Abstract
Tropical western Atlantic reefs have gradually shifted from being dominated by corals to being mainly covered by macroalgae. The mass-mortality of the sea urchin Diadema antillarum in the 80s and the slow to non-existent recovery exacerbated this shift. Chemical cues associated with these reefs are expected to have shifted too with potential negative effects on larval recruitment, possibly limiting recovery of important species like D. antillarum. In this study, we tested the effects of naturally derived biofilm and macroalgae species native to Caribbean coral reefs on the settlement rate of cultured D. antillarum larvae in two separate experiments. Crustose coralline algae (CCA) were included in both experiments, making it possible to compare settlement rates from both experiments. A biofilm of one week old yielded significantly lower settlement rates compared to two, four, and six weeks old biofilm and the highest settlement rate was found for CCA with over 62% of total larvae. All six tested macroalgae species resulted in settled larvae, with little significant difference between algal species, partly due to a high variation in settlement rates within treatments. Sargassum fluitans induced the highest settlement rate with 33%, which was not significantly different from CCA with 29%. We conclude that dominant macroalgae species likely to be encountered by D. antillarum on shifted reefs are no major constraint to settlement. Our findings increase the understanding of alternative stable state settlement dynamics for a keystone coral reef herbivore.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The once abundant sea urchin Diadema antillarum, hereafter Diadema, suffered from a mass-mortality event in 1983 and 1984, when 98% of the populations in the tropical western Atlantic died due to an unknown cause (Lessios 1988, 2016; Levitan et al. 2023). The loss of this key herbivore decreased grazing pressure, which, along with the historical overharvesting of herbivorous fishes and human-induced eutrophication of coastal waters, resulted in an increase of fleshy algae on reef communities (Bellwood et al. 2004; Hay 1984; Jackson and Kaufmann 1987). In the following decades, Diadema recovery has been limited and fragmented (Lessios 2016; Levitan et al. 2023). Reefs previously dominated by corals, have now shifted to be mainly dominated by macroalgae (Adam et al. 2015; Hughes et al. 1987, 2003). On reefs where Diadema is recovering, macroalgal cover is significantly reduced and crustose coralline algae (CCA) and open space increase, resulting in higher coral recruitment (Aronson and Precht 2000; Idjadi et al. 2010). Further recovery of this key species could increase the resilience of the tropical western Atlantic coral reefs (Precht and Precht 2015). Unfortunately, in 2022 a new die-off decimated Diadema populations in the Eastern Caribbean and the Greater Antilles (Hylkema et al. 2023).
Previous research identified a limited larval supply, a lack of larval settlement and low post-settlement survival as key factors constraining natural recovery (Feehan et al. 2016; Hylkema et al. 2022a; Miller et al. 2009; Vermeij et al. 2010). After the mass-mortality, larval supply was reduced due to lower numbers of individuals, resulting in a lower number of gametes and, therefore, less fertilized eggs (Feehan et al. 2016; Lessios 2005). Although this limited larval influx might still be limiting recovery in some regions, such as Florida and Mexico (Miller et al. 2009), larval supply has recovered to pre die-off densities on Curacao, Puerto Rico, Saba and St. Eustatius (Hylkema et al. 2022a; Vermeij et al. 2010; Williams et al. 2010). However, on most of these reefs, no significant recovery of Diadema density has been observed (Vermeij et al. 2010; Williams et al. 2010). This suggests that at locations that still have a significant larval supply, Diadema recovery is constrained by limited larval settlement success and/or high post-settlement mortality. Settlement can be hindered by low availability of suitable settlement substratum (Hylkema et al. 2022a) or missing the right settlement cues (Pilnick et al. 2023). Low post-settlement survival (Bechtel et al. 2006; Hylkema et al. 2022b; Williams et al. 2011) is the effect of a combination of decreased habitat complexity, low food availability, decreased shelter availability (Lee 2006; Rogers and Lorenzen 2016; Tuohy et al. 2020) and increased predation (Nedimyer and Moe 2006; The Nature Conservancy 2004).
Based on larval culture data, the pelagic larval stage of Diadema takes approximately 35 to 90 days for full growth and development (Eckert 1998; Pilnick et al. 2022, 2023; Wijers et al. 2023). This is followed by settlement, the phase in which the larvae attach to a hard substrate and metamorphose into a juvenile (Dworjanyn and Pirozzi 2008). Larval settlement is often induced by benthic communities that release chemical cues (McEdward and Miner 2001). The type of cues that are present are crucial (Doll et al. 2022; Mos et al. 2011; Swanson et al. 2004), therefore, changes in benthic communities can have major impacts on the settlement behavior of echinoid larvae (Doll et al. 2022). With the degradation of Caribbean coral reefs (Hughes et al. 2003; Jackson et al. 2014), coral and CCA cover decreased, while macroalgae cover increased (Mumby et al. 2006), changing the cue composition associated with these reefs (Remple et al. 2021). Biofilm, CCA and macroalgae are known cues for the settlement of sea urchin larvae (Mos et al. 2011; Swanson et al. 2004, 2006; Taniguchi et al. 1994).
Biofilm is the initial biological colonizer of new or cleaned surfaces, grows on almost all marine surfaces and is composed of a diverse mix of microorganisms, depending on the location and age of the biofilm (Hadfield 2011). It is known to induce larval settlement in a wide range of invertebrate species (Wieczorek and Todd 1998), including Diadema (Pilnick et al. 2021, 2023). Grazing Diadema create open bare spaces (Maciá et al. 2007), providing the opportunity for new biofilms to grow. As a consequence of the reduced grazing pressure, more mature biofilms can develop, ultimately leading to the subsequent overgrowth of reef substrates by fleshy macroalgae. Cultured Diadema larvae have been shown to settle on biofilm ranging from a few days—up to four weeks old (Eckert 1998; Pilnick et al. 2021, 2023; Wijers et al. 2023). In field trials Bak (1985) observed that Diadema settlement rates were higher on plates, cleaned every four to six months, compared to overgrown plates, suggesting that there is an optimum biofilm age. In more recent studies, settlement collectors were usually deployed for two weeks up to one month (Hernández et al. 2006; Hylkema et al. 2022a; Williams et al. 2010, 2011), both yielding a substantial amount of settlers. In contrast, collectors that were deployed for three months had a significantly lower settlement rate compared to collectors incubated for one month (Hylkema et al. 2022b). This suggests that the optimal age of biofilm for Diadema settlement is around three to four weeks, but this has not yet been experimentally evaluated.
Macroalgae and CCA induce settlement in a wide range of species, including sea urchins with highly variable settlement rates (Dworjanyn and Pirozzi 2008; Mos et al. 2011; Pearce and Scheibling 1991; Rowley 1989). CCA is a mixed group of calcifying algae associated with well-grazed reefs. They are the cement of reef structures (Kuffner et al. 2008) and provide settlement cues for corals and sea urchin larvae (Heyward and Negri 1999; Pearce and Scheibling 1990; Ritson-Williams et al. 2016). Macroalgae are now dominant on many Caribbean reefs and might influence larval settlement of Diadema. The Australian sea urchin Holopneustes purpurascens mainly settled on foliose red algae or on coralline turf algae in the wild and these also induced the highest rate of settlement in laboratory experiments (Swanson et al. 2006). For the green sea urchin Strongylocentrotus droebachiensis (widespread in the Northern hemisphere), red coralline algae resulted in high settlement rates, and relatively low rates when exposed to brown macroalgae (Pearce and Scheibling 1991). The more widespread and tropical species Tripneustes gratilla settled when exposed to a wide range of macroalgae and calcifying algae with variable results (Dworjanyn and Pirozzi 2008; Mos et al. 2011). Mos et al. (2011) found high settlement rates when exposed to Sargassum linearifolium and the calcifying red algae Corallina officinalis. Calcified algae are consistently among the most effective macroalgae for inducing settlement of a diverse range of sea urchin species. For non-calcified macroalgae, the effects are less clear and depend on the species tested.
Historically, the lack of consistent larval culture techniques limited the number of competent Diadema larvae available for research (Bielmyer et al. 2005; Eckert 1998; Leber et al. 2009; Nedimyer and Moe 2006). Recent advancements in larval culture methods now make it possible to produce 1000s of competent larvae that can be used to test the effect of cues on larval settlement (Pilnick et al. 2021, 2023; Wijers et al. 2023). Competent larvae are defined as larvae that reached a developmental point where they are capable of undergoing metamorphosis (Gosselin and Jangoux 1998). For Diadema, Pilnick et al. (2023) recently tested a range of settlement cues including biofilm, CCA and the calcifying macroalgae Halimeda sp. Halimeda sp. induced significantly more settlement compared to biofilm and CCA at intermediate rates. A comparison between the developmental time of biofilm, and different species of macroalgae on the settlement rate of Diadema, has not been tested yet.
In this study the settlement rate of cultured competent Diadema larvae was determined following exposure to biofilms with a range of ages, CCA and six species of fleshy macroalgae common on western Atlantic coral reefs. Fleshy macroalgae are indicative of the current prevailing benthic conditions on most reefs (Adam et al. 2015; Idjadi et al. 2010), whereas CCA is associated with well-grazed reef ecosystems (Belliveau and Paul 2002; Idjadi et al. 2010). Biofilm that is in an earlier stage of development, one to two weeks old (Shikuma and Hadfield 2005), may be indicative of a well-grazed reef with more bare surface (Idjadi et al. 2010; Maciá et al. 2007) for biofilm to colonize, whereas more mature biofilm can be associated with reefs characterized by limited herbivore activity. We hypothesize high (> 50%) larval settlement rates when exposed to CCA. As calcifying red algae yielded high settlement rates for a broad range of sea urchin species, we also expect high settlement rates for calcifying macroalgae species, and average (25–50%) settlement for the other macroalgae species tested (Dworjanyn and Pirozzi 2008; Mos et al. 2011; Swanson et al. 2006). For the biofilm, settlement is expected to peak on biofilm that has developed between three and four weeks (Bak 1985; Hylkema et al. 2022b; Miller et al. 2009).
This study unveils the settlement behavior of Diadema in response to a range of natural cues, related to the current and former composition of tropical western Atlantic reefs, to understand whether this could constrain natural Diadema settlement.
Material and methods
Gamete collection and larval rearing
Wild adult Diadema were collected from the Fort Bay Harbor on Saba, Caribbean Netherlands (N17.61635, W063.25140). Immediately after collection, a batch of 10 urchins was induced to spawn by immersing them in water at 31 °C (5 °C higher than the ambient seawater temperature) for a maximum of 30 min, following Pilnick et al. (2021). Gametes were collected using a 10-ml pipette, moved to a 1-L glass bottle, and checked under a microscope at 10 × 40 magnification for fertilization. The fertilized eggs (> 95% of total) were kept in 500-mL artificial seawater (ASW) (made with Tropic Marin® REEF-MIX sea salt and Water One® bottled drinking water, 35-g/L) on an Innova’40 shaker table for three days at a density of ~ 100 per ml. Three days post fertilization (DPF), larvae were diluted to a density of 1 larvae per ml and cultured in 500-mL ASW in 1-L bottles at 25.9 ± 0.1 °C, as described by Wijers et al. (2023). Larval competency was determined twice a week by scoring the presence of a mature rudiment in the larvae. When 600–900 competent larvae were available, the experiment was initiated.
Competent larval selection and measurements
Larvae were considered competent if they had a clearly developed external and/or internal rudiment, visible as white, instead of transparent tissue, with tube feet (Pilnick et al. 2023). Before the experiment started, competent larvae were separated from non-competent larvae and counted.
The larval culture facility on Saba, Caribbean Netherlands, had a limit of producing ~ 800 of competent Diadema larvae during each culture run. Therefore, the cues were tested in two separate settlement experiments, in the first half of 2022. Larvae used for the Biofilm experiment were 44 days old and 67 days old for the Macroalgae experiment, as even with similar culture methods, development time until competency can differ.
To compare body size and arm length between the competent larvae of the two different culture runs, pictures of 10 randomly selected larvae were taken with an Olympus Stereomicroscope SZ61. CellSense Image software was used to measure larval body length and width, from which body size was calculated following Wijers et al. (2023). Arm length was the length of the longest arm, as some larvae (partially) missed an arm or had slight differences in arm length.
Settlement setup
The setup of the settlement experiments was performed as described by Mos et al. (2011) with some adaptations. For each treatment, 10 replicate plastic petri dishes (Ø 5 cm), each containing 10 randomly selected competent larvae, were used to test settlement cues. Each petri dish contained 15-mL of autoclaved ASW, of which 50% was replaced daily, plus a potential settlement cue or autoclaved seawater as negative control. The total amount of settled (on petri dish and/or substrate), loose (not attached and healthy) and degraded (pale and brownish in color, often partly dissolving) larvae was determined after four days (Fig. 1). If in total less than 10 larvae were found per petri dish, those were scored as missing (presumed dead). Larvae were regarded as settled when they were attached to the substrate and had visible spine development. Settlers were left in the petri dish until the end of the experiment in the Biofilm experiment. However, after we sporadically observed dead settled urchins in the Biofilm experiment, we decided to daily remove settlers in the Macroalgae experiment. Settlement experiments were performed at 25.9 ± 0.1 °C (n = 20), with 12:12 L:D conditions using artificial lighting.
Treatment preparation
To form a natural biofilm and CCA layer, petri dishes were attached with a fishing line to a PVC structure following a coral nursery tree design. The tree was placed next to the dive site called “Diadema City”, Saba (N17.61468 W063.24888), at a depth of 9 m.
For the biofilm to ensure having dishes with varying biofilm ages, dishes were attached to the PVC structure weekly, starting two weeks prior to larval rearing.
For the CCA treatments, petri dishes were attached to the tree for four months and gently cleaned with a toothbrush to remove biofilm and filamentous algae every one to two weeks. Within 4 weeks, the first CCA started to show and two months later (at the start of the experiment), CCA covered most of the petri dish surface. Coralline algae species growing on these dishes were not identified, but looked very similar (Figs. S1b, S2b) and will generally be referred to as the CCA treatment. Before the CCA petri dishes were used for the settlement experiments, they were gently cleaned again to remove the biofilm and filamentous algae.
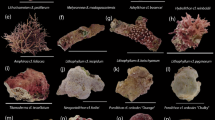
One day prior to the start of the macroalgae experiment (2nd of June 2022), six different species were collected and prepared. Dictyota pinnatifida, Halimeda incrassata and Lobophora variegata were collected at a depth of 11–13 m at the dive site “Ladder Bay” (N17.63636 W063.25633). Laurencia sp. and Padina pavonica were collected at the dive site “Big Rock Market” (N17.61279 W063.23801) at a depth of 12–14 m. Sargassum fluitans was collected floating at the surface at the dive site “Diadema City” (N17.61468 W063.24888). Algae were identified using standard keys as described by Littler and Littler (2000). All species were collected in separate containers and gently rinsed with ASW before cutting and adding ~ 3 cm2 of surface area to a clean petri dish (10 per exposure type).
Biofilm experiment
To test if a biofilm of different ages influenced settlement rates, we tested biofilms of one, two, four and six week (W) old. A total of 600 competent larvae, selected the 26th of January 2022, were used for the settlement experiment. The six treatments tested were Control, CCA, 1W, 2W, 4W and 6W (Fig. S1).
Macroalgae experiment
To test if macroalgae species influenced settlement rates, six different macroalgae species common on tropical western Atlantic reefs were tested. A total of 800 competent larvae, selected the 3rd of June 2022, were used for the settlement experiment. The eight treatments tested were Control, CCA, Lobophora, Laurencia, Dictyota, Padina, Halimeda and Sargassum (Fig. S2).
Data analyses
Statistical analyses were performed with R (R Core Team 2022), using R studio version 4.2.2. A Generalized Linear Model (GLM) was used to test if treatment had a significant effect on settlement for both the Biofilm and Macroalgae experiment. As settlement data were proportional (between 0 and 1, where 0 is no and 1 is 100% settlement), a binomial distribution was used (Zuur et al. 2009). When no settlement was observed, the number was set to 0.01 (1%) for the model to cope with the 0s in the data. To identify which treatments differed significantly (p < 0.05) from each other, Tukey’s post hoc tests were performed and adjusted for multiple comparisons. To test if body size and arm length of the competent larvae were different between both experiments, a Two-Sample t-Test was used. Statistical results (treatment effect per experiment, F values, z-ratios and p-values) for the settlement of the Biofilm and Macroalgae experiments are provided in the supplementary data (Tables S1–S4).
Results
Biofilm experiment
Settlement occurred in all treatments, except for the negative control (Fig. 2). Treatment had a significant effect on settlement rates (main test effect). Most settlement was found in the CCA treatment (positive control), in which 62 ± 12% (mean ± SD) of the larvae settled. Both the negative control (0 ± 0% settlement) and 1W (13 ± 15% settlement) treatment had significantly lower settlement rates compared to all other treatments. The 6W biofilm treatment (39 ± 15% settlement) had a lower settlement rate compared to both 2W and 4W, but this was not significant. In all treatments, except for 4W and 6W, most larvae that did not settle were loose and still healthy (Table 1). For treatments 4W and 6W, a large proportion of the larvae was either degraded (19–35%) or missing (12–17%) after 4 days.
Settlement of Diadema antillarum larvae exposed to a biofilm of one, two, four and six W (week(s)) old, CCA and a control with no cues, expressed as mean percentage of total ± SE (each treatment had n = 10 petri dish replicates, each containing 10 competent larvae). Treatments sharing the same letter do not differ significantly (P > 0.05)
Macroalgae experiment
All treatments, including the negative control, resulted in settlers (Fig. 3). Treatment had a significant effect on settlement rates (main test effect). Highest settlement was observed in the Sargassum treatment with 33 ± 16% (mean ± SD), followed by CCA with 29 ± 25%. Sargassum and CCA differed significantly from the control and Lobophora. Halimeda had more settlers compared to the control and Lobophora, but did not differ significantly from these treatments. Other treatments did not differ significantly from each other.
Settlement of Diadema antillarum larvae exposed to the macroalgae species Lobophora variegate (Lob), Laurencia sp. (Lau), Dictyota pinnatifida (Dic), Padina pavonica (Pad), Halimeda incrassate (Hal), and Sargassum fluitans (Sar) and CCA and a control with no cues, expressed as mean percentage of total ± SE (each treatment had n = 10 petri dish replicates, each containing 10 competent larvae). Treatments sharing the same letter do not differ significantly (P > 0.05)
At the end of the experiment, 90% of the control larvae still had not settled and remained loose (Table 1). For the macroalgae this was between 45 and 75% of all larvae. On average Lobophora and Laurencia had low settlement (6–14%) and high rates of degraded (28–32%) and missing (9–16%) larvae. In the CCA treatment, 12% of the larvae were missing, but this treatment had almost no degraded larvae (2%).
Larval production and morphology
The body size of the competent larvae used for the Macroalgae experiment was significantly higher than those used for the Biofilm experiment, and vice versa for arm length as shown in Table 2. Overall, the variation in body features was higher for the larvae used in the Macroalgae experiment. Some larvae from this culture run were observed with large body sizes, but the absence of discernible arms, and also larvae possessing notably elongated arms but having smaller bodies were observed.
Discussion
Our results show that competent Diadema larvae settled when exposed to a wide range of naturally occurring settlement cues. Overall settlement in the Macroalgae experiment was lower compared to the Biofilm experiment, possibly due to larvae with a lower fitness. Compared to other treatments, CCA induced high rates of settlement in both experiments.
We hypothesized that Diadema settlement would be highest on CCA and relatively young biofilm (one to two weeks old), and low on older biofilm (four to six weeks) and medium on macroalgae. As expected, CCA induced high rates of settlement. Biofilms of two and four weeks old had significantly higher settlement than biofilm of one week old. Biofilm of 6 weeks old resulted in a lower settlement rate, although this difference was only significant compared to 1 week old biofilm. Nevertheless, these results indicate that Diadema larvae settle in response to cues that are typically found in a habitat dominated by adults, as grazed surfaces result in bare substrate and CCA. This is a possible explanation for why juveniles are often found in close vicinity of adults (Hunte and Younglao 1988; Miller et al. 2007). These results are in line with Bak (1985), who observed more settlement on cleaned settlement plates. Rahim et al. (2004) found gradually increased settlement for the sea urchin Pseudocentrotus depressus, with increased biofilm age up to four weeks. For Anthocidaris crassispina, a bell-shaped curve was observed with 4 week old biofilm being most effective. Keough and Raimondi (1996) also found increased settlement for a range of marine invertebrates on a biofilm of four weeks old compared to no biofilm. Our results imply that with increasing age of biofilm of up to four weeks old, there is increased larval settlement, and no further increase in settlement with six week old biofilm.
Contrary to our hypothesis, settlement rates on most macroalgae, with the exception of Lobophora, did not differ significantly from settlement on CCA. These data suggest that changes in cue composition due to increased macroalgae cover are unlikely to result in lower natural settlement rates of Diadema. However, besides macroalgae, algal turfs are a dominant benthic component on many Caribbean reefs (Solandt and Campbell 2001; Williams 2021). Turf algae are multi-species assemblages of filamentous algae and are known to inhibit coral recruitment (Arnold et al. 2010; Harrington et al. 2010; Wells et al. 2021). This was not tested here for Diadema but is of interest for future research.
One limitation of our findings is the settlement observed in the negative control of the Macroalgae experiment. This suggests that certain larvae from this culture run had the capacity to settle without the need for specific cues. Diadema urchins have been observed to settle during larval culture (Wijers et al. 2023), suggesting that there are already some cues present. This could have been attributed to the settlement in the negative control. Notably, these larvae were older than those employed in the Biofilm experiment. Results of coral larvae settlement show that preference for specific cues decreases with age (Fulmore 2019). Nevertheless, the older larvae did not result in overall higher settlement rates. When examining our CCA settlement rates in the Biofilm experiment, they appeared to align with those observed by Pilnick et al. (2023). Nonetheless, it is important to note that in the Macroalgae experiment, both CCA and Halimeda exhibited settlement rates that were approximately half of those reported by Pilnick et al. (2023), suggesting that the overall fitness of the larvae was lower. It is unknown to what extent this depends on the egg or sperm quality of the parental sea urchins. Furthermore, despite identifying some morphological differences among larval culture runs, there was no corroborating evidence supporting the availability of greater energy reserves based on body size measurements. The large variance in settlement rates found here has also been reported for other sea urchin species (Mos et al. 2011; Wieczorek and Todd 1998). Diadema culture is still in its infancy (Pilnick et al. 2022; Wijers et al. 2023) and with increased experience variation in results may decrease. If settlement rates in the Macroalgae experiment were lower due to less fit larvae, it is possible to obtain Diadema larval settlement rates of > 50% in response to a range of naturally occurring macroalgae.
Larval influx, settlement rates, post-settlement survival and post-recruit survival have all been identified as factors possibly constraining natural recovery. The fact that a wide range of natural settlement cues resulted in metamorphosis, indicates that the transition from pelagic larvae to benthic urchins is not a major factor limiting Diadema recovery. Previous studies showed high settlement rates on multiple Caribbean islands (Hylkema et al. 2022a; Vermeij et al. 2010; Williams et al. 2009). At these locations (Hylkema et al. 2022b), post-settlement survival seems to be the limiting factor, especially when micro-predators can hide in the algae cover. For sea urchins in general, post-settlement processes have a larger influence on population dynamics compared to larval settlement preferences (Balch and Scheibling 2001). For the purple sea urchin Strongylocentrotus spp. Rowley (1989) also suggests post-settlement survival to be the driver for the amount of adults instead of settlement substrate. Our results indicate that this is also the case for Diadema, as previously shown by Vermeij et al. (2010), who found high settlement rates but no recovery of the local Diadema population. Low post-settlement survival rates could be the result of micropredation from small crabs and fireworms (Hylkema et al. 2022a), which appear to be more abundant on macroalgae covered reefs (Bechtel et al. 2006).
Another factor influencing the abundance of adult Diadema may be contemporary disease events. In some areas, Diadema have shown trajectories of population growth (Pusack et al. 2023). However, in early 2022, a die-off event producing similar symptoms at the animal level, but a different geographic pattern of spread relative to the 1983–84 mass mortality (Hylkema et al. 2023), resulted in rapid population declines of > 98% in some areas, with measurable effects on local benthic ecology (Levitan et al. 2023). The likely pathogen causing the 2022 event was rapidly identified (Hewson et al. 2023), providing hope for an improved understanding of Diadema disease dynamics moving forward. Thus, to restore Diadema populations, the focus should be on increasing post-settlement survival with techniques such as assisted natural recovery (Hylkema et al. 2022b) or increasing available habitat complexity (Bodmer et al. 2021).
The relatively high settlement induced by Sargassum seems positive, but may also represent a threat to the natural recovery of Diadema populations. Sargassum fluitans and Sargassum natans cover huge areas floating in the central Atlantic. During the last decades, blooms have become more frequent and, especially in spring and summer, large patches float into the Caribbean Sea (Wang et al. 2019). Spring and summer are also the peak settlement season of Diadema (Hylkema et al. 2022a; Williams et al. 2009, 2011). Our results indicate that Diadema larvae can potentially settle on the floating Sargassum patches in high numbers. This could be beneficial or detrimental. The Sargassum could function as a floating nursery for the juvenile urchins, comparable to the assisted natural recovery method (Hylkema et al. 2022b). However, it is likely that the juveniles face high mortality rates from the multitude of predators living in Sargassum mats (Alleyne et al. 2023; Martin 2016) and the Sargassum could never reach suitable habitat or wash ashore (Cabanillas-Teran et al. 2019; Wang et al. 2019).
In the older biofilm, and some of the macroalgae treatments, high rates of degraded and missing larvae were observed. An explanation for these high rates in Lobophora and Laurencia, might be the release of toxic compounds from these algae. Norris and Fenical (1982) found high levels of elatol, known to be toxic for fertilized sea urchin eggs in Laurencia obtusa. To obtain the ~ 3 cm2 of surface area, some macroalgae had to be cut, possibly releasing toxic compounds. Depleted oxygen in the petri dishes due to decaying or respiring algal material can also not be excluded, despite the daily 50% water exchanges. In future experiments, it is recommended to regularly measure water parameters in the petri dish. Furthermore, it cannot be excluded that the natural biofilm on the macroalgae could also influence larval settlement rates. Dworjanyn and Pirozzi (2008) found decreased larval settlement rates with macroalgae for T. gratilla after removal of the biofilm. However, in this study the macroalgae tested were used to represent reef conditions, therefore including their natural biofilm. Ultimately, our results revealed that, beyond the cues associated with well-grazed reefs, a diverse array of macroalgae, typically found in changing reefs with limited grazing, could induce settlement in competent Diadema larvae. Based on these findings, we expect that natural settlement cues on changing reefs are not a major factor in constraining the settlement of Diadema. Our examination here is limited to the assessment of settlement and metamorphosis processes. It is plausible, and indeed probable, that the presence of reefs dominated by fleshy macroalgae diminishes Diadema recruitment by imposing various constraints on post-settlement survival. The high settlement rates on Sargassum could have major implications, both positive and negative. Future research should study this further, as well as develop methods to enhance post-settlement survival.
Data availability
The datasets generated during and/or analyzed during the current study are available upon request.
References
Adam TC, Burkepile DE, Ruttenberg BI, Paddack MJ (2015) Herbivory and the resilience of Caribbean coral reefs: knowledge gaps and implications for management. Mar Ecol Prog Ser 520:1–20. https://doi.org/10.3354/meps11170
Alleyne KST, Small M, Corbin M, Vallès H, Oxenford HA (2023) Free-swimming fauna associated with influxes of pelagic sargassum: implications for management and harvesting. Front Mar Sci 10:1–12. https://doi.org/10.3389/fmars.2023.1090742
Arnold SN, Steneck RS, Mumby PJ (2010) Running the gauntlet: Inhibitory effects of algal turfs on the processes of coral recruitment. Mar Ecol Prog Ser 414:91–105. https://doi.org/10.3354/meps08724
Aronson RB, Precht WF (2000) Herbivory and algal dynamics on the coral reef at Discovery Bay, Jamaica. Limnol Oceanogr 45:251–255. https://doi.org/10.4319/lo.2000.45.1.0251
Bak RPM (1985) Recruitment patterns and mass mortalities in the Sea Urchin Diadema antillarum. In: Proceedings of 5th international Coral Reef Congress, vol 5, pp 267–272
Balch T, Scheibling RE (2001) Larval supply, settlement and recruitment in echinoderms. In: Jangoux M, Lawerence JM (eds) Echinoderm studies, vol 6. AA Balkema, Rótterdam, pp 1–83
Bechtel JD, Gayle P, Kaufman L (2006) The return of Diadema antillarum to Discovery Bay: patterns of distribution and abundance. In: Proceedings of the 10th International Coral Reef Symposium 37, vol 5, pp 367–375
Belliveau SA, Paul VJ (2002) Effects of herbivory and nutrients on the early colonization of crustose coralline and fleshy algae. Source Mar Ecol Prog Ser 232:105–114. https://doi.org/10.2307/24865155
Bellwood DR, Hughes TP, Folke C, Nyström M (2004) Confronting the coral reef crisis. Nature 429:827–833. https://doi.org/10.1038/nature02691
Bielmyer GK, Brix KV, Capo TR, Grosell M (2005) The effects of metals on embryo-larval and adult life stages of the sea urchin, Diadema antillarum. Aquat Toxicol 74:254–263. https://doi.org/10.1016/j.aquatox.2005.05.016
Bodmer MDV, Wheeler PM, Anand P, Cameron SE, Hintikka S, Cai W, Borcsok AO, Exton DA (2021) The ecological importance of habitat complexity to the Caribbean coral reef herbivore Diadema antillarum: three lines of evidence. Sci Rep 11:1–13. https://doi.org/10.1038/s41598-021-87232-9
Cabanillas-Teran N, Hernandez-Arana HA, Ruiz-Zarate MA, Vega-Zepeda A, Sanchez-Gonzalez A (2019) Sargassum blooms in the Caribbean alter the trophic structure of the sea urchin Diadema antillarum. PeerJ 2019:1–32. https://doi.org/10.7717/peerj.7589
Doll PC, Caballes CF, Hoey AS, Uthicke S, Ling SD, Pratchett MS (2022) Larval settlement in echinoderms: a review of processes and patterns. Oceanogr Mar Biol Annu Rev 60:433–494. https://doi.org/10.1201/9781003288602-9
Dworjanyn SA, Pirozzi I (2008) Induction of settlement in the sea urchin Tripneustes gratilla by macroalgae, biofilms and conspecifics: a role for bacteria? Aquaculture 274:268–274. https://doi.org/10.1016/j.aquaculture.2007.11.030
Eckert GL (1998) Larval development, growth and morphology of the sea urchin Diadema antillarum. Bull Mar Sci 63:443–451
Feehan CJ, Brown MS, Sharp WC, Lauzon-Guay J-S, AdaMS DK (2016) Fertilization limitation of Diadema antillarum on coral reefs in the Florida Keys. Ecology 97:1897–1904
Fulmore HS (2019) Desperate coral larvae? Behavioral responses to settlement cues in aging Agaricia agaricites Larvae (Master’s thesis). Nova Southeastern University
Gosselin P, Jangoux M (1998) From competent larva to exotrophic juvenile: a morphofunctional study of the perimetamorphic period of Paracentrotus lividus (Echinodermata, Echinoida). Zoomorphology 118:31–43. https://doi.org/10.1007/s004350050054
Hadfield MG (2011) Biofilms and marine invertebrate larvae: what bacteria produce that larvae use to choose settlement sites. Ann Rev Mar Sci 3:453–470. https://doi.org/10.1146/annurev-marine-120709-142753
Harrington L, Fabricius K, De’athNegri GA (2010) Recognition and selection of settlement substrata determine post-settlement survival in corals. Ecol Soc Am 85:3428–3437
Hay ME (1984) Patterns of fish and urchin grazing on Caribbean coral reefs: are previous results typical? Ecology 65:446–454. https://doi.org/10.2307/1941407
Hernández JC, Brito A, Cubero E, García N, Girard D, González-lorenzo G, Falcón JM (2006) Temporal patterns of larval settlement of Diadema antillarum (Echinodermata: Echinoidea) in the Canary Islands using an experimental larval collector. Bull Mar Sci 78:271–279
Hewson I, Ritchie IT, Evans JS, Altera A, Behringer D, Bowman E, Brandt M, Budd KA, Camacho RA, Cornwell TO, Countway PD, Croquer A, Delgado GA, Derito C, Duermit-moreau E, Francis-floyd RSG Jr, Henderson L, Hylkema A, Kellogg CA, Kiryu Y, Kitson-walters KA, Kramer P, Lang JC, Lessios H, Liddy L, Marancik D, Nimrod S, Patterson JT, Pistor M, Romero IC, Sellares-blasco R, Sevier MLB, Sharp WC, Souza M, Valdez-trinidad A, Van Der Laan M, Vilanova-cuevas B, Villalpando M, Von Hoene SD, Warham M, Wijers T, Williams SM, Work TM, Yanong RP, Zambrano S, Zimmermann A, Breitbart M (2023) A scuticociliate causes mass mortality of Diadema antillarum in the Caribbean Sea. Sci Adv 9:1–10. https://doi.org/10.1126/sciadv.adg3200
Heyward AJ, Negri AP (1999) Natural inducers for coral larval metamorphosis. Coral Reefs 18:273–279. https://doi.org/10.1007/s003380050193
Hughes TP, Reed DC, Boyle M-J (1987) Herbivory on coral reefs: community structure following mass mortalities of sea urchins. J Exp Mar Biol Ecol 113:39–59. https://doi.org/10.1016/0022-0981(87)90081-5
Hughes TP, Baird AH, Bellwood DR, Card M, Connolly SR, Folke C, Grosberg R, Hoegh-Guldberg O, Jackson JBC, Kleypas J, Lough JM, Marshall P, Nyström M, Palumbi SR, Pandolfi JM, Rosen B, Roughgarden J (2003) Climate change, human impacts, and the resilience of coral reefs. Science 1979(301):929–933. https://doi.org/10.1126/science.1085046
Hunte W, Younglao D (1988) Recruitment and population recovery of Diadema antillarum (Echinodermata; Echinoidea) in Barbados. Mar Ecol Prog Ser 45:109–119. https://doi.org/10.3354/meps045109
Hylkema A, Debrot AO, Pistor M, Postma E, Williams SM, Kitson-Walters K (2022a) High peak settlement of Diadema antillarum on different artificial collectors in the Eastern Caribbean. J Exp Mar Biol Ecol 549:151693. https://doi.org/10.1016/j.jembe.2022.151693
Hylkema A, Debrot AO, van de Pas EE, Osinga R, Murk AJ (2022b) Assisted natural recovery: a novel approach to enhance Diadema antillarum recruitment. Front Mar Sci 9:1–11. https://doi.org/10.3389/fmars.2022.929355
Hylkema A, Kitson-walters K, Kramer PR, Patterson JT, Roth L, Sevier MLB, Vega-rodriguez M, Warham MM, Williams SM, Lang JC (2023) The 2022 Diadema antillarum die-off event: comparisons with the 1983–1984 mass mortality. Front Mar Sci. https://doi.org/10.3389/fmars.2022.1067449
Idjadi JA, Haring RN, Precht WF (2010) Recovery of the sea urchin Diadema antillarum promotes scleractinian coral growth and survivorship on shallow Jamaican reefs. Mar Ecol Prog Ser 403:91–100. https://doi.org/10.3354/meps08463
Jackson JBC, Kaufmann KW (1987) Diadema antillarum was not a keystone predator in cryptic reef environments. Science 1979(235):687–689. https://doi.org/10.1126/science.235.4789.687
Jackson J, Donovan M, Cramer K, Lam V (2014) Status and trends of Caribbean coral reefs: 1970–2012. Global Coral Reef Monitoring Network, IUCN, Gland
Keough MJ, Raimondi PT (1996) Responses of settling invertebrate larvae to bioorganic films: effects of large-scale variation in films. J Exp Mar Biol Ecol 207:59–78. https://doi.org/10.1016/S0022-0981(96)02632-9
Kuffner IB, Andersson AJ, Jokiel PL, Rodgers KS, MacKenzie FT (2008) Decreased abundance of crustose coralline algae due to ocean acidification. Nat Geosci 1:114–117. https://doi.org/10.1038/ngeo100
Leber K, Lorenzen K, Main K, Moe M, Vaughan D, Capo T, Bardales A, Gillette P, Smith D, Frerer B (2009) Developing restoration methods to aid in recovery of a key herbivore, Diadema antillarum, on Florida coral reefs. Protect our reef Grants Porgram 2008/2009 Final Report
Lee SC (2006) Habitat complexity and consumer-mediated positive feedbacks on a Caribbean coral reef. Oikos 112:442–447. https://doi.org/10.1111/j.0030-1299.2006.14247.x
Lessios HA (1988) Population dynamics of Diadema antillarum (Echinodermata: Echinoidea) following mass mortality in Panamá. Mar Biol 99:515–526
Lessios HA (2005) Diadema antillarum populations in Panama twenty years following mass mortality. Coral Reefs 24:125–127. https://doi.org/10.1007/s00338-004-0443-5
Lessios HA (2016) The great Diadema antillarum die-off: 30 years later. Ann Rev Mar Sci 8:267–283. https://doi.org/10.1146/annurev-marine-122414-033857
Levitan DR, Besta RM, Edmunds PJ (2023) Sea urchin mass mortalities 40 y apart further threaten Caribbean coral reefs. Proc Natl Acad Sci 120:1–6
Littler DS, Littler MM (2000) Caribbean reef plants: an identification guide to the reef plants of Caribbean, Bahamas Florida and Gulf of Mexico. Offshore Graphics, Washington DC
Maciá S, Robinson MP, Nalevanko A (2007) Experimental dispersal of recovering Diadema antillarum increases grazing intensity and reduces macroalgal abundance on a coral reef. Mar Ecol Prog Ser 348:173–182. https://doi.org/10.3354/meps06962
Martin LM (2016) Pelagic Sargassum and its associated mobile fauna in the Caribbean, Gulf of Mexico, and Sargasso Sea. Master’s thesis, Texas A & M University
McEdward LR, Miner BG (2001) Echinoid larval ecology. Dev Aquac Fish Sci 32:59–78
Miller RJ, Adams AJ, Ebersole JP, Ruiz E (2007) Evidence for positive density-dependent effects in recovering Diadema antillarum populations. J Exp Mar Biol Ecol 349:215–222. https://doi.org/10.1016/j.jembe.2007.05.014
Miller MW, Kramer KL, Williams SM, Johnston L, Szmant AM (2009) Assessment of current rates of Diadema antillarum larval settlement. Coral Reefs 28:511–515. https://doi.org/10.1007/s00338-008-0458-4
Mos B, Cowden KL, Nielsen SJ, Dworjanyn SA (2011) Do cues matter? Highly inductive settlement cues don’t ensure high post-settlement survival in sea urchin aquaculture. PLoS ONE. https://doi.org/10.1371/journal.pone.0028054
Mumby PJ, Hedley JD, Zychaluk K, Harborne AR, Blackwell PG (2006) Revisiting the catastrophic die-off of the urchin Diadema antillarum on Caribbean coral reefs: fresh insights on resilience from a simulation model. Ecol Model 196:131–148. https://doi.org/10.1016/j.ecolmodel.2005.11.035
Nedimyer K, Moe MA (2006) Techniques development for the re-establishment of the long-spined Sea Urchin, Diadema antillarum on two small patch reefs in the upper Florida keys. Florida Keys Natl Mar Sanctuary, pp 285–318
Norris JN, Fenical W (1982) Chemical defense in tropical marine algae, in. In: Riitzler K, Macintyre IG (eds) The Atlantic barrier reef ecosystem at Carrie Bow Cay, Belize, I. Structure and communities. Smithsonian Institution Press, Washington, DC, pp 417–431
Pearce CM, Scheibling RE (1990) Induction of metamorphosis of larvae of the green sea urchin, Strongylocentrotus droebachiensis, by coralline red algae. Biol Bull 179:304–311. https://doi.org/10.2307/1542322
Pearce CM, Scheibling RE (1991) Effect of macroalgae, microbial films, and conspecifics on the induction of metamorphosis of the green sea urchin Strongylocentrotus droebachiensis (Müller). J Exp Mar Biol Ecol 147:147–162. https://doi.org/10.1016/0022-0981(91)90179-Z
Pilnick AR, Neil KLO, Moe M, Patterson JT (2021) A novel system for intensive Diadema antillarum propagation as a step towards population enhancement. Sci Rep. https://doi.org/10.1038/s41598-021-90564-1
Pilnick AR, Neil KLO, Dimaggio MA, Patterson JT (2022) Development of larviculture protocols for the long-spined sea urchin (Diadema antillarum) and enhanced performance with diets containing the cryptophyte Rhodomonas lens. Aquac Int. https://doi.org/10.1007/s10499-022-00945-0
Pilnick AR, Petrosino A, Hassan MM, Patterson JT (2023) Cue selection and ontogeny reveal larval settlement dynamics of the long-spined sea urchin Diadema antillarum, a keystone coral reef herbivore. Mar Biol. https://doi.org/10.1007/s00227-023-04290-5
Precht L, Precht W (2015) The sea urchin Diadema antillarum—keystone herbivore or redundant species? PeerJ Prepr. https://doi.org/10.7287/peerj.preprints.1565
Pusack TJ, Stallings CD, Albins MA, Benkwitt CE, Ingeman KE, Kindinger TL, Hixon MA (2023) Protracted recovery of long-spined urchin (Diadema antillarum) in the Bahamas. Coral Reefs 42:93–98. https://doi.org/10.1007/s00338-022-02321-z
R Core Team (2022) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Rahim SAKA, Li JY, Kitamura H (2004) Larval metamorphosis of the sea urchins, Pseudocentrotus depressus and Anthocidaris crassispina in response to microbial films. Mar Biol 144:71–78. https://doi.org/10.1007/s00227-003-1171-z
Remple KL, Silbiger NJ, Quinlan ZA, Fox MD, Kelly LW, Donahue MJ, Nelson CE (2021) Coral reef biofilm bacterial diversity and successional trajectories are structured by reef benthic organisms and shift under chronic nutrient enrichment. Biofilms Microbiomes. https://doi.org/10.1038/s41522-021-00252-1
Ritson-Williams R, Arnold SN, Paul VJ (2016) Patterns of larval settlement preferences and post-settlement survival for seven Caribbean corals. Mar Ecol Prog Ser 548:127–138. https://doi.org/10.3354/meps11688
Rogers A, Lorenzen K (2016) Does slow and variable recovery of Diadema antillarum on Caribbean fore-reefs reflect density-dependent habitat selection? Front Mar Sci 3:1–10. https://doi.org/10.3389/fmars.2016.00063
Rowley RJ (1989) Settlement and recruitment of sea urchins (Strongylocentrotus spp.) in a sea-urchin barren ground and a kelp bed: are populations regulated by settlement or post-settlement processes? Mar Biol 100:485–494
Shikuma NJ, Hadfield MG (2005) Temporal variation of an initial marine biofilm community and its effects on larval settlement and metamorphosis of the tubeworm Hydroides elegans. Biofilms 2:231–238. https://doi.org/10.1017/S1479050506002018
Solandt JL, Campbell AC (2001) Macroalgal feeding characteristics of the sea urchin Diadema antillarum Philippi at discovery bay, Jamaica. Caribb J Sci 37:227–238
Swanson RL, Williamson JE, De Nys R, Kumar N, Bucknall MP, Steinberg PD (2004) Induction of settlement of larvae of the sea urchin Holopneustes purpurascens by histamine from a host alga. Biol Bull 206:161–172. https://doi.org/10.2307/1543640
Swanson RL, De Nys R, Huggett MJ, Green JK, Steinberg PD (2006) In situ quantification of a natural settlement cue and recruitment of the Australian sea urchin Holopneustes purpurascens. Mar Ecol Prog Ser 314:1–14. https://doi.org/10.3354/meps314001
Taniguchi K, Kurata K, Maruzoi T, Suzuki M (1994) Dibromomethane, a chemical inducer of larval settlement and metamorphosis of the Sea Urchin Strongylocentrotus nudus. Fish Sci 60:795–796
The Nature Conservancy (2004) The Diadema workshop report. Report: The Diadema Workshop. March 19–20
Tuohy E, Wade C, Weil E (2020) Lack of recovery of the long-spined sea urchin Diadema antillarum Philippi in Puerto Rico 33 years after the Caribbean-wide mass mortality. PeerJ. https://doi.org/10.7717/peerj.8428
Vermeij MJA, Debrot AO, Van Der Hal N, Bakker J, Bak RPM (2010) Increased recruitment rates indicate recovering populations of the sea urchin Diadema antillarum on Curaçao. Bull Mar Sci 86:719–725
Wang M, Hu C, Barnes BB, Mitchum G, Lapointe B, Montoya JP (2019) The great Atlantic Sargassum belt. Science 364:83–87. https://doi.org/10.1126/science.aaw7912
Wells CD, Martínez-Quintana Á, Tonra KJ, Lasker HR (2021) Algal turf negatively affects recruitment of a Caribbean octocoral. Coral Reefs 40:1045–1053. https://doi.org/10.1007/s00338-021-02103-z
Wieczorek SK, Todd CD (1998) Inhibition and facilitation of settlement of epifaunal marine invertebrate larvae by microbial biofilm cues. Biofouling 12:81–118. https://doi.org/10.1080/08927019809378348
Wijers T, Hylkema A, Pilnick AR, Murk AJ, Patterson JT (2023) Novel cultivation method for the long spined sea urchin (Diadema antillarum: Philippi) results in high larval survival and settlement rates. Aquaculture. https://doi.org/10.1016/j.aquaculture.2022.738855
Williams SM (2021) The reduction of harmful algae on Caribbean coral reefs through the reintroduction of a keystone herbivore, the long-spined sea urchin Diadema antillarum. Restor Ecol. https://doi.org/10.1111/rec.13475
Williams SM, García-Sais JR, Capella J (2009) Temporal variation of early larval stages of the long-spined sea urchin Diadema antillarum in La Parguera, Puerto Rico. Caribb J Sci 45:110–117. https://doi.org/10.18475/cjos.v45i1.a14
Williams SM, Yoshioka PM, García Sais JM (2010) Recruitment pattern of Diadema antillarum in La Parguera, Puerto Rico. Coral Reefs 29:809–812. https://doi.org/10.1007/s00338-010-0633-2
Williams SM, García-sais JR, Yoshioka PM (2011) Spatial variation of Diadema antillarum settlement in La Parguera, Puerto Rico. Bull Mar Sci 87:531–540
Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM (2009) Mixed effects models and extensions in ecology with R. Springer Science & Business Media, Berlin, pp 1–4
Acknowledgements
The authors want to thank Femke van Nimwegen for helping with the pilot study of larval settlement in Leeuwarden, The Netherlands. We want to thank Kei Veenstra, Paul van Loon and Jan-Luca Mack for helping with the larval culture on Saba. Finally, we want to thank the Saba Conservation Foundation staff, especially Marijn van der Laan, for providing logistical support.
Funding
This study was conducted within the scope of the RAAK PRO Diadema project (project# RAAK.PRO03.005), partially funded by SIA, part of the Netherlands Organization for Scientific Research (NWO).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by TW, BvH and DM. The first draft of the manuscript was written by TW and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare there are no competing interests.
Ethics approval
No approval of research ethics committees was required to accomplish the goals of this study because experimental work was conducted with an unregulated invertebrate species.
Additional information
Responsible Editor: C. Caballes.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wijers, T., van Herpen, B., Mattijssen, D. et al. Implications of changing Caribbean coral reefs on Diadema antillarum larvae settlement. Mar Biol 171, 48 (2024). https://doi.org/10.1007/s00227-023-04368-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-023-04368-0