pacific and Caribbean. Primceton University Press, Princeton, New Jersey, USA. ISBN: 0-691-08995-7
Lesneski KC, D’Aloia CC, Fortin MJ, Buston PM (2019) Disentangling the spatial distributions of a sponge-dwelling fish and its host sponge. Mar Biol 166:66. https://doi.org/10.1007/s00227-019-3517-1
Lesser MP, Slattery M (2013) Ecology of Caribbean sponges: are top-down or bottom-up processes more important? PLoS One 8:e79799. https://doi.org/10.1371/journal.pone.0079799
Lesser MP, Slattery M (2018) Sponge density increases with depth throughout the Caribbean. Ecosphere 9(12):e02525. https://doi.org/10.1002/ecs2.2525
Lesser MP, Mueller B, Pankey MS, Macartney KJ, Slattery M, de Goeij JM (2020) Depth-dependent detritus production in the sponge Halisarca caerulea. Liminol Oceanogr 65:1200–1216
Lopez E, Britayev TA, Martin D, San Martin G (2001) New symbiotic associations involving Syllidae (Annelida: Polychaeta), with taxonomic and biological remarks on Pionosyllis magnifica and Syllis cf. armillaris. J Mar Biol Assoc UK 81(3):399–409. https://doi.org/10.1017/S0025315401004015
MacArthur RH, MacArthur JW (1961) On bird species diversity. ESA J 42:594–598
MacDonald C, Bridge TCL, Jones GP (2016) Depth, bay position and habitat structure as determinants of coral reef fish distributions: are deep reefs a potential refuge? Mar Ecol Prog Ser 561:217–231
MacDonald C, Tauati MI, Jones GP (2018) Depth patterns in microhabitat versatility and selectivity in coral reef damselfishes. Mar Biol 165:138. https://doi.org/10.1007/s00227-018-3396-x
Majoris JE, D’Aloia CC, Francis RK, Buston PM (2018a) Differential persistence favors habitat preferences that determine the distribution of a reef fish. Behav Ecol 29:429–439
Majoris JE, Francisco FA, Atema J, Buston PM (2018b) Reproduction, early development, and larval rearing strategies for two sponge-dwelling neon gobies. Elacatinus Lori and e Colini Aquac 483:286–295. https://doi.org/10.1016/j.aquaculture.2017.10.024
Maldonado M, Aguilar R, Bannister RJ, Bell, JJ, Conway KW, Dayton PK, Diaz C, Gutt J, Kelly M, Kenchington ELR, Leys SP, Pomponi SA, Rapp HT, Rützler K, Tendal OS, Vacelet J, Young CM (2017) Sponge grounds as key marine habitats: a synthetic review of types, structure, functional roles, and conservation concerns In: Bratmanti L, Gori A, Rossi S, Orejas C (eds) Marine animal forests: the ecology of benthic biodiversity hotspots. Springer. https://doi.org/10.1007/978-3-319-21012-4
McMurray SE, Stubler AD, Erwin PM, Finelli CM, Pawlik JR (2018) A test of the sponge-loop hypothesis for emergent Caribbean reef sponges. Mar Ecol Prog Ser 588:1–14
Menge BA (1976) Organization of the New England rocky intertidal community: role of predation, competition, and environmental heterogeneity Ecol. Monogr 46:355–393. https://doi.org/10.2307/1942563
Menge BA, Lubchenco J (1981) Community organization in temperate and tropical rocky intertidal habitats: prey refuges in relation to consumer pressure gradients. Ecol Monogr 51:429–450. https://doi.org/10.2307/2937323
Mortimer C, Dunn M, Haris A, Jompa J, Bell J (2021) Estimates of sponge consumption rates on an Indo-Pacific coral reef. Mar Ecol Prog Ser 672:123–140. https://doi.org/10.3354/meps13786
Moynihan JL, Hall AE, Kingsford MJ (2022) Interrelationships between soft corals and reef associated fishes on inshore reefs of the Great Barrier Reef. Mar Ecol Prog Ser 698:15–28. https://doi.org/10.3354/meps14160
Munday PL, Eyre PJ, Jones GP (2003) Ecological mechanisms for coexistance of colour polymorphism in coral-reef fish: an experimental evaluation. Oecologia 137(4):519–526
Munday PL, Jones GP, Pratchett MS, Williams AJ (2008) Climate change and the future for coral reef fishes. Fish Fish 9:261–285. https://doi.org/10.1111/j.1467-2979.2008.00281.x
Nagelkerken I, Blaber SJM, Bouillon S, Green P, Haywood M, Kirton LG, Meynecke JO (2008) The habitat function of mangroves for terrestrial and marine fauna: a review. Aquat Bot 89:155–185. https://doi.org/10.1016/j.aquabot.2007.12.007
Noonan SHC, Jones GP, Pratchett MS (2012) Coral size, health and structural complexity: effects on the ecology of a coral reef damselfish. Mar Ecol Prog Ser 456:127–137
Norbury G, Overmeire W (2019) Low structural complexity of non native grassland habitat exposes prey to higher predation. Ecol Appl. https://doi.org/10.1002/eap.1830
Painka ER (1973) The structure of lizard communities. Annu Rev Ecol Syst 4(1):53–74
Pawlik JR (2011) The chemical ecology of sponges on Caribbean reefs: natural products shape natural systems. BioScience 61:888–898
Pawlik JR, Loh TL, McMurray SE (2018) A review of bottom-up vs. top-down control of sponges on Caribbean fore-reefs: what’s old, what’s new, and future directions. PeerJ 2018:1–28
Pianka ER (1966) Latitudinal gradients in species diversity: a review of concepts. Am Nat 100(910):33–46
Pomponi SA, Diaz MC, Van Soest RWM, Bell LJ, Busutil L, Gochfeld DJ, Kelly M, Slattery M (2019) Sponges. In: Loya Y, Puglise KA, Bridge TCL (eds) Mesophotic coral ecosystems, coral reefs of the world 12. Springer, New York, pp 563–588
Powell A, Smith DJ, Hepburn LJ, Jones T, Berman J, Jompa J, Bell JJ (2014) Reduced diversity and high sponge abundance on a sedimented Indo-Pacific reef system: implications for future changes in environmental quality. PLoS One. https://doi.org/10.1371/journal.pone.0085253]
Powell A, Jones T, Smith DJ, Jompa J, Bell JJ (2015) Spongivory in the Wakatobi Marine National Park, southeast Sulawesi. Indonesia Pac Sci 69:487–508
Powell AM, Clarke E, Fruh E, Chaytor JD, Reiswig HM, Whitmire CE (2018) Characterizing the sponge grounds of Grays Canyon, Washington, USA. Deep Sea Res Part II 150(April):146–155. https://doi.org/10.1016/j.dsr2.2018.01.004
Pratchett M, Munday P, Wilson S, Graham N, Cinner J, Bellwood D, Jones G, Polunin N, Mcclanahan T (2008) Effects of climate-induced coral bleaching on coral-reef fishes, Ecological and economic consequences. In: Gibson R, Atkinson R, Gordon J (eds) Oceanography and Marine Biology. CRC Press, pp 251–296
Przeslawski R, Ahyong S, Byrne M, Wörheide G, Hutchings P (2008) Beyond corals and fish: the effects of climate change on noncoral benthic invertebrates of tropical reefs. Glob Change Biol 14(12):2773–2795. https://doi.org/10.1111/j.1365-2486.2008.01693.x
Ramsby BD, Hoogenboom MO, Smith HA, Whalan S, Webster NS (2018) The bioeroding sponge Cliona orientalis will not tolerate future projected ocean warming. Sci Rep 8:1–14
Rios R, Duffy JE (2007) A review of the sponge-dwelling snapping shrimp from Carrie Bow Cay, Belize, with description of Zuzapheus, new genus, and six new species (Crustacea: Decapoda: Alpheidae). Zootaxa 1602(1):1–89. https://doi.org/10.11646/zootaxa.1602.1.1
Roberts CM, McClean CJ, Veron JEN, Hawkins JP, Allen GR, McAllister DE, Mittermeier CG, Schueler FW, Spalding M, Wells F, Vynne C, Werner TB (2002) Marine biodiversity hotspots and conservation priorities for tropical reefs. Science 295(5558):1280–1284. https://doi.org/10.1126/science.1067728
Rohde S, Schupp PJ (2012) Growth and regeneration of the elephant ear sponge Ianthella basta (Porifera). Hydrobiologia 687:219–226. https://doi.org/10.1007/s10750-011-0774-5
Rovellini A, Dunn MR, Fulton EA, Webster NS, Smith DJ, Jompa J, Haris A, Berman J, Bell JJ (2019) Decadal variability in sponge abundance and biodiversity on an Indo-Pacific coral reef. Mar Ecol Prog Ser 620:63–76
Ryer CH, Stoner AW, Titgen RH (2004) Behavioral mechanisms underlying the refuge value of benthic habitat structure for two flatfishes with differing anti-predator strategies. Mar Ecol Prog Ser 268:231–243
Safriel UN, Ben-Eliahu MN (1991) The influence of habitat structure and environmental stability on the species diversity of polychaetes in vermetid reefs. In: Bell SS, McCoy ED, Mushinsky HR (eds) Habitat structure population and community biology series, vol 8. Springer, Dordrecht
Scharf FS, Manderson JP, Fabrizio MC (2006) The effects of seafloor habitat complexity on survival of juvenile fishes: species-specific interactions with structural refuge. J Exp Mar Biol Ecol 335(2):167–176
Schönberg CHL, Lim SC (2019) Psammobiosis and bioerosion: examining ecological strategies in sponges using the case example Coelocarteria singaporensis. Facies 65:1–11
Schönberg CHL, Hosie AM, Fromont J, Marsh L, O’Hara T (2016) Apartment-style living on a kebab sponge. Mar Biodivers 46:331–332
Seemann J, Yingst A, Stuart-Smith RD, Edgar GJ, Altieri AH (2018) The importance of sponges and mangroves in supporting fish communities on degraded coral reefs in Caribbean Panama. PeerJ. https://doi.org/10.7717/peerj.4455]
Spalding HL, Amando-Filho GM, Bahia RG, Ballatine DL, Fredericq S, Leichter JJ, Nelson WA, Slattery M, Tsuda RT (2019) Macroalgae. In: Loya Y, Puglise KA, Bridge TCL (eds) Mesophotic coral ecosystems. Coral reefs of the world, vol 12. Springer, New York, pp 507–536
Stella JS, Pratchett MS, Hutchings PA, Jones GP (2011) Coral-associated invertebrates: diversity, ecological importance and vulnerability to disturbance. Oceanogr Mar Biol 49:43–104
Stoner AW, Titgen RH (2003) Biological structures and bottom type influence habitat choices made by Alaska flatfishes. J Exp Mar Biol Ecol 292:43–59
Swierts T, Peijnenburg KTCA, de Leeuw C, Cleary DFR, Hornlein C, Seiawan E, Worheide G, Erpenbeck D, de Voogd NJ (2013) Lock, stock and two different barrels: comparing the genetic composition of morphotypes of the Indo-Pacific sponge Xestospongia testudinaria. PLoS One 8(9):e74396
Tyler JC, Böhlke JE (1972) Records of sponge-dwelling fishes, primarily of the Caribbean. Bull Mar Sci 22:601–642
Van Soest RWM, Boury-Esnault N, Vacelet J, Dohrmann M, Erpenbeck D, De Voogd NJ, Santodomingo N, Vanhoorne B, Kelly M, Hooper JNA (2012) Global diversity of sponges (Porifera). PLoS One 7(4):e35105. https://doi.org/10.1371/journal.pone.0035105
Veron JEN (2000) Corals of the world. Australian Institute of Marine Science and CRR Ald Pty Ltd, Townsville, Australia. ISBN: 978-0642322364
Veron JEN, Hoegh-Guldberg O, Lenton TM, Lough JM, Obura DO, Pearce-Kelly P, Sheppard CRC, Spalding M, Stafford-Smith MG, Rogers AD (2009) The coral reef crisis: The critical importance of <350 ppm CO2. Mar Poll Bull 58:1428–1436
Wahab MAA, Fromont J, Gomez O, Fisher R, Jones R (2017) Comparisons of benthic filter feeder communities before and after large-scale capital dredging program. Mar Pol Bull 122(1–2):176–193. https://doi.org/10.1016/j.marpolbul.2017.06.041
Wilkinson CR, Cheshire AC (1989) Patterns in the distribution of sponge populations across the central Great Barrier Reef. Coral Reefs 8:127–134
Wulff JL (1994) Sponge feeding by Caribbean angelfishes, trunkfishes and filefishes. In: van Soest RWM, Van Kempen TMG, Braekman JC (eds) Sponges in time and space. Balkema, Rotterdam, pp 265–271
Wulff JL (2005) Trade-offs in resistance to competitors and predators, and their effects on the diversity of tropical marine sponges. J Anim Ecol 74:313–321
Wulff JL (2006) Ecological interactions of marine sponges. Can J Zool 84:146–166. https://doi.org/10.1139/z06-019
Wulff JL (2012) Ecological interactions and the distribution, abundance, and diversity of sponges. Adv Mar Biol 61:273–344. https://doi.org/10.1016/B978-0-12-387787-1.00003-9
Acknowledgements
Thanks go to the staff at Mahonia Na Dari Research and Conservation Centre and the staff at Walindi Plantation Resort for logistical support. We greatly appreciate and acknowledge the traditional owners of the Tamare-Kilu reefs in Kimbe Bay for allowing both access to and use of their local reef resources in order to carry out this investigation. Thanks also go to Peter Doll and Kelsey Webber for their assistance with data collection in the field. Furthermore, we are grateful to three anonymous reviewers, whose constructive comments improved this manuscript.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. This work was supported by core funding to Geoffrey P Jones from the ARC Centre of Excellence for Coral Reef Studies (CE140100020).
Author information
Authors and Affiliations
Contributions
Amy G Coppock and Geoffrey P Jones conceived the ideas and designed methodology; Amy G Coppock collected the data; Amy G Coppock analysed the data; Amy G Coppock, Michael J Kingsford and Geoffrey P Jones led the writing of the manuscript. All authors contributed critically to the drafts and gave final approval for publication.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Ethical approval
All applicable international, national and institutional guidelines for the care and use of animals were followed. This work was conducted in compliance with the James Cook University Ethics Review Committee regulations (ethics approval number A2580).
Additional information
Responsible Editor: K. Clements.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Coppock, A.G., Kingsford, M.J. & Jones, G.P. Importance of complex sponges as habitat and feeding substrata for coral reef fishes. Mar Biol 171, 154 (2024). https://doi.org/10.1007/s00227-024-04467-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-024-04467-6