Abstract
Marine turtles exhibit temperature-dependent sex determination (TSD), and there is widespread concern that global warming is raising nest incubation temperatures, resulting in increasingly female-skewed sex ratios in “feminized” populations. We assessed the sex ratio of a mixed-stock aggregation of immature green turtles (Chelonia mydas) at a midocean developmental foraging ground in the Northwest Atlantic from 1975 to 2018. We used plasma testosterone concentrations, measured by radioimmunoassay (RIA) and calibrated by laparoscopy, to determine the sex of 2,724 green turtles captured 3,940 times in Bermuda (32°18′ N, 64°46′ W) waters. A logistic regression model correctly predicted the sex of 99.5% of turtles (189/190) with associated testosterone concentrations whose sex had been verified via laparoscopy. Empirical evaluation of the trend in sex ratio using four related data sets showed a significant increase (2.8–4.0% yr−1) in the sex ratio (F/M) over the course of the study. Using growth rates to predict the year of arrival of turtles in Bermuda, we estimated the sex ratio of recruiting cohorts over 4 decades. Mixed-stock analysis of mtDNA sequences of 602 turtles that recruited to Bermuda between 1970 and 2018 suggested that multiple, geographically dispersed rookeries contributed to the Bermuda aggregation making it regionally representative. Changes in rookery contributions and strong population increases at certain rookeries may partly explain the increasing trend in the sex ratio. But the steady rate of increase over decades and the increasing female percentage of arriving cohorts are consistent with impacts of global warming at source rookeries.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sex ratios are of fundamental importance in the demography of populations (Mrosovsky 1994; Mitchell et al. 2010). The biological mechanisms that determine sex in vertebrates are complex but are typically categorized as genotypic or temperature-dependent sex-determination mechanisms (respectively, GSD or TSD). In TSD species, sex is determined by incubation temperature during embryonic development (Bull 1983; Janzen 1994). This mechanism is not known to occur in birds, mammals, and amphibians and is of limited occurrence in fishes, but it is common among egg-laying reptiles (crocodilians, tuataras, some lizards, and most turtles) (Valenzuela and Lance 2004). Sex is determined over a narrow range of incubation temperatures during a thermosensitive period of embryonic development, leading to concerns about how global warming affects sex ratios of TSD species (Janzen 1994; Gibbons et al. 2000; Ospina-Alvarez and Piferer 2008; Lockley and Eizaguirre 2021).
Temperature-dependent sex determination in marine turtles was first postulated in 1978 (Owens and Hendrickson 1978) based on work with captive green turtles at a commercial turtle farm in the Cayman Islands. The hypothesis was later supported by laboratory experiments using the eggs and hatchlings from a single nest of a loggerhead turtle (Caretta caretta) (Yntema and Mrosovsky 1980). It is now well established that all marine turtles exhibit TSD (Wibbels 2003). Climate change-related research on marine turtles has mainly focused on effects of increasing temperatures on sex ratios via TSD, although numerous other threats from climate change have been identified, including habitat loss (especially loss of nesting beaches due to sea level rise) and changes in distribution, migration patterns, and reproductive success (Patricio et al. 2021, and references therein).
Most marine turtle populations studied at the nesting beach, where the primary sex ratio (i.e., the sex ratio of hatchlings) can be evaluated, have a female-skewed ratio although balanced and male-skewed ratios have also been reported (Wibbels 2003; Hays et al. 2014; Patricio et al. 2021). Secondary sex ratios (i.e., the sex ratio of immature animals), adult sex ratios, and operational sex ratios (i.e., the sex ratio of adult individuals that reproduce in a given season) have received far less study due to difficulties related to access, logistics, and sampling (Lagueux 1998; Campbell and Lagueux 2005; Hays et al. 2010, 2014; Braun McNeill et al. 2016; Arendt et al. 2021). The National Research Council (2010) identified the study of secondary and adult sex ratios as important in its assessment of ongoing research on sea turtle population status and trends.
Determination of primary sex ratios of hatchling marine turtles at the nesting beach is challenging for a host of reasons (Wibbels 2003; Wyneken and Lolavar 2015). Perhaps foremost is that it requires robust and sustainable sampling designs that can accommodate high spatial and temporal variability in factors that affect sex ratios (e.g., nest location, hatching success, temperature, and rainfall). Abiotic factors can vary within a nesting season, annually, and on multiyear time scales (e.g., El Niño–Southern Oscillation). Wyneken and Lolavar’s (2015) extensive review of the literature showed that most studies had involved small sample sizes, were short term (carried out during only 1 or 2 nesting seasons) and included data collection during only a part of the season and/or only in a portion of the nesting habitat in a rookery. Sex-determination methods for hatchlings (including histology of gonads of dead-in-the-nest or sacrificed hatchlings, laparoscopic examination of gonadal and accessory-duct characteristics of captive-reared hatchlings, molecular markers, and use of secondary and tertiary proxies such as temperature or incubation duration) all have their shortcomings, and few studies have included in situ validation where sex was confirmed (reviewed in Wyneken and Lolavar 2015; Fuentes et al. 2017; Lockley and Eizaguirre 2021; Patricio et al. 2021). In addition to global warming, other climate change-related factors that may influence sex ratios at the nesting beach such as moisture, rainfall, and extreme weather events (Horikoshi 1992; Houghton et al. 2007; Wyneken and Lolavar 2015; Lolavar and Wyneken 2017, 2020) have received relatively little attention compared to temperature, although they have demonstrated effects. Consequently, there is increasing concern that despite decades of attention, knowledge of primary sex ratios of marine turtles remains inadequate, especially at a time when these species, already listed as threatened or endangered, face formidable threats associated with global climate change (Wyneken and Lolavar 2015; Santidrián Tomillo and Spotila 2020; Lockley and Eizaguirre 2021; Patrício et al. 2021). With projected increases in incubation temperatures at nesting beaches, populations may become increasingly feminized (i.e., their sex ratios may become increasingly skewed toward females) and too few males may be produced to enable successful reproduction and population viability.
Challenges to determining primary sex ratios have led some researchers to focus instead on other life history stages. Secondary sex ratios measured on foraging grounds frequently differ from primary sex ratios (Hopkins-Murphy et al. 2003; Wibbels 2003; Delgado et al. 2010; Maffucci et al. 2013; Jensen et al. 2016) and tend to be less female skewed (Hopkins-Murphy et al. 2003; Wibbels 2003; Delgado et al. 2010). The lack of concordance is presumably due to factors such as high annual variability in nest numbers, hatching success, and sex ratios at source nesting beaches (Wibbels 2003; Wyneken et al. 2006; Casale et al. 2006; Bjorndal and Bolten 2008; Limpus et al. 2009). In addition, sex-specific differences in hatching success, emergence success, fitness, and survival rates (Wibbels 2003; Santidrián Tomillo et al. 2014) and variability of ocean currents affecting dispersal and recruitment (Wibbels 2003; Ascani et al. 2016; Monzón-Arguello et al. 2010) are likely to influence secondary sex ratios at foraging grounds.
Secondary sex ratio in marine turtles can be estimated via blood sampling of live-stranded or necropsy of dead-stranded turtles (Casale et al. 2006; de Almeida et al. 2021), but such studies may be biased by factors related to injury or mortality or the detectability of dead-stranded turtles. An exception may be sampling of turtles that strand during mass events (e.g., cold stunning) during which large aggregations may be affected (e.g., Foley et al. 2007; Avens et al. 2012). Studies of secondary sex ratios alternatively may involve in-water capture and sex determination (via plasma testosterone or laparoscopy, Wibbels 1999, 2003, and Wibbels et al. 2000) of live immature turtles.
Measuring secondary sex ratios at the foraging grounds has a distinct advantage over assessments at the nesting beach because foraging aggregations represent many cohorts of hatchling production and consequently, are a distillation of the highly variable factors associated with the nesting beach and subsequent dispersal (Wibbels et al. 1987a, 1991; Wibbels 2003; Delgado et al. 2010; Maffucci et al. 2013; Jensen et al. 2018). Studies of foraging-ground sex ratios also avoid the sampling problems associated with measuring operational sex ratios for marine turtles such as sex-specific reproductive periodicity and migration patterns of adults (Wibbels et al. 1991; Wibbels 2003), human-related effects on sex ratios, such as those resulting from selective harvesting (Wibbels 2003) or management practices (Jensen et al. 2016), and the difficult logistics of working with adult-size animals.
Our study assessed the secondary sex ratio of green turtles (Chelonia mydas) and its change over time at a developmental foraging ground in the Northwest Atlantic Ocean. We used plasma testosterone concentrations (titers), as measured by radioimmunoassay (RIA) and calibrated by laparoscopy of a subset of captured turtles, to determine sex. Earlier studies of sex ratios of sea turtle foraging aggregations (Casale et al. 2006; Delgado et al. 2010; Jensen et al. 2016, 2018) have recognized the importance of knowing what stocks are contributing to an aggregation, because source rookeries may be contributing differently, and these contributions are subject to change (Bjorndal and Bolten 2008; van der Zee et al. 2019). Thus, we also describe the genetic composition (via mitochondrial DNA and mixed-stock analysis) of the Bermuda aggregation and document changes in the contributions of source rookeries over time.
We had a particular advantage carrying out this study in Bermuda because 1) only one life stage of the green turtle (i.e., benthic immatures) is present at this oceanic site (Fig. 1) (Meylan et al. 2011, 2022); 2) an ongoing, long-term mark–recapture project, the Bermuda Turtle Project (BTP), has provided large annual samples of turtles over 27 yrs; 3) the samples comprised a wide range of sizes of immature turtles from multiple seagrass meadows that matched the size distribution known for the Bermuda aggregation (Meylan et al. 2011, 2022); and 4) widely dispersed C. mydas rookeries in the Northwest Atlantic contributed to the aggregation, making the sex ratio results broadly representative. The work also benefited from a partnership that allowed RIA analyses to be conducted in the same laboratory over nearly 3 decades of sample collection.
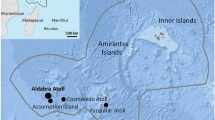
Location of Bermuda (32°18′ N, 64°46′ W), 1,050 km southeast of Cape Hatteras, North Carolina, USA. Labeled sites are locations at which > 25 captures of immature green turtles (Chelonia mydas) included in this study were made, 1976–2018 (total captures n = 3,940, individual turtles n = 2,724). Isobaths provided by the Bermuda Department of Environment and Natural Resources
Our study resulted in four related data sets (ranging in length from 27 to 40 yrs) that allowed an empirical evaluation of the trend in sex ratio of a mixed-stock aggregation of immature green turtles in the Northwest Atlantic. These data complement the primary sex ratio information available from the nesting beaches in this region and provide an important new perspective for the evaluation of the possible effects of global warming on marine turtles. We were able to elucidate the dynamics of interannual variation in secondary sex ratios over nearly 3 decades. By estimating the year in which turtles arrived in Bermuda using growth-rate data for turtles foraging at Bermuda, we were able to estimate the sex ratio of recruiting cohorts over 4 decades.
Materials and methods
Study site
Bermuda (32°18′ N, 64°46′ W) is located 1,050 km southeast of Cape Hatteras, North Carolina, USA (Fig. 1). It originated as a seamount on the Mid-Atlantic Ridge and now comprises a chain of islands (combined area, 56 km2) surrounded by the Bermuda Platform (775 km2 of shallow marine ecosystems). Green turtles (Chelonia mydas) were captured on one or more of 40 seagrass meadows around the Bermuda Platform, including inshore, nearshore, and offshore sites (Meylan et al. 2011, 2022). Sites at which at least 25 captures were made over the course of the study are labeled in Fig. 1. The Bermuda Platform is considered a single, mixed-stock, developmental foraging ground for immature green sea turtles.
Turtle captures
Turtles were captured one to seven times using an entrapment net (396–613 m × 6.1 m, mesh size of 10 × 10 cm) set in a circle on seagrass meadow foraging areas that were generally < 6.1 m deep. Captures that were part of this sex ratio study were made from 1976 to 2018 as part of an ongoing long-term study. Although new sampling localities were added over the years, a core group of sites was regularly sampled, including some original sites dating to 1975. Snorkelers patrolled the net, removed entangled turtles, and transferred them to a small boat. The turtles were then transferred to a larger research vessel for collection of data and biological samples (e.g., blood). Field sampling sessions typically lasted 5 to 10 days. Captures were made in all months of the year, but mostly during the summer months (i.e., June, July and August); after 2010, nearly all sampling was conducted in August.
Morphometrics
Every captured turtle was tagged and had a standard set of morphometric data collected except for repeated capture of a turtle during the same session. Minimum straight carapace length (SCLmin; Bolten 1999) was measured in cm along the midline from the center of the nuchal notch to the notch between the posteriormost pair of marginal scales.
Tagging
Captured turtles were tagged on the trailing edge of each front flipper (until 1992, only one tag was used) at a point just medial to the first large scale on the ventral side of the flipper. Tag size varied with turtle size and included Monel (style 1005–49) and Inconel (style 681) (National Band and Tag, Newport, Kentucky, USA), titanium (Stockbrands Co. Pty Ltd, Osborne Park, AUS), and plastic (Dalton Jumbo Ear Tag, Dal 8) tags. A reward message and return address (DEPT BIOL UF, GAINESVILLE FL USA) on the tag allowed the reporting of turtles that had departed Bermuda and were observed or captured outside of Bermuda, including those observed on nesting beaches. Beginning in 2001, an AVID (Norco, California, USA) passive induced transponder (PIT) tag was also inserted into the connective tissue between the radius and ulna of the left foreflipper.
Water temperatures
Bermuda’s developmental foraging ground for green turtles is the northernmost in the Northwest Atlantic that is occupied year-round (Meylan et al. 2011; Seminoff et al. 2015). Average monthly water temperature at a NOAA national data buoy (BEPB6) on Bermuda’s north coast ranged from 15.7 to 31.2 °C during the period October 2007–November 2019 (all available data from the buoy) (https://www.ndbc.noaa.gov/view_climplot.php?station=bepb6&meas=st). Because water temperature can affect the reliability of RIA in predicting the sex of marine turtles (Braun-McNeill et al. 2007; Hawkes et al. 2013), we took this parameter into consideration in our study. Water temperatures were available for 3138 of the 3163 captures at which blood plasma samples were collected for testosterone concentration analysis. Temperatures for 1990–1992 were monthly averages for sites on or near the north coast and were obtained from the Bermuda Institute for Ocean Science, the National Climate Data Center, and the Caribbean Coastal Marine Productivity program (CARICOMP). For 1993–2018, water temperature was measured at the approximate center of each set of the net during capture efforts. Temperatures were recorded to the nearest 1 °C using a mercury thermometer; most measurements were taken on the bottom, a small number at the surface. Where temperature was missing, substitute temperature values were derived from (in decreasing order of preference) a different set of the net at the same locality on the same day; at the same locality on the nearest possible date during the sampling session; or at an ecologically similar locality during the same sampling session.
Collection of samples for hormone and genetic analyses
A 4- to 7-ml sample of blood was collected from the dorsal cervical sinus (Owens and Ruiz 1980) using 18- to 22-gauge needles and Vacutainers coated with sodium heparin. An additional 1-ml sample of blood was collected in no-additive Vacutainers for genetic analysis and immediately transferred to a 10-ml plastic tube containing 9 ml of lysis buffer (Dutton 1996). Blood samples were held on board in coolers containing ice until centrifugation. Within 30 min to 5 h, blood samples collected for hormone analysis were centrifuged at high speed for 5 min; plasma was aliquoted into 2-ml cryovials. The blood plasma aliquots were held in a − 20 °C freezer for as long as 2 weeks in Bermuda and then transported to St. Petersburg, Florida, where they were stored at − 50 °C until being shipped on dry ice to College Station, Texas, or Charleston, South Carolina, for analysis. Blood samples preserved in lysis buffer were stored in an air-conditioned (± 22 °C) space except when at room temperature during transport.
When a blood sample was not obtained at capture, a biopsy of tissue for genetic analyses was taken from the trailing edge of a rear flipper with a 4.0-mm punch and preserved in 9 ml of SED (NaCl, EDTA = ethylene-diamine tetraacetic acid, and DMSO = dimethyl sulfoxide) buffer (Dutton 1996) or a saturated salt solution. Tissue samples were also stored in an air-conditioned (± 22 °C) space except when at room temperature during transport. Plasma samples were collected and analyzed from 1990 to 2016; however, some of the turtles sampled for plasma during this period had originally been caught as early as 1976 or were recaptured through 2018. Genetic samples were collected from 1990 to 2018.
Laparoscopy
Sex and maturity status were determined for a sample of green turtles via laparoscopy in 1990–1994 (Meylan et al. 2011) and in 2008, following the method described by Owens (1999). Laparoscopy allows the direct viewing of the gonads using an endoscope inserted into the body cavity through a small sleeve introduced with a trochar; illumination is provided by an external fiber-optic light source. All laparoscopies were conducted in an onshore laboratory. The laparoscopic data were used to calibrate the results of testosterone-titer RIAs run on the same animals so that sex could be predicted for other turtles using blood plasma testosterone titer alone. Sex was determined based on the appearance, texture, and color of the gonad following the criteria of Limpus and Reed (1985). Three maturity stages for males and four for females have been recognized for green turtles (Limpus and Reed 1985; Meylan et al. 2011), but only the first two stages of maturity were observed in green turtles in Bermuda. Sex, as assigned via laparoscopy at a single capture of an individual turtle, was thus known and available for use in all captures of that turtle, allowing use of multiple hormone titers for known sex individuals in the calibration. Having a large sample of turtles in this study with both laparoscopy-based and plasma testosterone-based sex determinations made it unnecessary to make inferences about sex assignments based on studies of green turtles elsewhere.
Other sex confirmation
The predicted sex (female) of five green turtles captured by the project and analyzed for blood plasma testosterone titer was independently verified by observation of those turtles on a nesting beach outside Bermuda (green turtles do not nest in Bermuda), documented via tag returns to the Archie Carr Center. The sex of another eight turtles captured by the project and analyzed for blood plasma testosterone titer was later verified during necropsy as part of the stranding program of the Bermuda Aquarium Museum and Zoo’s Wildlife Rehabilitation Center.
Plasma testosterone RIA protocol
All samples were analyzed in the same RIA laboratory (David Owens’s lab), located first at Texas A&M University, College Station, Texas, and then at the College of Charleston, Charleston, South Carolina. Samples were analyzed under a consistent RIA protocol throughout the study and were completed by a total of five assay technicians across the time series. The extended time series necessitated several adjustments to the assay, based primarily on the availability of reagents. Tritiated testosterone was purchased from New England Nuclear Life Sciences (1β, 2β H3(N); Boston, Massachusetts) and Perkin-Elmer Life and Analytical Sciences ([1,2,6,7-3H(N)]; Waltham, Massachusetts) and diluted with 0.05 M TRIS/gel buffer to yield 7000–12,000 counts per minute (cpm) per 100-µl aliquot, depending on binding efficiency with the testosterone antiserum. Testosterone antiserum was purchased from Cambridge Medical Diagnostics (catalog #R51226F; Billerica, Massachusetts), Endocrine Sciences (catalog #T3-125; Calabasas Hills, California), and Fitzgerald Industries (catalog #20R-TR018w; Acton, Massachusetts). As tested by the manufacturer, these testosterone antisera have cross-reactivity with steroids other than testosterone, so plasma testosterone concentrations resulting from the assay denote total androgens. Titration tests were used to achieve optimal percent binding (B/B0) of the antisera with the tritium delivered to each assay tube (i.e., ~ 50% B/B0, cpm standard:cpm “zero” standard). Radio-inert testosterone powder for preparation of standard curves was purchased from Steraloids (4-androsten-17β-ol-3-one; Wilton, New Hampshire) and Sigma-Aldrich (T-1500; St. Louis, Missouri). Samples were counted on a Wallac 1409, Wallac 1414, or TriCarb 3100 liquid scintillation counter (Perkin-Elmer, Waltham, Massachusetts).
Plasma testosterone concentration was determined according to the general RIA protocol described in Wibbels et al. (1987b), based on Coyotupa et al. (1972). One hundred eight assay runs were needed to process the three thousand two hundred twenty plasma samples collected over the 27 years; approximately forty individual plasma samples were extracted with each assay. The sea turtle plasma testosterone RIA protocol followed by the Owens laboratory has been described elsewhere (Coyotupa et al. 1972; Wibbels et al. 1987a, 1987b, 1989, 1990; Lee and Owens 2008), including notes on the importance of using laparoscopy to verify the sex assigned (Schroeder and Owens 1994; Wibbels et al. 2000). The assay has previously been used to analyze green turtle plasma samples (Owens et al. 1978; Wibbels et al. 1989, 1993). Plasma aliquots (generally 500–1000 µl) were extracted with 4 ml anhydrous diethyl ether, dried under nitrogen gas, and resuspended with 1 ml acetone. All samples were quantified in duplicate and were re-extracted if variation between duplicates was > 5%; two 400-µl aliquots were pipetted from the acetone and evaporated overnight. Each tube was reconstituted with 100 µl 0.05 M TRIS/gel buffer (pH = 7.2) and incubated in a water bath for 30 min at 37 °C. Following incubation, 50–100 µl of testosterone antiserum and 50–100 µl of tritiated testosterone (the volume used, depending on antiserum binding efficiency) were added to each sample tube, and tubes were incubated for 24 h at 4 °C. After incubation, to absorb unbound hormone, 1 ml of dextran-coated charcoal was added to all sample tubes, except those used to determine total cpm of tritium. All tubes were incubated for 15 min at 4 °C and centrifuged at 2,300 rpm at 4 °C for 15 min. Each supernatant was poured into a scintillation vial and 4 ml of Ecolume scintillation cocktail added. All vials were counted for 60 s on a liquid scintillation counter.
Plasma testosterone concentrations in pgml−1 were calculated from raw scintillation counts per minute using a standard curve included within each assay, and data were processed via a parallel-line analysis software (RIAMENU) based on a four-parameter logistic data-reduction program. Standard tubes with 100 μl of a known concentration of radio-inert testosterone (ranging from 19.5 to 1250 pg 100 μl−1) were freshly prepared for each assay run. The testosterone concentration in each sample was corrected by multiplying the extracted volume by the extraction efficiency and the fraction aliquoted from the reconstituted sample (40%). Extraction efficiency was calculated via addition of tritiated testosterone to aliquots of green turtle plasma (i.e., samples collected from individual turtles) before extraction with diethyl ether. In addition, two procedural blanks (i.e., 1000-μl aliquots of H2O) were extracted in each assay run to monitor any changes in background cpm. Control plasma samples (pooled sea turtle plasma; two to four samples included per assay) were extracted with each assay run to monitor intra- and inter-assay variability. Mean extraction efficiency (± standard error) was 89.0% ± 0.35 across all assays and all laboratory technicians. The mean intra-assay coefficient of variation (CV) was 9.3% ± 0.48, and the mean inter-assay CV was 18.7% ± 2.2. Mean sensitivity of the assay was 10.6 pg tube−1 ± 0.45 based on 90% binding (B/B0). Binding between 20 and 80% B/B0 (i.e., linear portion of binding curve) for each of two sample replicates was considered most accurate, and samples were re-extracted with smaller plasma volumes, when possible, to achieve optimal binding.
Sex ratio estimation
We used 190 turtles of known sex (as determined by laparoscopy) for which testosterone titers were available for one or more captures to develop models for assigning sex to 2,946 turtles that had not been laparoscoped but for which testosterone titers were available. We tested mixture models, logistic regression models, and a Bayesian logistic regression model to determine how best to assign sex to turtles for which only a plasma testosterone titer was available. For all models, plasma testosterone titers were log-transformed to minimize the effects of heteroscedasticity. All models gave similar results in terms of assigning sex to the turtles; we focused primarily on the results of the non-Bayesian logistic regression model (Tables 1, 2). Once we estimated the sex for each turtle using the testosterone titer, we compiled linear regression models to test for changes in sex ratio over time. We used the binconf R function from the Hmisc package to calculate binomial confidence intervals for the sex ratio for each year (Brown et al. 2001; R Core Team 2020; Harrell et al. 2021).
We used the glm function in the R base package (R Core Team 2020) to fit logistic regression models using the data from turtles that had been laparoscoped (hereafter, laparoscoped turtles) with known sex and plasma testosterone titer. We then used the fitted model to assign sex to each turtle for which a plasma testosterone value was available. We used AICc to select the best model out of a group of candidate models that included all possible combinations of plasma testosterone titer, water temperature, and turtle size as covariates for a total of seven candidate models (Burnham and Anderson 2002). In addition, to check for the influence of water temperature on plasma testosterone levels, we re-ran the logistic regression models using only turtles that had been captured in waters between 26 and 30 °C. Twenty-six degrees was conservatively chosen as the lower temperature limit for this analysis because it was well above the 24 °C limit noted, for loggerhead turtles, as free of temperature effects on plasma testosterone titer (Braun-McNeill et al. 2007). For the logistic regression output, each turtle was assigned a probability of being male, which ranges between 1 and 0. Sex of turtles for which the probability of being male was > 0.50 were deemed male, the others female. After assigning sex to each capture of a turtle for which a plasma testosterone titer was available, we assigned sex to other captures of that turtle during which a blood sample had not been taken, based on measured testosterone titers for that turtle; if a turtle was captured and tested more than once, we assigned sex using the mean testosterone titer. When a turtle was captured more than once in a year, the first plasma testosterone titer associated with that turtle in that year was used in annual sex ratio calculations; other captures were removed from the sex ratio data set. Based on laparoscopy results or size of the turtles, we could be confident that all turtles in the study were immature at all captures, supporting these data treatment decisions (see maturity profiles in Meylan et al. 2011: Tables 4 and 5).
We used the normalmixEM function in the mixtools R package (Benaglia et al. 2009) to assign each turtle to either a male or a female group via a mixture model. Mixture models assume that the sample data have underlying subpopulations that differ with respect to a sampled parameter and then assign each observation to a subpopulation. We specified lambda (λ) to be the starting value for the mixing proportions, based on the proportions of male and female turtles observed in the laparoscoped turtles. This method does not directly make use of the laparoscoped turtles of known sex.
To compare our model results with those of a modeling method used previously for assigning sex to turtles using plasma testosterone titers, we used the jagsUI package in R to fit a Bayesian logistic model of the form (p[i]) = beta0 + beta_H * H1[i] where p[i] is the probability of being female, beta0 is the intercept term, beta_H is the coefficient for testosterone titer, and H1[i] is the value of testosterone in the laparoscoped turtles. We ran 40,000 iterations with 4 chains with a burn-in of 10,000. For additional information on this model, see Allen et al. (2015).
We estimated the year of arrival (recruitment) to Bermuda for all individual turtles used in this study based on our long-term observation that green turtles arrive in Bermuda at approximately 25 cm SCLmin and grow at an average rate of 2.5 cm yr−1 (Meylan et al. 2011). Arrival year was estimated as the year of capture (the year that the turtle was measured, and the plasma testosterone sample taken) minus the quantity ([size at sampling − 25 cm]/2.5). For turtles that had been captured multiple times, the estimated year of arrival was averaged and rounded to the nearest year.
We modeled plasma testosterone titer as a function of turtle size separately for males and females using linear regression with robust standard errors due to the data not meeting the assumption of homoscedasticity. We used the lm R function (Base R stats package) to generate the model and then used the coeftest function from the lmtest R package (Zeileis and Hothorn 2002) to calculate standard errors that are robust to heteroscedasticity using a covariance matrix that does not assume constant variance.
We assembled four related data sets to analyze our accumulated observations: A) only captures of green turtles for which a blood plasma sample was collected at that capture and for which a measurement of plasma testosterone titer was generated; B) all captures of individual green turtles for which sex had been assigned via plasma testosterone titer for at least one capture; C) only captures of green turtles where a blood plasma sample was collected that same day and water temperatures were 26–30 °C; D) for every green turtle for which sex had been assigned via plasma testosterone titer for at least one capture and an estimated year of arrival (recruitment) in Bermuda was available. Data sets A and B were kept separate to ensure that bias potentially introduced by including turtles with multiple captures (e.g., males and females potentially having differing capture vulnerability due to behavior or residency times) was minimized. Data set C was designed to address potential water temperature impacts. Data set D allowed us to remove turtle size as a factor influencing annual sex ratios and extend the time period over which sex ratios could be examined.
To provide an estimate of the sex ratio of immature green turtles in Bermuda in the latest years of the study, we combined data on the predicted number of turtles of each sex from the 4 most recent years of data available for each data set. This was done to provide a final estimate of feminization but also to compensate for annual variation in sex ratios.
Genetic laboratory analyses
Mitochondrial DNA (mtDNA) sequence data were from 602 green turtles captured as described above (n = 555) or recovered via the stranding program (n = 47) of the Wildlife Rehabilitation Center of the Bermuda Aquarium Museum and Zoo during 1990–2018. Samples were processed by Eckerd College genetics classes during 1995–2020. DNA was extracted from blood samples or tissue samples using Qiagen DNeasy kits following manufacturer’s instructions. DNA purity and concentration was tested after extraction using a Nanodrop 8000. Through 2005, PCR amplifications used primers LTCM2 and HDCM2 to generate a ~ 500-bp sequence. Subsequently, the size of the sequenced fragment was extended to ~ 840 bp using primers H950 and LTE9 (Abreu-Grobois et al. 2006). Sequences were generated by the Biotechnologies for the Ecological, Evolutionary, and Conservation Sciences (BEECS) Genetic Analysis Core, now a part of the Interdisciplinary Center for Biotechnology Research, University of Florida, during 1995–2011, and by Eurofins Genomics LLC during 2012–2020. Sequence quality was assessed by examination of a chromatogram, and clean sequences were aligned using Multalin (Corpet 1988); unknowns were aligned with standards from the Archie Carr Center (https://accstr.ufl.edu/resources/mtdna-sequences/). All mtDNA haplotypes identified in Bermuda for this project had been registered by other researchers. All genetic analyses were performed using the short sequence. Variation in genetic makeup of the Bermuda foraging aggregation over 5 decades was investigated using Arlequin Version 3.5 (Excoffier et al. 2005). To test for population (aggregation) differentiation due to changes in the genetic structure over the course of the study, fixation index (Fst) values were computed from haplotype frequencies of short fragments observed during 5 individual decades (1970–1979, 1980–1989, 1990–1999, 2000–2009, 2010–2019) of estimated arrival (recruitment) in Bermuda. Significance levels for differentiation were tested by comparison to values generated from 10,000 random permutations.
Mixed-stock analysis
To determine which source rookeries may have contributed to the Bermuda foraging aggregation, we performed a mixed-stock analysis (Bolker et al. 2003). Frequencies of mtDNA haplotypes at the foraging ground (one orphan haplotype, Cm-A29.1, excluded) were compared to known haplotype frequencies from 24 possible Atlantic source nesting beaches (rookeries) to estimate the most likely combination of rookery contributions to the foraging aggregation of immature green turtles in Bermuda. Analyses were run using the short (~ 500 bp) mtDNA sequences and the Mixstock R package (Bolker 2015) in a many-to-one mixed-stock analysis (R Core Team 2020). Specifically, we used the mcmc (Markov chain Monte Carlo) function in the Mixstock R package (Bolker et al. 2003), which uses Gibbs sampler with 50,000 iterations, a burn-in of 5000, and a thinning rate of 4. We used the Gelman–Rubin criterion to check for chain convergence (Gelman and Rubin 1992). Ninety-five-percent confidence intervals were derived by calculating the 97.5% and 2.5% quantiles of the posterior distribution. Baseline rookery size data for Atlantic beaches were taken from Naro-Maciel et al. (2017: Supplementary Table 1), with updates from Shamblin et al. (2018: Table S2; 2020: Supplementary Material) and van der Zee et al. (2019: Appendix 1). Nesting-female abundance for Dry Tortugas, Florida, was calculated using the method of Shamblin et al. (2018), with annual nest totals for 2015–2019 provided by the Florida Fish and Wildlife Conservation Commission (FWC). Analyses for all years combined and by estimated decade of arrival in Bermuda were performed with and without a population size prior (weighting factor). However, because of the long time frame of this study and our understanding that most rookeries have experienced large changes in abundance over this time period (see Discussion), we emphasize results without priors in Results and Discussion.
Results
Sex determinations
Sex was determined via laparoscopy or predicted based on plasma testosterone titer for 2,724 individual green turtles (Chelonia mydas) captured 3,940 times in Bermuda waters during 1976–2018. The sex of 191 individual green turtles was assessed via laparoscopy; 114 females and 76 males (1.5 F:1.0 M) were identified. Sex could not be determined for one individual. All turtles were assessed to be immature (Stage 1 or 2). These 190 immature turtles were captured a combined 293 times; 218 of those captures (25.1–77.1 cm SCLmin; mean ± SD: 53.15 ± 12.92) (Fig. S1a) were of turtles for which plasma testosterone titers had been measured, and therefore could be used to calibrate sex assignment for turtles that were not laparoscoped but for which plasma testosterone titers were available. Captures of turtles that were not laparoscoped included turtles ranging in size from 22.3 to 78.6 cm SCLmin (mean ± SD: 45.11 ± 11.90, n = 2,918 (size missing for 28 captures) (Fig. S1b). In total, plasma testosterone titer was available for 2,510 immature turtles (2,946 captures) that were not laparoscoped; these turtles were captured a total of 3,587 times, including captures for which no sample was collected for testosterone titer.
Plasma testosterone titers for laparoscoped turtles ranged from 8.7 to 157 pg ml−1 for females and from 172 to 26,680 pg ml−1 for males except for one male for which the titer was only 88.4 pg ml−1 (Fig. 2). The distributions of plasma testosterone titers associated with all captures of laparoscoped green turtles (n = 218) and with all captures of turtles whose sex was predicted solely by plasma testosterone titer (n = 2946) were very similar (Fig. 3a, b). The distributions of titers for females and males determined in this study were nearly discrete with only a small amount of overlap (Fig. S2). Male titers showed a significant increase with size (linear regression, adjusted r2 = 0.1727, F1,1103 = 23.15, p < 0.0001); female titers decreased slightly but not significantly with size (linear regression, adjusted r2 = 0.001283, F1,2026 = 3.605, p = 0.08).
Log of plasma testosterone titer (pg ml−1) for 190 immature green turtles (Chelonia mydas) captured in Bermuda, 1990–2013, for which sex was determined by laparoscopy (n = 218 captures). Red = female, blue = male. The highest testosterone titer for females was 157 pg ml−1; the lowest testosterone titer for males was 172 pg ml−1 except for one 28.6-cm SCLmin male with a titer of 88.4 pg ml.−1
All laparoscopies were performed on turtles captured in summer, but some of those turtles were recaptured and sampled for testosterone in other seasons. Water temperatures ranged from 19 to 29.5 °C for captures of turtles that were laparoscoped (n = 218); for 92.6% of these captures, water temperature was ≥ 26 °C (Fig. S3a). Captures of turtles that were not laparoscoped (n = 2,920) were made in waters ranging in temperature from 17.6 to 30.5 °C; for 79.2% of these captures, water temperature was also ≥ 26 °C (Fig. S3b).
Results for the three types of models considered for use to predict immature green turtle sex were similar. The maximum testosterone titer for immature females was 152 pg ml−1 for the mixture model, 188 pg ml−1 for the Bayesian logistic regression model, and 168 pg ml−1 for the logistic regression model. The three models agreed with each other on sex prediction for all but 2 of 218 captures of laparoscoped turtles (i.e., of known sex) for which testosterone titers were obtained; the mixture model and the Bayesian model each disagreed once with the other two models. For 72 (2.4%) non-laparoscoped turtles, testosterone titers were 152–188 pg ml−1 (the immature female testosterone concentration maximum range for all three models), which could have predicted conflicting sex assignment, depending on model. We elected to use the logistic regression models for the results presented in the remainder of this manuscript due to its ease of model selection compared with Bayesian methods as well as the flexibility of the logistic regression model compared with that of the mixture model. The logistic regression model correctly assigned sex to all captures of laparoscoped turtles except for the single male with a testosterone titer of 88.4 pg ml−1 (within the immature female range), giving an accuracy of sex prediction of 99.5%.
Using logistic regression, we developed models to test the possibility that water temperature and turtle size (SCLmin) influenced testosterone titer. AICc values indicated that a model with a single predictor of testosterone titer was the best and that the addition of water temperature and SCLmin did not improve the model more than the penalty for including the additional term (Table 1).
Because most (n = 205, 94%) captures of laparoscoped turtles that were tested for testosterone titer occurred at water temperatures between 26 and 30 °C, we tested for an effect of water temperature on our model-fitting capabilities. We re-ran our model selection process using the logistic regression models with only turtles sampled at water temperatures between 26 and 30 °C. The testosterone-only model was still the best fit (Table 2).
Sex predictions with the logistic regression model agreed in 100% of instances for laparoscoped turtles for which plasma testosterone had been measured more than once over their capture history. Sample sizes were (a) n = 16 when 2 plasma testosterone titers had been measured; (b) n = 4 for 3 titers; (c) n = 1 for 4 titers; and (d) n = 1 for 5 titers.
For non-laparoscoped turtles for which plasma testosterone had been measured more than once over their capture history, nearly all sex predictions agreed; sample sizes were (a) n = 304, 2 titers; (b) n = 52, 3 titers; (c) n = 9, 4 titers; and (d) n = 4, 5 titers. There was disagreement as to predicted sex for 24 turtles that were captured a total of 60 times (< 1.0% of the 2,510 turtles for which plasma testosterone was measured) resulting in more than one available titer. Data on those turtles were removed from additional analyses. For 4 of these 24 turtles, testosterone titers were near the value at which the model switched from predicting a female to predicting a male. Other cases of disagreement were possibly the result of labeling errors, either in the field or the laboratory.
Sex ratios
The trend in estimated annual sex ratio (and 95% confidence interval) of immature green turtles on the foraging grounds in Bermuda (1990–2018) using all captures of known or predicted sex is shown in Fig. 4a. The sex ratio became more female-skewed over time increasing 2.8% annually (p = 0.024). It varied from 0.95 in 1991 to 3.16 in 2007, with an overall ratio for 1990–2018 of 1.85 F:1 M (Table S1: Data set B). The sex ratio for the four most recent years (2015–2018) of data combined was 1.68 F:1 M (62.7% female). Similar trends in annual sex ratio were observed for two related data sets, captures of turtles with a sex assigned via a plasma testosterone sample collected at capture that year, and the same data set restricted to captures at water temperatures of 26–30 °C (Fig. S4, Table S1: Data sets A,C).
Trend in estimated annual sex ratio (with 95% confidence interval) of immature green turtles (Chelonia mydas) in Bermuda. a Sex ratio by year of capture, 1990–2018, n = 3,817 captures. Sex determination based on laparoscopy and/or plasma testosterone titer at any capture(s) of an individual turtle (Table S1: Data set B). Sex ratio for 2012 was based on 1 turtle for which a titer was measured for the first time and 47 turtles for which sex had been determined during other years; sex ratios for 2017 (n = 49 recaptures) and 2018 (n = 52 recaptures) were based exclusively on previously assigned sex determinations. b Sex ratio by estimated year of arrival, 1975–2015, n = 2,654 turtles (Table S1: Data set D)
The trend in estimated annual sex ratio (and 95% confidence interval) for 2654 immature green turtles captured in Bermuda, based on estimated year of arrival (1975–2015), is shown in Fig. 4b. These sex ratios represent annual cohorts of turtles approximately 25 cm in size (SCLmin). The sex ratio ranged from 0.71 F:1 M in 1975 to 3.57 F:1 M in 2013. The overall sex ratio for 1975–2015 was 1.80 F:1 M; the sex ratio for the most recent 4 years of data (2012–2015) combined was 2.1 F:1 M. Annual sample size (annual cohort) of turtles over the 40 years averaged 64.7 (SD = 19.6, range = 30–98, Table S1: Data set D). The sex ratio became more female dominated over time, increasing 3.8% annually (p < 0.001).
Table S2 summarizes the predicted sex composition of the Bermuda green turtle developmental foraging aggregation over all years and at the end of the study (last 4 years combined) for all four related data sets. It also gives the estimated rate of increase in the sex ratio (feminization) over all years.
Origin of the foraging aggregation
A total of 533 mtDNA control-region sequences generated from individual Bermuda green turtles were assigned to 27 long (740 bp) haplotypes and an additional 69 were assigned to 6 short (~ 500 bp) haplotypes (Table S3). The combined results for 18 short haplotypes (Tables S3, S4) were used in all analyses. The four most common Atlantic haplotypes, Cm-A1, Cm-A3, Cm-A5, and Cm-A8, dominated the Bermuda aggregation across all years. However, the relative mix changed significantly over 5 decades (Fig. S5, Table S5). Cm-A3 predominated in the first 2 decades (Cm-A1 was entirely absent in the 1970–1979 sample). By 1990–1999, Cm-A1 became a dominant haplotype. Haplotypes in the category “other FL/MX” showed the same increase seen in Cm-A1. Two less common haplotypes, Cm-A5 and Cm-A8, declined in relative abundance over the 5-decade period.
Table S4 shows the distribution of short mtDNA haplotypes of green turtles at the 24 rookeries (23 in the Atlantic and one in the Mediterranean) that are available in the published literature and could contribute to the Bermuda foraging aggregation. These were used in the mixed-stock analyses. Results for all years combined indicated possible contributions from all 24 rookeries (Fig. 5) with only minor differences for major contributors between runs with and without a size prior, but with small rookeries showing little to no contribution with a size prior. Mixed-stock results for all years combined (with and without priors) are shown in Table S6.
Estimated proportional contribution of 24 green turtle (Chelonia mydas) rookeries to the Bermuda developmental foraging aggregation based on many-to-one mixed stock analysis (Bolker et al. 2007), genetic samples (n = 600) collected during 1990–2018. Estimated contributions and 95% upper CI are shown for MSA results with and without a rookery size prior. See also Table S6
Five decadal samples for estimation of rookery contributions varied in size from 39 for individuals estimated to have arrived on the Bermuda Platform in 1970–1979 to 195 for individuals arriving in 2000–2009 (Fig. 6, Table S7). Decadal analyses without priors suggested that 18 rookeries were among the 10 most likely to have contributed to the aggregation during at least 1 decade. Contributions from major source rookeries varied significantly among decades. Estimates from samples from the’70 s and’80 s were dominated by turtles from Tortuguero, Costa Rica, differing from those from the’90 s,’00 s and’10 s, in which turtles from beaches in Florida and Mexico provided contributions larger in size than that from Costa Rica.
Temporal variation in estimated rookery contributions to the Bermuda green turtle (Chelonia mydas) developmental foraging aggregation over 5 decades. Estimates and 95% upper CI are given for 18 rookeries that were among the 10 largest contributors (among 24) in at least 1 decade (Table S7). Results are from many-to-one mixed stock analysis (Bolker et al. 2007) by decade of arrival (see methods), without priors. Rookeries are East Central Florida (ECFL), Tortuguero, Costa Rica (TOCR), Scorpion (Alacranes) Reef, Mexico (SRMX), Quintana Roo, Mexico (QRMX), western Bay of Campeche, Mexico (WCMX), Guanahacabibes, Cuba (CUBA), Dry Tortugas, Florida (DTFL), Cayman Islands (CAY), French Guiana (FRGU), Aves Island, Venezuela (AVES), eastern Bay of Campeche, Mexico (ECMX), Southeast Florida (SEFL), Guinea-Bissau (GUBI), Bioko, (BIOKO), Fernando de Noronha, Brazil (FDNB), Cayos Arcas, Mexico, (CAMX), Guadeloupe (GUAD), and Buck Island, US Virgin Islands (BIUS)
Discussion
Sex determinations
We have documented a significant trend toward feminization of a mixed-stock foraging aggregation of immature green turtles. The trend is based on measurement of plasma testosterone titer in turtles, calibrated by a large sample of turtles of known sex (via laparoscopy), and samples collected over 27 years. The size range of laparoscoped green turtles (Chelonia mydas) was nearly the same as that of turtles whose sex was predicted by plasma testosterone titer. The laparoscoped turtles were on average slightly larger, because the laparoscopies in the 1990s were focused on larger turtles, to address the question of maturity status of green turtles in Bermuda (Meylan et al. 2011); laparoscopies conducted in 2008 were performed on smaller individuals to broaden the size range in the study. The size distribution of turtles included in the present sex ratio study represented the entire Bermuda developmental foraging aggregation (Meylan et al. 2011: Fig. 5A, 2022: Fig. 3). All were judged to be immature based on laparoscopy (n = 190) or size (22.3–78.6 cm SCLmin) (see Meylan et al. 2011). Having a single life stage represented proved to be a large advantage for our study because the distributions of male vs female testosterone titers were nearly discrete, and the observed trends associated with size (significantly increasing in males, insignificantly decreasing in females) served to separate the two distributions at larger size classes. Testosterone levels are known to rise in reproductively active green turtles in association with migration and mating (Wibbels et al. 1992; Blanvillain et al. 2011) complicating sex ratio studies in which both immatures and matures are present.
Our logistic regression model correctly predicted, from plasma testosterone titer, the sex of 189 of 190 (99.5%) turtles whose sex had been confirmed by laparoscopy. For individual turtles for which multiple hormone samples were analyzed, there was total agreement in sex predictions for 22 laparoscoped turtles from which 53 testosterone analyses were available, and for 369 non-laparoscoped turtles from which 820 testosterone analyses were available, corroborating the high consistency of the methodologies on which this study is based. Sex predictions were independently confirmed via two other lines of evidence. Five of seven turtles tagged in Bermuda as immatures and known to have subsequently nested in Costa Rica, Florida, or Mexico (Meylan et al. 2011, 2014, Bermuda Turtle Project, unpublished data) had been sampled for plasma testosterone in Bermuda, and all were predicted to be females. In addition, eight turtles sampled for plasma testosterone at capture in Bermuda stranded dead at a later date, and their sex was determined during necropsy; all eight hormone-based sex predictions were confirmed.
Sex ratios
Our data suggest that the aggregation of immature green turtles captured on the foraging grounds in Bermuda during our study consisted of more females than males, and the sex ratio (F/M) increased over time (Fig. 4). These patterns also held for both the data set that was restricted to captures at which plasma testosterone was collected that day (Fig. S4a), and the data set that was restricted to water temperatures of 26–30 °C (Fig. S4c, Table S2).
Previous studies have suggested that exposure of turtles to low water temperatures can confound RIA results. RIAs of green turtles captured during a cold-stunning event in Florida showed no clear separation of values that would allow males and females to be distinguished (Schroeder and Owens 1994). Studying loggerheads (C. caretta) in North Carolina, Braun-McNeill et al. (2007) found that RIA correctly identified the sex of male loggerheads caught in water at temperatures ≥ 24 °C but was unreliable in a second group of turtles captured when temperatures were ≤ 16 °C. Hawkes et al. (2013) reported a positive relationship between testosterone level and both date and sea surface temperature for hawksbills (Eretmochelys imbricata) captured at Anegada, British Virgin Islands. Because our laparoscopies were performed during the summer, plasma testosterone was analyzed for only a few laparoscoped turtles captured in cool water. Therefore, we could not exclude the effect of temperature as a factor in our RIA results. We addressed this issue using a restricted data set with only turtles captured (and testosterone samples collected) at water temperatures of 26–30 °C (Table S1: Data set C). For this restricted data set, the sex ratio (F/M) over all years (1990–2016) and over the last 4 years (2013–2016) combined was nearly identical to that for all water temperatures. Further supporting our assessment that cool water temperatures did not affect the outcome of our RIA analyses, 18 (11 females, 7 males) of the 519 Bermuda turtles for which plasma testosterone titer was measured at captures made at temperatures < 24 °C were recaptured and retested, with 100% agreement in sex prediction. Braun-McNeill et al. (2007) experienced problems with testosterone analyses of loggerheads captured at temperatures ≤ 16 °C but had reliable results for a group captured at ≥ 24 °C. Our lowest water temperature at capture was 17.6 °C.
Comparison of sex ratios by 5- or 10-cm size categories is commonly used in sex ratio studies on foraging grounds to evaluate change over time (e.g., Chaloupka & Limpus 2001; Braun McNeill et al. 2016). But this method was not considered useful for our study because the sex ratio of turtles within specific size categories could have changed over the extensive time frame of the study. Furthermore, in recent years, turtles in small size categories (i.e., those hatched more recently) account for much more of the aggregation than in earlier years of the Bermuda mark–recapture study (Meylan et al. 2022), and they would overly contribute to the sex ratios of small size categories while large size categories would be overly represented by turtles born decades ago. We addressed the issue of change in sex ratio over time by analyzing sex ratios by estimated year of arrival (see methods), which removes size as a factor (Fig. 4b). The growth rate of 2.5 cm yr−1 (Meylan et al. 2011), used to estimate year of arrival, is consistent with growth rates calculated from 9,690 growth increments (including 845 from Bermuda) for green turtles at 30 localities in the Atlantic (Bjorndal et al. 2017). Growth rates by 10-cm category for turtles with sizes represented in Bermuda (i.e., ~ 20–80 cm) ranged from 2.3 to 3.8 cm yr−1 SCLnt (straight carapace length notch-to-tip; Bjorndal et al. 2017: Appendix S3). We expect Bermuda to be on the lower end of that range because of lower winter water temperatures; our use of SCLmin vs. SCLnt would also result in a slightly lower estimate of growth rate. The use of a single growth rate to represent all sizes of turtles does not capture size-related differences, but error is reduced by the fact that turtles were recaptured after different time intervals, some spanning multiple size categories.
When examined by year of arrival, the estimated percent female over the 40 years (1975–2015) and annual change in sex ratio were comparable to those in the other data sets in the study (Table S2). But this latter measure was more variable from year to year (Table S1: Data set D), and the percent female over the most recent 4 years combined was higher than that for annual cohorts of mixed sizes. Green turtles recruiting to Bermuda in 2012–2015 were estimated to be 68.1% female, the highest percent female for the 4 most recent years of data of any of our four data sets (Table S2).
The sex ratio of the C. mydas developmental foraging aggregation in Bermuda varied from year to year over the nearly 3 decades of testosterone sample collection (Table S1: Data sets A, B). Annual variation has been documented on other foraging grounds (e.g., C. mydas: Chaloupka and Limpus 2001; C. caretta: Delgado et al. 2010; Maffucci et al. 2013; Braun McNeill et al. 2016), although in most sex ratio studies, samples have been collected during only one or a few years. For C. mydas, the longest study that had sufficient samples to evaluate annual variation was carried out over 8 years, in the southern Great Barrier Reef in Australia (Chaloupka and Limpus 2001). Sex ratios were tracked separately for juveniles, subadults, and adults and demonstrated high annual variation. In a 7-yr study of C. mydas on foraging grounds in the Southwest Atlantic, no significant changes in sex ratio were observed (de Almeida et al. 2021). Annual variation on the foraging grounds is likely largely due to recruitment-related variation (Casale et al. 2006; Delgado et al. 2010) but may also reflect sex-specific differences in survivorship after recruitment to the foraging grounds (Wibbels 2003) and in emigration (Delgado et al. 2010). The size at which turtles emigrate from the Bermuda Platform has been decreasing since at least as early as 1976 (Meylan et al. 2022); whether males and females emigrate at different sizes has not been determined.
Contributions to the Bermuda foraging aggregation from source rookeries
The benthic developmental foraging aggregation of C. mydas on the Bermuda Platform, like those studied elsewhere in the Atlantic (e.g., Lahanas et al. 1998; Luke et al. 2004; Bass et al. 2006; Foley et al. 2007; Proietti et al. 2009; van der Zee et al. 2019) appears to be a mixed stock from multiple source rookeries. Four common and widespread Atlantic haplotypes (Cm-A1, Cm-A3, Cm-A5 and Cm-A8) are well represented in Bermuda although their relative abundance varied significantly over the decades of our study (Table S5, Fig. S5).
Mixed-stock analysis (MSA) is currently the best tool for examining changes in source contributions that would explain the observed changes in haplotype frequencies in a foraging aggregation. However, results of MSAs are known to have large associated error, in part due to the sharing of common haplotypes among multiple rookeries (Bowen et al. 2007). Also, for our long-term study, the question of changes in the genetic structure of rookeries must be considered. Early work on the genetics of green turtles in the Atlantic recognized the possibility that the genetic structure of rookeries might change over evolutionary time (Enclada et al. 1996). However, subsequent studies failed to detect genetic structuring over shorter time scales at three of the largest green turtle rookeries in the Atlantic Ocean. Bjorndal et al. (2005) failed to detect structuring over two seasons at Tortuguero, Costa Rica; Formia et al. (2007) did not detect structuring over a 4-year period at Ascension Island; and Stahelin et al. (2022) did not detect significant structuring at Melbourne Beach, Florida, between samples collected before 2000 and samples collected 2016–2018. Velez-Zuazo et al. (2008) suggested that natal homing and remigration interval modulation promote temporal genetic stability in sea turtle rookeries.
Given these caveats, mixed-stock analyses of decadal samples from Bermuda (without priors) suggested that 18 of the rookeries included in our study were among the top 10 contributors to the aggregation during at least 1 decade of our study (Fig. 6, Table S7). Contributions from major source rookeries varied markedly with a single significant shift, during the 1990s, from domination by Tortuguero, Costa Rica, to a broader pattern of contribution, with East Central Florida and three rookeries (combined) in Mexico (Quintana Roo, western Bay of Campeche, and Scorpion Reef) exceeding the contribution of Costa Rica during the 2000s and 2010s. The timing of this shift in sources agrees with annual changes observed at Inagua, Bahamas, where, during 1992–2003, the relative contribution from Costa Rica decreased while those from Mexico and Florida increased (Bjorndal and Bolten 2008: Fig. 2). This change in major sources resulted in a significant change in the genetic structure of the Bermuda aggregation (Table S5).
The most likely source rookeries estimated to have contributed to the Bermuda foraging aggregation from 1970 to 2019 (Tables S6, S7) are widely distributed across the Atlantic, spanning 26° of latitude and 105° of longitude (Fig. 7). They belong to both regional management units (RMUs) for green turtles in the Atlantic Ocean (North Atlantic, South Atlantic) (Wallace et al. in press). The source rookeries estimated to have contributed most significantly are in the North Atlantic Distinct Population Segment (DPS) (U.S. Fish and Wildlife Service and National Oceanic and Atmospheric Administration 2016; Seminoff et al. 2015); rookeries in the South Atlantic Distinct Population Segment contributed less. The possibility that the Mediterranean Distinct Population Segment contributes appears remote. Although the common Mediterranean haplotype, Cm-A13, is known from Bermuda (n = 5), detection of this haplotype at low frequency on Florida beaches (Shamblin et al. 2015), a major contributor to the Bermuda aggregation, suggests that this is the likely origin of this haplotype.
Green turtle (Chelonia mydas) rookeries (n = 18) in the Atlantic Ocean that were among the ten largest estimated contributors to the Bermuda developmental foraging aggregation in at least 1 decade between 1970 and 2019 (Table S7). Results are from many-to-one mixed-stock analyses (Bolker et al. 2007) by decade of arrival (see methods), without priors. See Fig. 6 for rookery abbreviations. Bioko not shown
Results of the mixed-stock analyses reported here are corroborated by nesting events involving Bermuda-tagged turtles observed at three rookeries that were estimated to have made major contributions to the aggregation during this study. Three females tagged as immatures in Bermuda nested on the east coast of Florida, two nested at Tortuguero, Costa Rica, and two nested in Quintana Roo, Mexico (Meylan et al. 2014; Bermuda Turtle Project, unpublished data). Contribution to the Bermuda aggregation from nesting beaches along the western Bay of Campeche is supported by the recapture in a Texas estuary of a Bermuda-tagged subadult green turtle with a mitogenomic haplotype (Cm-A1.1.1) associated with that Mexican rookery (Shamblin et al. 2017; Meylan et al. 2020). Contribution from rookeries in the East Caribbean (Aves Island, Buck Island) is supported by the occurrence in Bermuda of the long haplotype Cm-A5.2 (Shamblin et al. 2012). Our results also agree broadly with mixed-stock analyses performed using genetic samples from immature green turtles studied at benthic developmental sites in Florida and the Bahamas. In general, these sites show large contributions from Costa Rica, Florida, and Mexico, with variable contributions from Cuba and relatively small contributions from southern Caribbean and southern and eastern Atlantic rookeries (Lahanas et al. 1998; Bass and Witzell 2000; Foley et al. 2007; Bjorndal and Bolten 2008; Naro-Maciel et al. 2017).
Rookery recovery, or at least population increases, could have played a role in changes observed in the genetic composition of the Bermuda foraging aggregation during 1970–2019, i.e., changes could be due to increasing output from rookeries that had provided minimal input before 1990 but that had recovered sufficiently during the 1990s to significantly change the genetic makeup of the Bermuda aggregation. Conservation efforts appear to have increased green turtle populations in East Central Florida, Tortuguero, western Bay of Campeche, Quintana Roo, Aves Island, French Guiana, and Guinea-Bissau (Zurita et al. 2003; Chaloupka et al. 2008; Catry et al. 2002; Azanza et al. 2013; Seminoff et al. 2015; Garcia-Cruz et al. 2015; Broderick and Patricio 2019; Valdivia et al. 2019; Guzmán-Hernández et al. 2022; Restrepo et al. 2023). The timing of nesting increases at rookeries that are likely to be contributing has varied. Tortuguero has seen significant conservation effort since 1955, and increases were observed as early as the 1970s (Seminoff et al. 2015: Figs. 5.4). An increasing trend continued through 2008 after which the trend became negative (Restrepo et al. 2023). Nesting increases at other rookeries estimated to be contributing began in later decades (Seminoff et al. 2015: Figs. 5.7 and 7.2; Garcia-Cruz et al. 2015: Fig. 3D; Valdivia et al. 2019: Fig. 4; Zurita et al. 2003: Fig. 3; Guzmán-Hernández et al. 2022: Table 1).
The magnitude of population increase has varied across rookeries. Annual increases have been as high as 75.7% for beaches that make up Florida’s Index Nesting Beach Survey (which includes the Archie Carr National Wildlife Refuge) during 1989–2016 (Valdivia et al. 2019). Estimates of the annual increase in number of nests at 11 beaches in Mexico bordering the Gulf of Mexico and the Caribbean Sea ranged from 4.2 to 16.5% for different intervals during 1990–2019 (Guzmán-Hernández et al. 2022). The annual increase was 2.9–14% at five beaches studied in French Guiana, Suriname, and Guinea-Bissau for different time intervals during 1988–2017 (Broderick and Patricio 2019: Supplemental Table 1).
Given the increases in the level of hatchling production at rookeries with increasing populations, one might expect to see changes in the sex ratio of turtles recruiting to Bermuda due to differences in the sex ratio of hatchlings produced at those rookeries. Primary sex ratio has been estimated at five of the rookeries that are estimated to contribute to the Bermuda developmental foraging aggregation (Table 3A). Studies have ranged from 1 to 8 yrs, and sample sizes from 27 to 687 nests and 131 to 1,100 hatchlings (Table 3A). Percent female varied widely, from 10.1% to 90%, among these studies, the highest recorded in Florida and Cuba, and the lowest at Tortuguero, although results of the studies at Tortuguero in 1980 and 1986–1988 differed significantly (10.1 and 40.6% female vs. 67% female (Spotila et al. 1987; Horikoshi 1992). Horikoshi (1992) attributed the low value in 1986 to above-average rainfall (3871 mm in 6 mo) and extreme events (141–178 mm 24 h−1) that lowered sand temperatures and concluded that the sex ratio recorded that year may have been atypical. Rainfall, including extreme events, has been recognized as a significant factor in studies of primary sex ratios involving multiple sea turtle species (Godfrey et al. 1996; Houghton et al. 2007; Lolavar and Wyneken 2015, 2017, 2020; Laloë et al. 2021). No recent evaluation of the sex ratio of green turtles being produced at Tortuguero is available.
We note that Florida and Cuba rookeries both appear to have had highly female-skewed primary sex ratios in studies conducted from 1980 to 2018 (Table 3a), a period for which our genetic results suggest that those rookeries were contributing more hatchlings to the Bermuda aggregation than they had contributed earlier. Primary sex ratios of loggerheads in Florida (which use many of the same beaches as green turtles) have been reported to be highly female skewed (Mrosovsky and Provancha 1989, 1992; Wyneken and Lolavar 2015). We are unaware of published studies of the primary sex ratio of C. mydas in Mexico, but hawksbill turtles at three nesting beaches in Campeche and the Yucatan were predicted, based on incubation temperature, to have a female-dominated sex ratio (Flores-Aguirre et al. 2020). The pivotal temperatures estimated for green turtles, hawksbills, and loggerheads fall within a narrow range (Wibbels 2003). It is possible that increased contributions from female-skewed rookeries may account for at least some of the change in sex ratio observed in the green turtle aggregation in Bermuda but the trend is nearly linear while rookery abundance trends (and potential hatchling contributions) have varied strongly in timing and magnitude. Analysis of genetic structure suggests a single significant change in major sources during the middle decade of our study.
Feminization
In the sea turtle literature, the term feminization has been used to describe the pattern of increasingly female-skewed primary sex ratios at nesting beaches (Fuentes et al. 2010; Patricio et al. 2021, and references therein). Yet we are unaware of nesting beach studies that document trends in empirically measured hatchling sex ratios (e.g., increasing female percent over multiple seasons) or nest incubation temperatures. Rather, feminization has been predicted primarily by models using proxies for direct evaluation of sex ratios, such as the duration of incubation or air, sea, or sand temperature during the middle third of incubation, in concert with pivotal temperature curves (Wyneken and Lolavar 2015). Fuentes et al. (2010) found no significant change in estimated incubation temperatures at seven key green turtle rookeries in Australia over 18 years (1990–2008). At Australian green turtle rookeries, Jensen et al. (2018) extrapolated primary sex ratios over time using sex ratios of immature turtles captured on a foraging ground using size class (as a proxy for age), growth rates, and genetic information. de Almeida et al. (2021) used sex ratios of green turtles that stranded in Brazil over 7 yrs as a proxy for foraging-ground sex ratios and used genetic information to relate those ratios to primary sex ratios at contributing rookeries.
Here, we report significant feminization of a foraging aggregation of immature green turtles in Bermuda based on data collected over 27 years. In terms of time frame, the most similar study involving immature C. mydas that suggested an increasing female-skewed sex ratio was a comparison of sex ratios measured at the same site in Australia in 1983–1984 (Limpus and Reed 1985) and again in 1985–1992 (Chaloupka and Limpus 2001), which, in the first period, showed equal male-to-female ratios and in the second, showed female-skewed ratios. Sanchez (2013) examined sex ratios of immature green turtles at three sites in eastern Florida (2011–2012) and reported a ratio skewed toward females (3.2F:1 M) that did not differ from the ratio found in an investigation in the late 1990s but that did differ from a pilot study conducted in the late 1980s. In contrast, shorter-term studies, conducted 20 years apart, of sex ratios of immature green turtles at the Yaeyama Islands, Japan, showed no significant change (Kameda et al. 2019). A 7-year study of green turtles on foraging grounds in Brazil detected no significant change in sex ratio (de Almeida et al. 2021).
Our results show that, as of 2018, the Bermuda developmental foraging aggregation was only moderately dominated by females relative to other aggregations of immature C. mydas reported in the literature. Relatively few studies of secondary sex ratios of C. mydas have been conducted in the Atlantic (Table 3b); the majority in this ocean basin have been conducted on loggerheads and hawksbills (see Arendt et al. 2021). The percent female in Atlantic feeding-ground studies of C. mydas ranged from 58.6 to 84.8% (Table 3b), which is comparable to percent female ranging from 51 to 80.8% reported for green turtles from outside the Atlantic as reviewed by Allen et al. (2015). Studies published after that review include those by Pilcher et al. (2015), Godoy et al. (2016), Jensen et al. (2016, 2018), and Kameda et al. (2019), all of which were conducted in the Indian Ocean or the Pacific Ocean. Sex ratios in these later studies showed an overall moderate skew toward females, although an extreme skew (99.1% of juveniles, 99.8% of subadults) toward females was observed in the Howick Islands (Australia) for animals originating from the northern Great Barrier Reef (Jensen et al. 2018).
The significant and sustained trend toward an increasingly female-skewed sex ratio in the Bermuda aggregation over multiple decades merits continued attention. Sex ratios (and associated trends) reported at the foraging grounds are considered more likely to be accurate than those at the nesting beaches because they condense many years of highly variable and difficult-to-measure hatchling production (Wibbels et al. 1987a, 1991; Wibbels 2003; Jensen et al. 2018). In the case of Bermuda, the trend towards an increasingly female-dominated sex ratio can have important consequences because it reflects the output of multiple rookeries that span a wide geographic area and that are of critical importance to recovery of this species in the West Atlantic (Seminoff et al. 2015).
Feminization of the Bermuda C. mydas foraging aggregation is likely due to a combination of factors. Changes in rookery contributions and strong population increases at certain rookeries may partly explain the increasing trend in the sex ratio (F/M). But the steady rate of increase over decades and the increasing female percentage of arriving cohorts are consistent with the impact of global warming at source rookeries. Other hypotheses, such as the feminizing effects of environmental contaminants that disrupt endocrine systems, should also be considered (see review in Barraza et al. 2021). Barraza et al. (2021) concluded that evidence of contaminants affecting sea turtle sex ratios was inconclusive but the topic warranted further research.
Relevant to identifying the cause of the increasingly female-skewed sex ratio in the green turtle aggregation in Bermuda, we offer the observation that the steady increase (Fig. 4), suggests a linear driver, likely temperature. The pattern is more consistent with global warming at contributing rookeries than with changes in the aggregation’s genetic composition. We saw no evidence of a major shift in sex ratios resulting from the single significant change in genetic composition that occurred during the 1990s, i.e., the increase in the sex ratio (F/M) appears to be taking place continuously over time and across a broad geographic area.
Furthermore, cohorts of turtles recruiting to Bermuda (at the size of 25 cm SCLmin) during 1975–2015 became increasingly dominated by females. This pattern is consistent with the hypothesis that global warming is the cause (Limpus et al. 2009) and has been noted in other studies. Sanchez (2013), at study sites in eastern Florida, observed that the probability that an immature green turtle captured in their study would be female was inversely related to its size. Jensen et al. (2018), studying immature green turtles captured on the Howick Islands feeding grounds, also found a negative relationship between size and probability of a turtle being female. Using growth rates and genetic information, they related highly female-skewed sex ratios to beaches that had experienced severely warm weather during the period in which they would have hatched.
It remains largely unknown how secondary sex ratios of marine turtles translate to adult or operational sex ratios over their many decades of life. Operational sex ratios for populations of four species are reviewed by Santidrián Tomillo and Spotila (2020); the two estimates for green turtles are from the Mediterranean and are both skewed toward males.
Considerable controversy surrounds the threat to marine turtles posed by highly female-skewed sex ratios that have been estimated on nesting beaches. Wyneken and Lolavar (2015) argue that knowledge of primary sex ratios based on nesting beach studies remains highly uncertain, and they suggest that there is resilience in the system that has not yet been considered. Hays et al. (2010, 2014, 2022) suggest that differential reproductive periodicity (i.e., male turtles breed more often than females) and polyandry could help compensate for sex ratios that are highly skewed toward females. If growth rates of males and females are equal, maturation of males at a smaller size, at least in some populations (Godley et al. 2002; Meylan et al. 2011; Figgener et al. 2022), could also provide a buffer against extremely female-skewed sex ratios in populations (Hays et al. 2022). Modeling by Arendt et al. (2021) suggests that the neritic recruitment age for loggerheads in the eastern United States was influenced by the Atlantic Multidecadal Oscillation, resulting in the synchrony of adult male and adult female abundances across decades. They concluded that the population-level sex ratio was more plastic than would be predicted solely by nesting data. Some have posited that, to an extent, a skew toward females in species with temperature-dependent sex determination is an advantage in a warming climate (Santidrián Tomillo and Spotila 2020; Hays et al. 2014, 2017), but it is generally acknowledged that there may be a tipping point below which the number of males would be too small to support reproduction (e.g., Santidrián Tomillo et al. 2015; Hays et al. 2017; Jensen et al. 2018).
Long-term monitoring of sex ratios in marine turtles should be broadly implemented to detect significant trends that may affect reproductive rates (Delgado et al. 2010; Jensen et al. 2016, 2018). We concur with previous authors (e.g., Wibbels 2003) that secondary sex ratios likely provide a better window into the understanding of sex ratios of the general population than do primary sex ratios. In addition, the logistics of sampling large numbers of juvenile turtles is far simpler than those of sampling adults. We have good confidence that the measurement of plasma testosterone titer is a reliable empirical method to predict sex. To avoid the complications involved with working with radioactive materials (i.e., tritium), RIA analysis for sex ratios of marine turtles is gradually being replaced by the ELISA, or enzyme-linked immunosorbent assay (Allen et al. 2015; Jensen et al. 2018), which can also be used to measure plasma testosterone. Plasma testosterone samples continue to be collected by the Bermuda Turtle Project providing a continuing opportunity to evaluate sex ratios of Northwest Atlantic populations of the green turtle.
Data availability
Data used in this study are presented in the published article, supplementary files, the FWC Digital Library https://f50006a.eos-intl.net/F50006A/OPAC/Details/Record.aspx?BibCode=5724420 or are available from the corresponding author upon reasonable request.
Change history
29 March 2024
A Correction to this paper has been published: https://doi.org/10.1007/s00227-024-04428-z
05 March 2024
A Correction to this paper has been published: https://doi.org/10.1007/s00227-024-04409-2
References
Abreu-Grobois A, Horrocks J, Formia A, Dutton P, LeRoux R, Velez-Zuazo X, Soares L, Meylan P (2006) New mtDNA d-loop primers which work for a variety of marine turtle species may increase the resolution of mixed stock analyses. In: Frick M, Panagopoulou A, Rees A, Williams K (eds) 26th annual symposium on Sea Turtle Biology and Conservation, book of abstracts. International Sea Turtle Society, Athens, p 179
Allen CD, Robbins MN, Eguchi T, Owens DW, Meylan AB, Meylan PA, Kellar NM, Schwenter JA, Nollens HH, LeRoux RA, Dutton PH, Seminoff JA (2015) First assessment of the sex ratio for an East Pacific green sea turtle foraging aggregation: validation and application of a testosterone ELISA. PLoS ONE 10:e0138861. https://doi.org/10.1371/journal.pone.0138861
Arendt MD, Schwenter JA, Owens DW, Valverde RA (2021) Theoretical modeling and neritic monitoring of loggerhead Caretta [Linnaeus, 1758] sea turtle sex ratio in the southeast United States do not substantiate fears of a male-limited population. Glob Chang Biol 27:4849–4859. https://doi.org/10.1111/gcb.15808
Ascani F, Van Houtan KS, Di Lorenzo E, Polovina JJ, Jones TT (2016) Juvenile recruitment in loggerhead sea turtles linked to decadal changes in ocean circulation. Glob Chang Biol 22:3529–3538. https://doi.org/10.1111/gcb.13331
Avens L, Goshe LR, Harms CA, Anderson ET, Hall AG, Cluse WM, Godfrey MH, Braun-McNeill J, Stacy B, Bailey R, Lamont MM (2012) Population characteristics, age structure, and growth dynamics of neritic juvenile green turtles in the northeastern Gulf of Mexico. Mar Ecol Prog Ser 458:213–229. https://doi.org/10.3354/meps09720
Azanza JA, Ibarra ME, Gonzalez G, Abreu FA, Eckert K, Espinosa G, Oyama K (2013) Nesting ecology of Chelonia mydas (Testudines: Cheloniidae) on the Guanahacabibes Peninsula. Cuba Rev Biol Trop 61:1935–1945
Barraza AD, Finlayson KA, Leusch FD, van de Merwe JP (2021) Systematic review of reptile reproductive toxicology to inform future research directions on endangered or threatened species, such as sea turtles. Environ Pollut 286:117470. https://doi.org/10.1016/j.envpol.2021.117470
Bass AL, Witzell WN (2000) Demographic composition of immature green turtles (Chelonia mydas) from the East Central Florida coast: evidence from mtDNA markers. Herpetologica 56:357–367
Bass AL, Epperly SP, Braun J, Owens DW, Patterson RM (1998) Natal origin and sex ratios of foraging sea turtles in the Pamlico-Albemarle Estuarine Complex. In: Epperly SP, Braun J (eds) Proceedings of the 17th annual sea turtle symposium NOAA Tech Memo NMFS-SEFSC-415, pp 137–138
Bass AL, Epperly SP, Braun-McNeill J (2006) Green turtle (Chelonia mydas) foraging and nesting aggregations in the Caribbean and Atlantic: impact of currents and behavior on dispersal. J Hered 97:346–354. https://doi.org/10.1093/jhered/esl004
Benaglia T, Chauveau D, Hunter DR, Young D (2009) mixtools: an R package for analyzing finite mixture models. J Stat Softw 32:1–29
Bjorndal KA, Bolten AB (2008) Annual variation in source contributions to a mixed stock: implications for quantifying connectivity. Mol Ecol 17:2185–2193. https://doi.org/10.1111/j.1365-294X.2008.03752.x
Bjorndal KA, Bolten AB, Troeng S (2005) Population structure and genetic diversity in green turtles nesting at Tortuguero, Costa Rica, based on mitochondrial DNA control region sequences. Mar Biol 147:1449–1457. https://doi.org/10.1007/s00227-005-0045-y
Bjorndal KA, Bolten AB, Chaloupka M, Saba VS, Bellini C, Marcovaldi MA, Santos JB, Bortolon LFW, Meylan AB, Meylan PA, Gray JA, Hardy R, Brost B, Bresette M, Gorham JC, Connett S, Crouchley BVS, Dawson M, Hayes D et al (2017) Ecological regime shift drives declining growth rates of sea turtles throughout the West Atlantic. Glob Chang Biol 23:4556–4568. https://doi.org/10.1111/gcb.13712
Blanvillain G, Owens DW, Kuchling G (2011) Hormones and reproductive cycles in turtles. In: Hormones and reproduction of vertebrates, Elsevier, Inc. pp. 277–303.
Bolker B, Okuyama T, Bjorndal K, Bolten A (2003) Sea turtle stock estimation using genetic markers: accounting for sampling error of rare genotypes. Ecol Appl 13:763–775. https://doi.org/10.1890/1051-0761(2003)013%5B0763:STSEUG%5D2.0.CO;2
Bolker B, Okuyama T, Bjorndal KA, Bolten AB (2007) Incorporating multiple mixed stocks in mixed stock analysis: “many-to-many” analyses. Mol Ecol 16:685–695. https://doi.org/10.1111/j.1365-294X.2006.03161.x
Bolker B (2015) Mixstock: mixed stock analysis in R. R package version 0.9.9
Bolten AB (1999) Techniques for measuring sea turtles. In: Eckert KL, Bjorndal KA, Abreu-Grobois FA, Donnelly M (eds) Research and management techniques for the conservation of sea turtles. IUCN/SSC Marine Turtle Specialist Group Publication No. 4, pp 110–114
Bolten AB, Bjorndal KA, Grumbles JS, Owens DW (1992) Sex ratio and sex-specific growth rates of immature green turtles, Chelonia mydas, in the southern Bahamas. Copeia 1992:1098–1103. https://doi.org/10.2307/1446647
Bowen BW, Grant WS, Hillis-Starr Z, Shaver DJ, Bjorndal KA, Bolten AB, Bass AL (2007) Mixed-stock analysis reveals the migrations of juvenile hawksbill turtles (Eretmochelys imbricata) in the Caribbean Sea. Mol Ecol 16:49–60. https://doi.org/10.1111/j.1365-294X.2006.03096.x
Braun McNeill J, Avens L, Goodman Hall A, Goshe LR, Harms CA, Owens DW (2016) Female-bias in a long-term study of a species with temperature-dependent sex determination: Monitoring sex ratios for climate change research. PLoS ONE 11:e0160911. https://doi.org/10.1371/journal.pone.0160911
Braun-McNeill J, Epperly SP, Owens DW, Avens L, Williams E, Harms CA (2007) Seasonal reliability of testosterone radioimmunoassay (RIA) for predicting sex ratios of juvenile loggerhead (Caretta caretta) turtles. Herpetologica 63:275–284
Broderick A, Patricio A (2019) Chelonia mydas (South Atlantic subpopulation). The IUCN red list of threatened species 2019: e.T142121866A142086337. https://doi.org/10.2305/IUCN.UK.2019-2.RLTS.T142121866A142086337.en
Brown LD, Cai TT, DasGupta A (2001) Interval estimation for a binomial proportion (with discussion). Stat Sci 16:101–133
Bull JJ (1983) Evolution of sex determining mechanisms. Benjamin/Cummings, Menlo Park, CA
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information–theoretic approach. In: Burnham KP, Anderson DR (eds) Model selection and multimodel inference. Springer, New York, pp 98–100
Calderón-Peña R, Betancourt-Avila R, Rodríguez-Fajardo E, Martínez-González Y, Azanza-Ricardo J (2020) Sex ratio of the green sea turtle Chelonia mydas (Testudines: Cheloniidae) hatchlings in the Guanahacabibes Peninsula. Cuba. Rev Biol Trop 68:777–784. https://doi.org/10.15517/rbt.v68i3.39033
Campbell CL, Lagueux CJ (2005) Survival probability estimates for large juvenile and adult green turtles (Chelonia mydas) exposed to an artisanal marine turtle fishery in the western Caribbean. Herpetologica 61:91–103. https://doi.org/10.1655/04-26
Casale P, Lazar B, Pont S, Tomás J, Zizzo N, Alegre F, Badillo J, Di Summa A, Freggi D, Lackovic G, Raga JA, Lucio Rositani L, Nikola Tvrtkovic N (2006) Sex ratios of juvenile loggerhead sea turtles Caretta in the Mediterranean Sea. Mar Ecol Prog Ser 324:281–285. https://doi.org/10.3354/meps324281
Catry P, Barbosa C, Indjai B, Almeida A, Godley BJ, Vié J-C (2002) First census of the green turtle at Poilão, Bijagós Archipelago, Guinea-Bissau: the most important nesting colony on the Atlantic coast of Africa. Oryx 36:400–403. https://doi.org/10.1017/S0030605302000765
Chaloupka M, Limpus C (2001) Trends in the abundance of sea turtles resident in southern Great Barrier Reef waters. Biol Conserv 102:235–249. https://doi.org/10.1016/S0006-3207(01)00106-9
Chaloupka M, Bjorndal KA, Balazs GH, Bolten AB, Ehrhart LM, Limpus CJ, Suganuma H, Troëng S, Yamaguchi M (2008) Encouraging outlook for recovery of a once severely exploited marine megaherbivore. Glob Ecol Biogeogr 17:297–304. https://doi.org/10.1111/j.1466-8238.2007.00367.x
Corpet F (1988) Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res 16:10881–10890. https://doi.org/10.1093/nar/16.22.10881
Coyotupa J, Parlow AF, Abraham GE (1972) Simultaneous radioimmunoassay of plasma testosterone and dihydrotestosterone. Anal Lett 5:329. https://doi.org/10.1080/00032717208066108
de Almeida JPFA, dos Santos RG, Mott T (2021) Sex ratios and natal origins of green turtles from feeding grounds in the Southwest Atlantic Ocean. ICES J Mar Sci 78:1840–1848. https://doi.org/10.1093/icesjms/fsab093
Delgado C, Canario AVM, Dellinger T (2010) Sex ratios of loggerhead sea turtles Caretta during the juvenile pelagic stage. Mar Biol 157:979–990. https://doi.org/10.1007/s00227-009-1378-8
Dutton PH (1996) Methods for collection and preservation of samples for sea turtle genetic studies. In: Bowen BW, Witzell WN (eds) Proceedings of the international symposium on sea turtle conservation genetics. NOAA Tech Memo. NMFS-SEFSC-396, pp 17–24
Enclada SE, Lahanas PN, Bjorndal KA, Bolten AB, Miyamoto MM, Bowen BW (1996) Phylogeography and population structure of the Atlantic and Mediterranean green turtle Chelonia mydas: a mitochondrial DNA control region sequence assessment. Mol Ecol 5:473–483. https://doi.org/10.1111/j.1365-294X.1996.tb00340.x
Excoffier L, Laval G, Schneider S (2005) Arlequin ver. 3.0: an integrated software package for population genetics data analysis. Evol Bioinform Online 1:47–50
Figgener C, Bernardo J, Plotkin PT (2022) Marine turtles are only minimally sexually size dimorphic, a pattern that is distinct from most nonmarine aquatic turtles. Ecol Evol 12:e8963. https://doi.org/10.1002/ece3.8963
Flores-Aguirre CD, Díaz-Hernández V, Salgado Ugarte IH, Sosa Caballero LE, Méndez de la Cruz FR (2020) Feminization tendency of hawksbill turtles (Eretmochelys imbricata) in the western Yucatán Peninsula, Mexico. Amphib Reptile Conserv 14:190–202
Foley AM, Singel KE, Dutton PH, Summers TM, Redlow AE, Lessman J (2007) Characteristics of a green turtle (Chelonia mydas) assemblage in northwestern Florida determined during a hypothermic stunning event. Gulf Mex Sci 25:131–145. https://doi.org/10.18785/goms.2502.04
Formia A, Broderick AC, Glen F, Godley BJ, Hays GC, Bruford MW (2007) Genetic composition of the Ascension Island green turtle rookery based on mitochondrial DNA: implications for sampling and diversity. Endanger Species Res 3:145–158. https://doi.org/10.3354/esr003145
Fuentes MMPB, Hamann M, Limpus CJ (2010) Past, current and future thermal profiles of green turtle nesting grounds: Implications from climate change. J Exp Mar Biol Ecol 383:56–64. https://doi.org/10.1016/j.jembe.2009.11.003
Fuentes MM, Monsinjon J, Lopez M, Lara P, Santos A, Marcovaldi MA, Girondot M (2017) Sex ratio estimates for species with temperature-dependent sex determination differ according to the proxy used. Ecol Modell 365:55–67. https://doi.org/10.1016/j.ecolmodel.2017.09.022
García-Cruz MA, Lampo M, Peñaloza CL, Kendall WL, Solé G, Rodríguez-Clark KM (2015) Population trends and survival of nesting green sea turtles Chelonia mydas on Aves Island, Venezuela. Endanger Species Res 29:103–116. https://doi.org/10.3354/esr00695
Gelman A, Rubin DB (1992) Inference from iterative simulation using multiple sequences. Stat Sci 7:457–511. https://doi.org/10.1214/ss/1177011136
Gibbons JW, Scott DE, Ryan TJ, Buhlmann KA, Tuberville TD, Metts BS, Greene JL, Mills T, Leiden Y, Poppy S, Winne CT (2000) The global decline of reptiles, déjà vu amphibians. Bioscience 50:653–666. https://doi.org/10.1641/0006-3568(2000)050%5B0653:TGDORD%5D2.0.CO;2
Godfrey MH, Mrosovsky N, Barreto R (1996) Estimating past and present sex ratios of sea turtles in Suriname. Can J Zool 74:267–277. https://doi.org/10.1139/z96-033
Godley BJ, Broderick AC, Frauenstein R, Glen F, Hays GC (2002) Reproductive seasonality and sexual dimorphism in green turtles. Mar Ecol Prog Ser 226:125–133. https://doi.org/10.3354/meps226125
Godoy DA, Smith ANH, Limpus C, Stockin KA (2016) The spatio-temporal distribution and population structure of green turtles (Chelonia mydas) in New Zealand. N Z J Mar Freshwater Res 50:549–565. https://doi.org/10.1080/00288330.2016.1182034
Gorham JC, Bresette MJ, Guertin JR, Shamblin BM, Nairn CJ (2016) Green turtles (Chelonia mydas) in an urban estuary system: Lake Worth Lagoon, Florida. Fla Sci 79:14–27
Guzmán-Hernández V, del Monte-Luna P, López-Castro MC, Uribe-Martínez A, Huerta-Rodríguez P, Gallegos-Fernández SA, Azanza-Ricardo J, Martínez-Portugal RC, Barragán-Zepeda AK, Quintana Pali GP, Martín-Viaña YF, Gómez-Ruíz PA, Acosta-Sánchez HH, López-Hernández M, Castañeda-Ramírez DG, Ortíz A, Cuevas E (2022) Recovery of green turtle populations and their interactions with coastal dune as a baseline for an integral ecological restoration. Acta Bot Mex 129:e1954. https://doi.org/10.21829/abm129.2022.1954
Harrell FE Jr with contributions from Charles Dupont et al. (2021) Hmisc: Harrell miscellaneous. R package version 4.5–0. https://CRAN.R-project.org/package=Hmisc
Hawkes LA, McGowan A, Godley BJ, Gore S, Lange A, Tyler CR et al (2013) Estimating sex ratios in Caribbean hawksbill turtles: testosterone levels and climate effects. Aquat Biol 18:9–19. https://doi.org/10.3354/ab00475
Hays GC, Fossette S, Katselidis KA, Schofield G, Gravenor MB (2010) Breeding periodicity for male sea turtles, operational sex ratios, and implications in the face of climate change. Conserv Biol 24:1636–1643. https://doi.org/10.1111/j.1523-1739.2010.01531.x
Hays GC, Mazaris AD, Schofield G (2014) Different male vs. female breeding periodicity helps mitigate offspring sex ratio skews in sea turtles. Front Mar Sci 1:43. https://doi.org/10.3389/fmars.2014.00043
Hays GC, Mazaris AD, Schofield G, Laloe JO (2017) Population viability at extreme sex-ratio skews produced by temperature-dependent sex determination. Proc Royal Soc B Biol Sci 284:20162576. https://doi.org/10.1098/rspb.2016.2576
Hays GC, Shimada T, Schofield G (2022) A review of how the biology of male sea turtles may help mitigate female-biased hatchling sex ratio skews in a warming climate. Mar Biol 169:89. https://doi.org/10.1007/s00227-022-04074-3
Hopkins-Murphy S, Owens DW, Murphy T (2003) Ecology of immature loggerheads on foraging grounds and adults in internesting habitat in the eastern United States. In: Bolten A, Witherington B (eds) Loggerhead sea turtles. Smithsonian Books, Washington, pp 79–92
Horikoshi K (1992) Egg survivorship and primary sex ratios of green turtles, Chelonia mydas, at Tortuguero, Costa Rica. Dissertation, University of Florida
Houghton JDR, Myers AE, Lloyd C, King RS, Isaacs C, Hays GC (2007) Protracted rainfall decreases temperature within leatherback turtle (Dermochelys coriacea) clutches in Grenada, West Indies: ecological implications for a species displaying temperature dependent sex determination. J Exp Mar Biol Ecol 345:71–77. https://doi.org/10.1016/j.jembe.2007.02.001
Janzen FJ (1994) Climate change and temperature-dependent sex determination in reptiles. Proc Natl Acad Sci USA 91:7487–7490. https://doi.org/10.1073/pnas.91.16.7487
Jensen MP, Pilcher N, FitzSimmons NN (2016) Genetic markers provide insight on origins of immature green turtles Chelonia mydas with biased sex ratios at foraging grounds in Sabah, Malaysia. Endanger Species Res 31:191–201. https://doi.org/10.3354/esr00763
Jensen MP, Allen CD, Eguchi T, Bell IP, LaCasella EL, Hilton WA, Hof CAM, Dutton PH (2018) Environmental warming and feminization of one of the largest sea turtle populations in the world. Curr Biol 28:154–159. https://doi.org/10.1016/j.cub.2017.11.057
Kameda K, Suzuki K, Kuroyanagi K, Takase M, Matsuda K, Noda J (2019) Comparison of green turtle Chelonia mydas sex ratios at two time-points over 20 years at a foraging ground in Yaeyama Islands, Ryukyu Archipelago, Japan. Endanger Species Res 38:127–134. https://doi.org/10.3354/esr00944
Lagueux CJ (1998) Marine turtle fishery of Caribbean Nicaragua: human use patterns and harvest trends. Dissertation, University of Florida
Lahanas PN, Bjorndal KA, Bolten AB, Encalada SE, Miyamoto MM, Valverde RA, Bowen BW (1998) Genetic composition of a green turtle (Chelonia mydas) feeding ground population: evidence for multiple origins. Mar Biol 130:345–352. https://doi.org/10.1007/s002270050254
Laloë JO, Tedeschi JN, Booth DT, Bell I, Dunstan A, Reina RD, Hays GC (2021) Extreme rainfall events and cooling of sea turtle clutches: implications in the face of climate warming. Ecol Evol 11:560–565. https://doi.org/10.1002/ece3.7076
Lee AM, Owens DW (2008) Correction of an error in the testosterone radioimmunoassay for evaluating sex ratios and reproductive status of sea turtles. In: Kalb H, Rohde A, Gayheart K, Shanker K (eds) Proceedings of the 25th annual symposium on sea turtle biology and conservation. NOAA Tech Memo. NMFS-SEFSC-582, pp 63–64
Limpus CJ, Reed PC (1985) The green turtle (Chelonia mydas) in Queensland: a preliminary description of the population structure in a coral reef feeding ground. In: Grigg GC, Shine R, Ehmann H (eds) Biology of the Australasian frogs and reptiles. Royal Zoological Society of New South Wales, Sydney, pp 47–52
Limpus CJ, Bell IP, Miller JD (2009) Mixed stocks of green turtles foraging on Clack Reef, northern Great Barrier Reef identified from long-term tagging studies. Mar Turt Newsl 123:3–5
Lockley EC, Eizaguirre C (2021) Effects of global warming on species with temperature-dependent sex determination: bridging the gap between empirical research and management. Evol Appl 14:2361–2377. https://doi.org/10.1111/eva.13226
Lolavar A, Wyneken J (2015) Effect of rainfall on loggerhead turtle nest temperatures, sand temperatures and hatchling sex. Endanger Species Res 28:235–247. https://doi.org/10.3354/esr00684
Lolavar A, Wyneken J (2017) Experimental assessment of the effects of moisture on loggerhead sea turtle hatchling sex ratios. Zool 123:64–70. https://doi.org/10.1016/j.zool.2017.06.007
Lolavar A, Wyneken J (2020) The impact of sand moisture on the temperature–sex ratio responses of developing loggerhead (Caretta caretta) sea turtles. Zool 138:125739. https://doi.org/10.1016/j.zool.2019.125739
Luke K, Horrocks JA, LeRoux RA, Dutton PH (2004) Origins of green turtle (Chelonia mydas) feeding aggregations around Barbados, West Indies. Mar Biol 144:799–805. https://doi.org/10.1007/s00227-003-1241-2
Maffucci F, D’Angelo I, Hochscheid S, Ciampa M, De Martino G, Travaglini A, Treglia G, Bentivegna F (2013) Sex ratio of juvenile loggerhead turtles in the Mediterranean Sea: Is it really 1:1? Mar Biol 160:1097–1107. https://doi.org/10.1007/s00227-012-2160-x
Meylan PA, Meylan AB, Gray JA (2011) The ecology and migrations of sea turtles, 8. Tests of the developmental habitat hypothesis. Bull Am Mus Nat Hist 357:1–70. https://doi.org/10.1206/357.1
Meylan A, Iturbe I, Zurita J, Harrison E, Gray J, Meylan P (2014) Green turtles tagged in benthic developmental habitat in Bermuda nest in Mexico and Costa Rica. Mar Turt Newsl 141:15–17
Meylan A, Hardy RF, Meylan P, Gray J, Shamblin B, Frandsen HR, Shaver DJ (2020) Chelonia mydas (Green Sea Turtle) Developmental Migration. Herpetol Rev 51:107
Meylan PA, Hardy RF, Gray JA, Meylan AB (2022) A half-century of demographic changes in a green turtle (Chelonia mydas) foraging aggregation during an era of seagrass decline. Mar Biol 169:1–20. https://doi.org/10.1007/s00227-022-04056-5
Mitchell NJ, Allendorf FW, Keall SN, Daugherty CH, Nelson NJ (2010) Demographic effects of temperature-dependent sex determination: Will tuatara survive global warming? Glob Chang Biol 16:60–72. https://doi.org/10.1111/j.1365-2486.2009.01964.x
Monzón-Argüello C, López-Jurado LF, Rico C, Marco A, López P, Hays GC, Lee PL (2010) Evidence from genetic and Lagrangian drifter data for transatlantic transport of small juvenile green turtles. J Biogeogr 37:1752–1766. https://doi.org/10.1111/j.1365-2699.2010.02326.x
Mrosovsky N (1994) Sex ratios of sea turtles. J Exp Zool 270:16–27. https://doi.org/10.1002/jez.1402700104
Mrosovsky N, Provancha J (1989) Sex ratio of loggerhead sea turtles hatching on a Florida beach. Can J Zool 67:2533–2539. https://doi.org/10.1139/z89-358
Mrosovsky N, Provancha J (1992) Sex ratio of hatchling loggerhead sea turtles: data and estimates from a 5-year study. Can J Zool 70:530–538. https://doi.org/10.1139/z92-080
Mrosovsky N, Dutton PH, Whitmore CP (1984) Sex ratios of two species of sea turtle nesting in Suriname. Can J Zool 62:2227–2239. https://doi.org/10.1139/z84-324
Naro-Maciel E, Hart KM, Cruciata R, Putman NF (2017) DNA and dispersal models highlight constrained connectivity in a migratory marine megavertebrate. Ecography 40:586–597. https://doi.org/10.1111/ecog.02056
National Research Council (2010) Assessment of sea-turtle status and trends: integrating demography and abundance. Demography. National Academy Press, Washington, D.C. http://books.nap.edu/openbook.php?record_id=12889&page=R1
Ospina-Álvarez N, Piferrer F (2008) Temperature-dependent sex determination in fish revisited: prevalence, a single sex ratio response pattern, and possible effects of climate change. PLoS ONE 3:e2837. https://doi.org/10.1371/journal.pone.0002837
Owens DW (1999) Reproductive cycles and endocrinology. In: Eckert KL, Bjorndal KA, Abreu-Grobois FA, Donnelly M (eds) Research and management techniques for the conservation of sea turtles. IUCN/SSC Marine Turtle Specialist Group Publication 4, pp 119–123
Owens DW, Hendrickson JR (1978) Endocrine studies and sex ratios of the green sea turtle, Chelonia mydas. In: Henderson G (ed) Proceedings of the Florida and interregional conference on sea turtles, Jensen Beach, Florida. Fla Mar Res Pub 33, pp. 12–14
Owens DW, Ruiz GT (1980) New methods of obtaining blood and cerebrospinal fluid from marine turtles. Herpetologica 36:17–20
Owens DW, Hendrickson JR, Lance V, Callard IP (1978) A technique for determining sex of immature Chelonia mydas using a radioimmunoassay. Herpetologica 34:270–273
Patrício AR, Marques A, Barbosa C, Broderick AC, Godley BJ, Hawkes LA, Rebelo R, Regalla A, Catry P (2017) Balanced primary sex ratios and resilience to climate change in a major sea turtle population. Mar Ecol Prog Ser 577:189–203. https://doi.org/10.3354/meps12242
Patrício AR, Hawkes LA, Monsinjon JR, Godley BJ, Fuentes MM (2021) Climate change and marine turtles: recent advances and future directions. Endanger Species Res 44:363–395. https://doi.org/10.3354/esr01110
Pilcher NJ, Al-Maslamani I, Williams J, Gasang R, Chikhi A (2015) Population structure of marine turtles in coastal waters of Qatar. Endang Species Res 28:163–174. https://doi.org/10.3354/esr00688
Proietti MC, Lara-Ruiz P, Reisser JW, Pinto LDS, Dellagostin OA, Marins LF (2009) Green turtles (Chelonia mydas) foraging at Arvoredo Island in southern Brazil: genetic characterization and mixed stock analysis through mtDNA control region haplotypes. Genet Mol Biol 32:613–618. https://doi.org/10.1590/S1415-47572009000300027
R Core Team (2020) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. https://www.R-project.org/
Restrepo J, Webster EG, Ramos I, Valverde RA (2023) Recent decline of green turtle Chelonia mydas nesting trend at Tortuguero, Costa Rica. Endanger Species Res 51:59–72. https://doi.org/10.3354/esr01237
Rogers MM (2013) Hatchling sex ratios and nest temperature–sex ratio response of three South Florida marine turtle species (Caretta L., Chelonia mydas L., and Dermochelys coriacea V.). Master’s Thesis, Florida Atlantic University
Sanchez C (2013) Sex ratios of juvenile green turtles (Chelonia mydas) in three developmental habitats along the coast of Florida. Master’s Thesis, University of Central Florida
Santidrián Tomillo P, Spotila JR (2020) Temperature-dependent sex determination in sea turtles in the context of climate change: uncovering the adaptive significance. BioEssays 42:2000146. https://doi.org/10.1002/bies.202000146
Santidrián Tomillo P, Oro D, Paladino FV, Piedra R, Sieg AE, Spotila JR (2014) High beach temperatures increased female-biased primary sex ratios but reduced output of female hatchlings in the leatherback turtle. Biol Conserv 17:71–79. https://doi.org/10.1016/j.biocon.2014.05.011
Santidrián Tomillo P, Genovart M, Paladino FV, Spotila JR, Oro D (2015) Climate change overruns resilience conferred by temperature-dependent sex determination in sea turtles and threatens their survival. Glob Chang Bio 2:2980–2988. https://doi.org/10.1111/gcb.12918
Schroeder BA, Owens DW (1994) Sex ratio of immature green turtles in an East Central Florida developmental habitat. In: Schroeder BA, Witherington BE (eds) Proceedings of the 13th annual symposium on sea turtle biology and conservation. NOAA Tech Memo NMFS-SEFSC-341, pp 157–161
Seminoff JA, Allen CD, Balazs GH, Dutton PH, Eguchi T, Haas H, Hargrove SA, Jensen M, Klemm DL, Lauritsen AM, MacPherson SL (2015) Status review of the green turtle (Chelonia mydas) under the U.S. Endangered Species Act. NOAA Tech Memo. NOAA-NMFS-SWFSC-539, pp 1–571
Shamblin BM, Bjorndal KA, Bolten AB, Hillis-Starr ZM, Lundgren IAN, Naromaciel E, Nairn CJ (2012) Mitogenomic sequences better resolve stock structure of southern Greater Caribbean green turtle rookeries. Mol Ecol 21:2330–2340. https://doi.org/10.1111/j.1365-294X.2012.05530.x
Shamblin BM, Bagley DA, Ehrhart LM, Nairn CJ (2015) Mitochondrial repeat haplotypes suggest local origins for CM-A13 green turtles foraging in Florida. Mar Turt Newsl 146:11–13
Shamblin BM, Dutton PH, Shaver DJ, Bagley DA, Putman NF, Mansfield KL, Ehrhart LM, Peña LJ, Nairn CJ (2017) Mexican origins for the Texas green turtle foraging aggregation: a cautionary tale of incomplete baselines and poor marker resolution. J Exp Mar Biol Ecol 488:111–120. https://doi.org/10.1016/j.jembe.2016.11.009
Shamblin BM, Witherington BE, Hirama S, Hardy RF, Nairn CJ (2018) Mixed stock analyses indicate population-scale connectivity effects of active dispersal by surface-pelagic green turtles. Mar Ecol Prog Ser 601:215–226. https://doi.org/10.3354/meps12693
Shamblin BM, Hart KM, Martin KJ, Ceriani SA, Bagley DA, Mansfield KL, Ehrhart LM, Nairn CJ (2020) Green turtle mitochondrial microsatellites indicate finer-scale natal homing to isolated islands than to continental nesting sites. Mar Ecol Prog Ser 643:159–171. https://doi.org/10.3354/meps13348
Spotila JR, Standora EA, Morreale SJ, Ruiz GJ (1987) Temperature dependent sex determination in the green turtle (Chelonia mydas): effects on the sex ratio on a natural nesting beach. Herpetologica 43:74–81
Stahelin GD, Hoffman EA, Quintana-Ascencio PF, Reusche M, Mansfield KL (2022) Incorporating distance metrics and temporal trends to refine mixed stock analysis. Sci Rep 12:20569. https://doi.org/10.1038/s41598-022-24279-2
U.S. Fish and Wildlife Service and National Oceanic and Atmospheric Administration (2016) Endangered and threatened wildlife and plants; final rule to list eleven distinct population segments of the green sea turtle (Chelonia mydas) as endangered or threatened and revision of current listings under the Endangered Species Act. Federal Register 81(66) / Wednesday, April 6, 2016.
Valdivia A, Wolf S, Suckling K (2019) Marine mammals and sea turtles listed under the U.S. Endangered Species Act are recovering. PLoS ONE 14:e0210164. https://doi.org/10.1371/journal.pone.0210164
Valenzuela N, Lance V (eds) (2004) Temperature-dependent sex determination in vertebrates. Smithsonian Books, Washington. https://doi.org/10.5479/si.9781944466213
van der Zee JP, Christianen MJ, Nava M, Velez-Zuazo X, Hao W, Bérubé M, van Lavieran H, Hiwat M, Berzins R, Chevalier J, Chevalier D, Lankester M, Bjorndal K, Bolten A, Becking L, Palsbøll PJ (2019) Population recovery changes population composition at a major southern Caribbean juvenile developmental habitat for the green turtle, Chelonia mydas. Sci Rep 9:1–11. https://doi.org/10.1038/s41598-019-50753-5
Vélez-Zuazo X, Ramos WD, van Dam RP, Diez CE, Abreu-Grobois A, McMillan WO (2008) Dispersal, recruitment and migratory behavior of a hawksbill sea turtle population. Mol Ecol 17:839–853. https://doi.org/10.1111/j.1365-294X.2007.03635.x
Wallace BP, Posnik ZA, Hurley BJ, DiMatteo AD, Bandimere A, Rodriguez I, Maxwell SM, Meyer L, Brenner H, Jensen MP, LaCasella EL, Shamblin BM, Abreu-Grobois FA, Stewart KR, Dutton PH, Barrios-Garrido H, Dalleau M, Dell’Amico F, Eckert KL, FitzSimmons N, García-Cruz M, Martins S, Mobaraki A, Mortimer JA, Nel R, Phillott AD, Pilcher NJ, Putman N, Rees AF, Rguez-Baron JM, Swaminathan A, Seminoff JA, Turkozan O, Vargas SM, Vernet PD, Vilaça ST, Whiting SD, Hutchinson BJ, Casale P, Mast RB (2023) Marine turtle regional management units 2.0: an updated framework for conservation and research of wide-ranging megafauna species. Endang Species Res. https://doi.org/10.3354/esr01243
Wibbels T (1999) Diagnosing the sex of sea turtles in foraging habitats. In: Eckert KL, Bjorndal K, Abreu-Grobois A, Donnelly M (eds) Research and management techniques for the conservation of sea turtles. IUCN/SSC Marine Turtle Specialist Group Publication No. 4, pp 139–143
Wibbels T (2003) Critical approaches to sex determination in sea turtles. In: Lutz PL, Musick JA, Wyneken J (eds) The biology of sea turtles, vol 2. CRC Press. Boca Raton, Florida, pp 103–134
Wibbels T, Owens DW, Amoss M (1987a) Seasonal changes in serum testosterone titers of loggerhead sea turtles captured along the Atlantic Coast of the United States. In: Witzell W (ed) Ecology of east Florida sea turtles. NOAA Tech Rept NMFS 53, pp 59–64
Wibbels T, Owens DW, Morris YA, Amoss M (1987b) Sexing techniques and sex ratios for immature loggerhead sea turtles captured along the Atlantic Coast of the United States. In Witzell W (ed) Ecology of east Florida sea turtles. NOAA Tech Rept NMFS 53, pp 65–74
Wibbels T, Owens DW, Limpus CJ, Amoss M (1989) Field testing of a sexing technique for immature sea turtles. In: Ogren LH (ed) Proceedings of the second Western Atlantic turtle symposium, Puerto Rico. NOAA Tech Memo NMFS-SEFC-226, pp 349–350
Wibbels T, Owens DW, Limpus CJ, Reed PC, Amoss MS (1990) Seasonal changes in serum gonadal steroids associated with migration, mating, and nesting in the loggerhead sea turtle (Caretta caretta). Gen Comp Endocrinol 79:154–164. https://doi.org/10.1016/0016-6480(90)90099-8
Wibbels T, Martin RE, Owens DW, Amoss MS (1991) Female-biased sex ratio of immature loggerhead sea turtles inhabiting the Atlantic coastal waters of Florida. Can J Zool 69:2973–2977. https://doi.org/10.1139/z91-419
Wibbels T, Owens DW, Licht P, Limpus C, Reed PC, Amoss MS Jr (1992) Serum gonadotropins and gonadal steroids associated with ovulation and egg production in sea turtles. Gen Comp Endocrinol 87:71–78. https://doi.org/10.1016/0016-6480(92)90151-9
Wibbels T, Balazs GH, Owens DW, Amoss MS (1993) Sex ratio of immature green turtles inhabiting the Hawaiian archipelago. J Herpetol 27:327–329. https://doi.org/10.2307/1565155
Wibbels T, Owens DW, Limpus CJ (2000) Sexing juvenile sea turtles: Is there an accurate and practical method? Chelonian Conserv Biol 3:756–761
Wyneken J, Lolavar A (2015) Loggerhead sea turtle environmental sex determination: implications of moisture and temperature for climate change–based predictions for species survival. J Exp Zool (Mol Devel Evol) 3248:295–314. https://doi.org/10.1002/jez.b.22620
Wyneken J, Epperly S, Heppell S, Crowder L (2006) The importance of annual variation in understanding sea turtle sex ratios. In: Frick M, Panagopoulou A, Rees AF, Williams K (eds) 26th annual symposium on Sea Turtle Biology and Conservation, book of abstracts. International Sea Turtle Society, Athens, pp 336–337
Yntema CL, Mrosovsky N (1980) Sexual differentiation in hatchling loggerheads (Caretta caretta) incubated at different controlled temperatures. Herpetologica 36:33–36
Zeileis A, Hothorn T (2002) Diagnostic checking in regression relationships. R News 2(3):7–10
Zurita JC, Herrera R, Arenas A, Torres ME, Calderón C, Gómez L, Alvarado JC, Villavicencio R (2003) Nesting loggerhead and green sea turtles in Quintana Roo, Mexico. In: Seminoff J (compiler) Proceedings of the 22nd annual symposium on sea turtle biology and conservation. NOAA Tech Memo NMFS-SEFSC-503, pp 125–127
Acknowledgements
We thank the numerous staff and volunteers from the Bermuda Zoological Society, the Bermuda Aquarium Museum and Zoo, and the Sea Turtle Conservancy who have assisted our work over many years. Ian Walker, Richard Winchell, and David Godfrey were particularly instrumental in making the Bermuda Turtle Project a long-term success. We thank the more than 200 students from 41 countries who participated in the Bermuda Turtle Project’s International Sea Turtle Biology and Conservation course and assisted with field work. We thank the W. Galbraith, E. Harvey, and G. Wardman families for hosting project staff. Gaëlle Blanvillain, Janice Grumbles, Michelle Lee, and Rhonda Patterson assisted with RIA analyses. We thank Guy Bradley, Whitney Bullock, Sarah Duncan, Jen Gilkey, Bill Roess, Brian Zielinski, and students at Eckerd College, as well as Ginger Clark at BEECS Genetic Analysis Core, University of Florida, for help with genetic analyses. Paul Kubilis contributed many useful ideas to the analyses of the sex ratio data in early years. The Archie Carr Center coordinated international tag returns. We thank Jeffrey Seminoff and an anonymous reviewer for helpful comments on the manuscript; Bland Crowder and Allen Foley commented on earlier versions. For DO and JS, this is contribution 592 of the Grice Marine Laboratory of the College of Charleston.
Funding
Funding or in-kind support was provided by H. Clay Frick, the Helen Clay Frick Foundation, the Sea Turtle Conservancy, the Atlantic Conservation Partnership, the Bermuda Zoological Society, the Florida Fish and Wildlife Conservation Commission, and Eckerd College. Open access fees were paid by the Florida Fish and Wildlife Conservation Commission, Fish and Wildlife Research Institute.
Author information
Authors and Affiliations
Contributions
Conceived and designed study: AM, PM, DO; conducted field work, hormone sampling, laparoscopies: AM, PM, RH, JG, DO; sample and data management: AM, PM, BB, RH, BT, JS; laboratory analyses: DO, JS (RIA); SD, DF, LC (genetics); data analyses and interpretation: AM, PM, BT; modeling and statistics: BT, PM, AM; writing: AM, PM, BT, DO, JS; all authors contributed to final version.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
Research permits were granted by the Bermuda Government (successively, the departments of Agriculture and Fisheries; Conservation Services; and Environment and Natural Resources; most recent permit 2018071309). Blood and tissue samples were exported to the United States from Bermuda under a series of CITES permits (most recent, 18BM0005) issued by the Bermuda Government to the FWC; imports into the United States were covered by a series of U.S. CITES permits issued to the FWC (most recent, 18US48288C/9). No IACUC agreement was required for this work; however, the same methods are used by ABM and PAM in Panama and have been approved by the Smithsonian Tropical Research Institute’s IACUC committee, most recently STRI ACUC 2020–0414-2023. David Owens’ laparoscopy contribution in 2008 was approved by the College of Charleston IACUC-2008–020.
Additional information
Responsible Editor: P. Casale.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: In this article Fig. 4 and some text was wrongly appeared. It has been corrected. Also, the supplementary file has been updated.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Meylan, A.B., Brost, B., Conrad, L.J. et al. Feminization of a mixed-stock foraging aggregation of immature green turtles (Chelonia mydas), 1975–2018. Mar Biol 171, 11 (2024). https://doi.org/10.1007/s00227-023-04320-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-023-04320-2