Abstract
Coastal ecosystems act as spawning grounds, settlement areas or nurseries for a diversity of fish species. Today, these ecosystems are under various anthropogenic stressors. One of these disturbances is the structural modification of the shallow littoral by coastal infrastructure such as ports, piers, marinas and roads. Here, we investigate whether there are differences between modified (artificial) and non-modified (natural) habitats in central Greece in terms of the fish assemblage structure, species diversity and richness, fish abundance and the proportion of juveniles. We carried out underwater visual census from September 2020 to August 2021 across two habitats (natural and artificial) in three locations of central Greece. The fish community and Shannon diversity index did not vary across habitat type; however, both species richness and total abundance were significantly higher in artificial habitats. The abundance of five (out of ten examined in total) taxa was significantly affected by habitat type. Higher abundance in the artificial habitat in the warmer or more productive months was found, possibly relevant to food availability. The significance of habitat interactions with location and the temporal component in community composition and species abundance models indicated intense heterogeneity in species-specific occupancy and may be linked to the type and characteristics of artificial habitats. Modeling of the percentage of small-sized individuals indicated patterns of juvenile occupancy requiring further investigation about the potential role of artificial habitats for some species. Future research should examine the impact of specific artificial habitat types on population dynamics and investigate the implications of artificial habitat construction for conserving fish diversity and maintaining coastal ecosystem functioning.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Coastal areas comprise important ecosystems because of characteristics such as their high productivity and diversity (Airoldi and Beck 2007; Seitz et al. 2014). A high diversity of species is found in coastal habitats, as these often play an important role for their life cycle as, e.g., spawning or nursery grounds (Costanza et al. 1997; Somarakis and Machias 2002; Kiparissis et al. 2008; Tiralongo et al. 2021). The shallowest part of the coastal zone, the shallow littoral, is located at the interface of its neighboring marine and terrestrial environments and thus has unique environmental characteristics (Ray 1991). Shallow littoral habitats can, therefore, be crucial environments for fish (Valesini et al. 2004) and may serve as settlement areas for various fish species (Biagi et al. 1998). Predation avoidance is another important function of these areas, especially for fish juveniles (e.g., Mavraki et al. 2016; Munsch et al. 2017). In addition, areas of the shallow littoral can have a role as nursery grounds making them critical for the conservation of fish diversity and even the maintenance of fisheries resources (Nagelkerken et al. 2015; Cheminée et al. 2021). It has even been demonstrated that the shallow littoral can play distinct roles in different ontogenetic stages of one species, e.g., functioning as a spawning ground and as a refuge until the metamorphosis of larvae to juveniles—with absence of individuals between these ontogenetic stages from this habitat (Polte et al. 2017).
Coastal areas constitute the marine zones most impacted by anthropogenic pressures (Islam and Tanaka 2004; Halpern et al. 2008). The types of impacts are diverse, and their level is unprecedented today (Crain et al. 2009). Degradation has often modified coastal habitats resulting in their failure to operate as nurseries, feeding or spawning grounds (Beck et al. 2001; Worm et al. 2006). Consequently, it is of utmost importance to determine the impact of anthropogenic stressors, especially on the communities of the shallow littoral habitats that, due to their proximity to land, are the most prone to be affected, as wide-scale disturbances affecting the relative abundance of habitat types may have a significant effect on biodiversity (Fraschetti et al. 2001). Considering the importance of this environment for fish life cycle, it is important to examine the impacts of stressors throughout the annual cycle in order to associate them with specific phases of the biology of populations or aspects of their ecology.
One of the most evident disturbances on the coastal zone is its construction and structural modification, as the intensifying coastal urbanization has resulted in the transformation of the coastal seascape worldwide. For example, about 19% of the European Union’s coastline is affected by alterations in seabed habitats due to urbanization, port and flood protection infrastructures, boating, or land reclamation (EEA 2019). In Europe, especially in the Mediterranean Sea, the coastal strip has been modified by human infrastructure and activity (Airoldi and Beck 2007). The impact of the resulting constructions such as breakwaters and jetties, piers, marinas, coastal walls and defenses or roads may be the physical modification of natural habitats or their fragmentation (Bulleri and Chapman 2010). Coastal infrastructures are usually built without prior assessment of their potential impacts on the physiology, behavior and dynamics of marine populations (Pastor et al. 2013). Depending on the surrounding habitats, the effects can be diverse (Bulleri 2005) and may even add novel habitat to the coastal zone, e.g., when built on soft substrates (Guidetti 2004) or interact with other anthropogenic stressors like light pollution affecting the distribution and behavior of coastal populations (Georgiadis et al. 2014).
Shoreline modifications associated with artificial habitat have a variety of physical and biological effects such as changes in substrate, slope, water depth, circulation and shoreline vegetation. These affect fish in aspects such as behavior, feeding, diet, predation avoidance, mortality, growth, spawning, habitat partitioning between sizes and ontogenetic shifts (e.g., Duffy-Anderson and Able 1999, 2001; Toft et al. 2007; Munch et al. 2014, 2016). For example, poor feeding conditions and suboptimal foraging are experienced by fish distributed under or around piers, potentially linked to lower growth and higher mortality rates (Duffy-Anderson and Able 1999). Shoreline modification affects the availability and consumption of epibenthic prey (Morley et al. 2012) or may even result in fish switching from benthivory to planktivory (Munch et al. 2015). For species like Pseudopleuronectes americanus, juvenile feeding is suppressed under piers despite the presence of sufficient prey, suggesting that piers are unsuitable long-term juvenile habitats (Duffy-Anderson and Able 2001). Egg deposition of intertidal spawners may also be affected, as shown by a reduction of Menidia menidia egg densities in artificial habitats (Balouskus and Targett 2012). Furthermore, artificial shoreline replacing natural spawning beaches with impervious, unvegetated substrate may result in increased embryonic mortality due to excessive exposure to sunlight, heat and dryness (Rice 2006). Due to the existence of shoreline modifications that truncate the littoral zone, fish that would typically be spread-out over a broad area may aggregate against the shoreline instead (Toft et al. 2007). As smaller fish generally tend to occupy shallower depths where predators are less abundant, the replacement of natural small inclination with steeper inclination may result in the lack of protective shallows and the presence of larger predators (Munsch et al. 2016).
Various works have investigated the impact of constructions such as seawalls, piers, bridges and ripraps on fish abundance (Toft et al. 2007; Able et al. 2013; Pastor et al. 2013; Grothues et al. 2016) or on assemblage composition and diversity (Bilkovic and Roggero 2008; Guidetti 2004; Munch et al. 2014; Riofrío-Lazo et al. 2022). Overall, as the modified habitat creates novel conditions, this may result in decreases (e.g., Hendon et al. 2000) or increases (Pastor et al. 2013) to the abundance of a specific taxon, which may in turn affect the entire community. Nekton density, species richness, and density of dominant species are higher along natural beach shorelines relative to adjacent artificial riprap (Torre and Targett 2016). Multi-annual monitoring has linked lower annual stability of fish communities to artificial substrate (Scyphers et al. 2015). Juvenile fish assemblages may also be affected by artificial habitat as a consequence of factors such as structural complexity, shading or water depth (Duffy-Anderson et al. 2003). For a more detailed account of the impacts of shoreline armoring and modifications see Munsch et al. (2017). From the above, it is evident that the effects of artificial habitat on fish abundance can be diverse and their impacts not necessarily direct or straightforward (e.g., Pastor et al. 2013 document that artificial defense structures can act as artificial nurseries but may also cause the loss of the nursery function of neighboring habitats like lagoons). Therefore, before setting specific questions about the functional effect of artificial habitat on fish populations and their fitness, it is essential to study the fish community over anthropogenic habitat. Considering the importance of the shallow littoral for juvenile fish, it is essential to better understand the patterns of association between juvenile and adult fish and anthropogenic habitats.
The aim of this work is to investigate whether there are differences between modified (artificial) and non-modified (natural) habitats in central Greece in terms of: (a) the structure of fish assemblages, (b) total fish abundance, species diversity and species richness, (c) abundance of the most dominant fish taxa and (d) the ratio of juveniles, and to document whether these differences are consistent across the temporal component and between different sites.
Methodology
Sampling
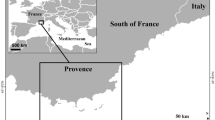
We sampled with underwater visual census from September 2020 to August 2021 in three locations of the western Korinthiakos Gulf: Rio, Arachovitika and Psathopirgos (Fig. 1). The locations were selected after preliminary coastal observations and dives in order to ensure that in each one a coastal transect would be feasible in two separate habitats acting as sampling stations: one with a natural coastal substrate and coastline and another one with an artificial/anthropogenic coastal substrate. In Rio, the anthropogenic substrate was the wall of the local fortress (built during the fifteenth century), in Arachovitika, it was a cement pier of the local marina enhanced with boulders for support and in Psathopirgos, it was the coastal fortification built next to the beach road. In each location, a coastal strip transect parallel to the artificial substrate was determined with a length of 30 m and a width of 2.5 m and constituted the structurally modified habitat. Within a distance of 10–50 m from the edge of this station, a transect parallel to the natural coastline and with equal dimensions was determined as the natural habitat. The close proximity of the two habitats (within each location) allowed for the maximum possible similarity between them in all aspects (e.g., waves, current regime, and exposure) apart from the habitat origin and type under the planned experimental design. All sampling stations had a depth ranging from 1.5 m to 4.0 m. Overall, we sampled two transects per month in each station, resulting to the following sampling plan:
A Location of the three sampling locations in continental central Greece (Ara: Arachovitika, Psa: Psathopirgos) and drone photos of sampling locations with an approximate delineation of the strip transect of each habitat (yellow: natural, orange: artificial), specifically B Rio, C Arachovitika, and D Psathopirgos
As the coastal fish communities vary with time of day (Willis et al. 2006; Samourdani and Tzanatos 2022), all transects were sampled after noon (from 14:00 to 17:00). During sampling, the observer carried out the visual census while snorkeling across the transect length. Each strip transect was outlined with two 30 m measuring tapes that were unfolded and laterally connected with ropes of 2.5 m length to keep the transect dimensions (all anchored with lead weights to maintain their position on the seabed. The diver would enter the sea, set up the transect and then exit the transect area and remain inactive nearby for ~ 10’ to allow the fish that might have been disturbed to resettle and resume their previous behavior. Transects on both habitats (natural-artificial) of the same location were performed on the same day.
In each transect, the observer would swim over the delineated area at a speed of ~ 5 m per minute, that has been documented as suitable for visual census of fish (Clynick et al. 2008), even though optimal speed depends on the species to be observed and their mobility (De Girolamo and Mazzoldi 2001). The observer would note each grouping of fish of the same taxon that constituted a clearly identifiable association of individuals (e.g., behaving with some sort of coordination or with a clear spatial or behavioral interaction) as a group, hence during a transect more than one group of the same species might be observed. The abundance of individual fish in a group was noted as a code with the following distinct categories: Α: 1 (the “group” consisting of a solitary individual not interacting with other conspecifics), Β: 2, C: 3, D: 4, Ε: 5, F: 6–10, G: 11–20, H: 21–30, Ι: 31–50, J: 51–100 and K: > 100 individuals. For each fish taxon, three size groups were identified, namely: small, medium and large. The size groups’ limits for each species were determined in preliminary observations as approximate sizes that the observer defined, was trained to identify and could easily distinguish; they are presented in Table 1. Each fish group observed was characterized accordingly with regard to the dominant size (in almost all cases fish individuals within a group belonged to a single size group). As we did not have a means of verification of our visual size assignment, in cases of ambiguity (when the fish looked to be on the size limit between two groups), we followed a conservative approach placing the individual or group to the medium size. Total taxon abundance was estimated by adding the abundances of all the taxon size categories observed in the transect.
Analysis
The Bray–Curtis similarity matrix of the littoral fish community composition was analyzed using the Permutational analysis of variance (PERMANOVA) in order to detect whether there were significant differences in community composition between different locations, habitats and sampling months (including the explanatory variable interactions). Habitats were considered to have a fixed effect while the effect of locations and months was considered as random in the analysis. We also used the similarity percentage analysis (SIMPER) to attribute the observed dissimilarity across habitats to specific taxa.
Regarding total abundance and community composition, total fish abundance (sum of the abundance of all taxa), Shannon diversity index and species richness (number of species) recorded in each transect were analyzed through generalized additive models (GAMs). GAMs are non-parametric generalizations of the multiple linear regression having fewer limitations on the underlying data distribution (Hastie and Tibshirani 1990). Date was used as a continuous explanatory variable, while location (Rio, Arachovitika, Psathopirgos) and habitat (Anthropogenic and Natural) were used as categorical explanatory variables. Habitat was considered as a fixed effect in the models, while location was considered to have a random effect (varying intercept) on our response variables. In addition to assessing a main temporal effect in the GAM models, a habitat specific temporal effect was also examined. Intrinsic variance among the three different locations that potentially differentiate the temporal effect or the effect of habitat was incorporated in the models by applying a varying smoother by location. These model adjustments are equivalent to the two-way interactions examined in the PERMANOVA (date × habitat, date × location, habitat × location, respectively) thus enabling the approximate estimation of the relevant p-values (Wood 2013). These varying smoothers are in effect (but not in the strict sense) equivalent to a three-way interaction, as a different relationship can be estimated for each combination of factors, but no three-way interaction (as a tensor smooth product of the three covariates) probability is estimated. Temporal correlation, implied by the repeated nature of the transects across the sampling stations, was examined by evaluating residual autocorrelation—with a lag from 2 up to 5 previous sampling dates (which corresponds to up to two months lag)—in the models. Temporal autocorrelation was not found to be significant enough to incorporate a correlation structure in our models.
To determine the factors affecting the abundance fluctuations of individual taxa, the abundance of the most important ten taxa (in descending order: Chromis chromis, Atherina sp., Boops boops, Coris julis, Sarpa salpa, Diplodus sargus, Tripterygion sp., Diplodus vulgaris, Diplodus annularis, Oblada melanura) were also modeled through GAMs. Habitat, location, date and their first-level interactions were included in the models as potential explanatory variables with a model configuration similar to the one described above.
Finally, to determine the effect of these factors on juveniles, we modeled the effect of location, habitat and date on the percentage of juveniles for the most dominant taxa. For this, we divided the abundance of the “small” size class of each taxon with the total abundance of that taxon within each transect. Even though the small size class, as it was recorded during visual census, does not necessarily correspond exactly to the young-of-the-year size class of each taxon, it was considered as an adequate representation of the abundance of the juveniles of the respective species. For some species, either the “small” size class was rarely recorded (the frequency of observation of the “small” size was very low) or the abundance of “small” sized individuals in comparison to the total abundance was very low (resulting in very low percentage values); therefore, we did not analyze the taxa whose juveniles were: (a) recorded in less than 20 (out of the total 144) transects or (b) whose juvenile abundance represented less than 20% of the total abundance. As a result, Generalized Additive Modeling was carried out for the juvenile percentages of the following taxa: C. chromis, C. julis, D. sargus, D. vulgaris and Tripterygion sp.
All GAM analyses were carried out in R language (R Development Core Team 2021), using the library mgcv (Wood 2006). The multivariate analyses of fish community were carried out in PRIMER 6 (Clarke and Gorley 2006; Anderson et al. 2008). Over-fitting in GAMs was avoided by limiting the maximum number of degrees of freedom in the splines to four by setting the number of knots used in the smoother to five (Zucchetta et al. 2010). The selected suitable distributions for the response variables were the Negative Binomial for species abundance (with a log link), the Binomial for juvenile percentages (with a logit link) and a Poisson–Gamma mixture (Tweedie family with a log link) for the Shannon diversity and species richness indices. Model selection was performed automatically (during model fitting in mgcv package) by imposing an extra penalty on the null space of spline basis on top of the range space penalty (Marra & Wood 2011). This way all models retained all aforementioned explanatory variables and their interactions, for consistency, while the double penalty approach could select out not significant covariate smooths. For all models, predicted values of the response variables were computed and presented for the three-way interaction of the covariates: date, habitat and location (extensive partial effects of the covariates and their first-level interactions are presented in the Supplement). The level of statistical significance for all inferential tests was set at α = 0.05.
Results
Littoral fish community
In total, 37 fish taxa were recorded (Supplementary Table S1). The most abundant taxa were: C. chromis (mean abundance of individuals per transect, m = 21.2, standard deviation, s = 42.7), Atherina sp. (m = 18.4, s = 51.7), B. boops (m = 9.8, s = 30.8), C. julis (m = 7.2, s = 6.8) and S. salpa (m = 4.7, s = 16.1), while those most frequently recorded were: C. julis, (frequency of observation out of 144 transects, f = 113), C. chromis (f = 103), Serranus scriba (f = 79), D. vulgaris (f = 75) and Blenniidae (f = 74).
The PERMANOVA indicated a complex, three-way interaction between location, habitat and month (Table 2). Post-hoc pairwise comparisons between habitats for each location and month indicated that, over the entire sampling period, the two habitats differed significantly in community composition ten times in Arachovitika, three times in Psthopyrgos and five times in Rio (Supplementary Table S2). According to SIMPER results, from the ten times at which community composition differed between natural and anthropogenic habitat in Arachovitika, C. chromis was among the five most important taxa eight times, Atherina sp. seven times, D. annularis and Gobiidae six times each, C. julis and Tripterygion sp. four times each, D. sargus, O. melanura and S. salpa each three times, Blenniidae and B. boops twice each and D. vulgaris and Mullus barbatus once each. In Psathopyrgos, only a few species contributed to dissimilarity between habitats in the three months that these were documented, namely: C. chromis all three times, Tripterygion sp. twice and Atherina sp., D. sargus and Mugilidae one time each. Finally, in Rio, from the five times of different community composition between natural and anthropogenic habitat, C. chromis was among the five most important species for dissimilarity all five times, C. julis and D. vulgaris four times, Atherina sp. and Mugilidae two times each and S. salpa, B. boops and L. mormyrus one time each.
Total abundance, diversity and richness
All explanatory variables date, habitat and location (but not their interactions) had a significant effect on total fish abundance (Table 3). Total abundance increased with date (Suppl. Figure 1), was higher in artificial (m = 109.5, s = 78.4) than natural habitats (m = 69.4, s = 68.8) and the highest values were recorded in Arachovitika (m = 110.6, s = 78.3), while the lowest were found in Rio (m = 73.3, s = 72.0), as also indicated in the model predictions of total abundance temporal dynamics for each habitat by location (Fig. 2A).
Shannon diversity index H varied across locations and dates and also there was a significant interaction of habitat and date (Table 3). Diversity was higher in August–September and lower in January–February with the highest values recorded in Arachovitika and the lowest in Rio (Suppl. Figure 2). A steady temporal increase of diversity was observed in artificial habitats, while in the natural ones, two local maxima appeared in November also evident in the pattern of model predictions in Fig. 2B.
Statistically significant differences in species richness were documented across all main factors and also for the interaction of location and date (Table 3). Overall, species richness was minimal in January and maximum in September, higher in artificial than natural habitats and the highest in Arachovitika and the lowest in Rio. However, different patterns in species richness dynamics were documented across locations (Suppl. Figure 3), resulting in predictive models of consistently higher richness in artificial habitats, albeit at different levels and with small differences in the temporal pattern across the three locations (Fig. 2C).
Abundance of dominant species
Neither any of the factors date, habitat and location, nor any of their interactions had a significant effect on the abundance of Atherina sp. (Table 4, Supplementary Fig. 4, Fig. 3A). Despite a non-significant effect, there was a temporal trend in this species abundance that was low in November (no individuals of this taxon were recorded in December) and maximum in May (m = 74.6, s = 115.8). Only date had a significant effect on the abundance of B. boops (Table 4, Supplementary Fig. S5). B. boops also had the highest abundances in Psathopirgos (m = 16.2, s = 36.6), but the lowest ones were in Arachovitika (m = 2.8, s = 9.3). The lowest abundances of this species were recorded from July to November, while the highest was recorded in May (m = 31.1, s = 61.1) resulting in model predictions of maximum abundances from March to May depending on the location (Fig. 3B). C. chromis abundance was found to vary significantly across locations and habitats and also depended on their interaction and the interaction of location with date (Table 4). It had significantly higher abundance in the artificial habitats (m = 34.3, s = 53.2) than the natural ones (m = 8.2, s = 22.2). The highest abundance of this species was found in Arachovitika (m = 31.0, s = 56.1), while the lowest was recorded in Psathopirgos (m = 8.8, s = 25.8). The lowest abundance of this species was recorded in February (m = 4.1, s = 5.5), while high abundances were found in April (m = 48.1, s = 75.9), September (m = 44.6, s = 81.1) and October (m = 41.1, s = 61.3). The interaction plots indicated higher abundances of this species in artificial habitats in Arachovitika and Rio, but slightly lower in Psathopirgos. There, the interaction with time also hinted at complex dynamics (Supplementary Fig. 5). Consequently, the predictive model indicated constantly higher abundance in the artificial habitat is Arachovitika and Rio, but lower in Psathopirgos (Fig. 3C). Significant differences in the abundance of C. julis were found in the different habitats and across time and also on the interaction of location with time (Table 4). Higher abundances of C. julis were documented in natural habitats. The maximum abundances of this species were recorded in July (m = 11.5, s = 6.9) and August (m = 10.3, s = 8.6), while the lowest were in April (m = 4.7, s = 5.3). The interaction of location with date showed a rather complex pattern in Rio (Supplementary Fig. S7). As a result, contrasting patterns between Arachovitika and Rio were documented between the artificial and the natural habitat (in Arachovitika higher abundance in the artificial habitat while in Rio the opposite) in the predictive model (Fig. 3D); in Psathopyrgos the initial higher abundances observed in the artificial habitat ended with the confidence intervals of the two habitat types highly overlapping.
Generalized Additive Model predictions of the abundance of most important fish taxa based on the partial effects of Habitat, Location and Date and their interactions. Shaded lines indicate 95% confidence intervals. Day number = 0: 25/9/2020 (first sampling day). A Atherina sp., B Boops boops, C Chromis chromis, D Coris julis, E Diplodus annularis, F Diplodus sargus, G Diplodus vulgaris, H Oblada melanura, I Sarpa salpa, J Trypterygion sp.
Habitat, date and all interactions had a significant effect on the abundance of D. annularis (Table 4). Its abundance was higher in artificial habitats (m = 4.6, s = 7.0) than natural ones (m = 1.1, s = 2.5). It showed a complex temporal trend with minimal abundance in April (m = 0.5, s = 1.0) and maximum in August (m = 5.7, s = 7.9). However, all interaction types indicated that the impact of each factor varied across the levels of the other factors indicating complex dynamics in space and time (Suppl. Fig. S8). As a result, the predictive models gave differing effect of artificial versus natural habitat in the three locations (higher abundance over the artificial habitat in Arachovitika and Rio, lower in Psathopirgos) but still indicated minimal abundances in March–May and maximal in August (Fig. 3E). Habitat, date and their interaction had a significant effect on the abundance of D. sargus (Table 4). This species had higher abundance in the anthropogenic (m = 6.6, s = 11.0) over the natural habitat (m = 2.5, s = 4.0). Similarly, its maximum abundance was recorded in July (m = 12.4, s = 7.9), but the lowest abundance was in November (m = 0.1, s = 0.3). The interaction plot indicated a pattern demonstrating higher abundance in the natural habitat until December and then a switch to the artificial habitat from March onwards (Suppl. Fig. S9). The resulting predictive models demonstrated higher species abundance in artificial habitats from March to September in Arachovitika and Rio, contrary to a higher abundance in natural habitat in Psathopyrgos (Fig. 3F). Date, location and both interactions of date with location and habitat had a significant effect on the abundance of D. vulgaris (Table 4). Abundance was higher in Rio (m = 3.9, s = 6.8) and Arachovitika and lower in Psathopirgos (m = 1.5, s = 2.7). The temporal pattern showed an increase of abundance with time (Suppl. Figure S10). However, the interaction plots indicated higher abundance in the natural habitat in December, then in the artificial habitat in March and again in the natural habitat in July–August and different temporal patterns across locations. The above resulted in a common pattern of the predictive models across all areas with a local maximum in natural habitats in December, then switching to artificial habitats in March–April and the highest abundances occurring in July and August (Fig. 3G).
The abundance of O. melanura was found to be significantly affected by date and the interactions of location with both date and habitat (Table 4). The lowest abundances were documented in November (reaching zero across all sampling transects and remaining low until February) and peaking in May (maximum m = 9.6, s = 28.6) and in August (Suppl. Figure S11). The interaction plots indicated contrasting abundances for different habitat type across locations (artificial habitat in Arachovitika, natural habitat in Rio) and also a different timing albeit with a similar pattern interchanging from high to low and again from high to low abundances in all locations. The final predictive model showed higher abundance in the artificial habitat in Arachovitika contrary to the other two locations and an increased abundance in the beginning of the sampling scheme and through the summer months (Fig. 3H). The abundance of S. salpa was found to vary across date and the interactions of location with habitat and date (Table 4). Abundance was low from September to January (zero records in December) and progressively increased to high values with a maximum in June (m = 17.7, s = 40.0). A similar interaction pattern of location and habitat to the one of O. melanura was found, while the timing interaction showed a different timing across Psathopyrgos and Rio, while in Arachovitika this species abundance just followed the main pattern (Suppl. Figure S12). Consequently, the predictive model indicated long periods of absence of this species from the littoral and an increase in abundance with a variable onset across locations and high abundance in the warmer months (Fig. 3I). Finally, the abundance of Tripterygion sp. was found to fluctuate across all factors and their interactions (Table 4). This species had a higher abundance in anthropogenic habitats (m = 7.1, s = 10.7) rather than natural ones (m = 0.9, s = 2.4). Its abundance was higher in Arachovitika (m = 3.9, s = 6.8) and Psathopirgos and the lowest was recorded in Rio (m = 0.4, s = 1.3). The temporal evolution showed an initial increase to maximum abundance in November (m = 10.6, s = 16.5) remaining high until February and then a decrease leading to zero recorded abundances in August (Suppl. Figure S13). The interaction plots indicated, again, the importance of artificial habitat in Arachovitika contrary to the natural one in Rio. The natural habitat initially recorded higher abundance of this species, but the temporal pattern changed from March onwards. Differing temporal patterns across locations were also documented. As a result, the artificial habitat hosted more fish of this species in all locations in the predictive models with especially high abundances from December to March and declining afterwards (Fig. 3J).
Juvenile abundance
The percentage of C. chromis juveniles was found to vary across date, location and the interaction of date with location and habitat (Table 5). Juvenile percentage was high from March to June (months of relatively low overall abundance) and was the highest in Arachovitika (where the species was most abundant). The percentage of juveniles followed a markedly different pattern in Psathopirgos, compared to the other two locations (Supplementary Figure S14). In natural habitats, juvenile ratios peaked in May–June, while in artificial habitats, they peaked in September. The predictive models indicated that juveniles reached their highest percentages in artificial habitats around February–March in Arachovitika and Rio, while in Psathopyrgos, there was a high overlap in the percentages appearing over the two habitat types for most of the year (Fig. 4A). C. julis juvenile percentage varied significantly across habitats and depended on the interaction of location with date (Table 5). Juvenile percentages were generally higher in natural habitats and their temporal dynamics were different pattern in Rio compared to the other two locations (Supplementary Figure S15). According to the predictive model (Fig. 4B), the ratio of juveniles was higher in the natural habitat in Arachovitika and Psathopyrgos (but lower than in the artificial habitat in Rio, albeit with a high overlap between the two types. All factors (date, habitat and location) had an effect on D. sargus juvenile percentages (Table 5). Juvenile percentage fluctuations also followed the species overall pattern: higher juvenile ratio was documented in the artificial than in the natural habitat, with higher percentages in Arachovitika and Psathopirgos (Supplementary Figure S16). The temporal dynamics showed an increasing trend from the beginning to the end of the sampling period. The predictive model showed a consistent higher ratio of this species juveniles in artificial habitats in all three locations with increasing trends from January–February onwards (Fig. 4C). The percentage of juveniles of D. vulgaris varied across date and its interaction with location (the effect of habitat p = 0.055, indicating a tendency for artificial habitat, but not at significant levels––Table 5). There was a gradual increase of the percentage of juveniles with time and there were differences in the timing between the three locations (Suppl. Figure S16). The predictive models indicated zero or low juvenile percentages in the first 5 months followed by an abrupt (in Arachovitika and Psathopirgos) or steady (in Rio) increase in the following months (Fig. 4D). Habitat and location had a significant effect on the percentage of Tripterygion sp. juveniles (Table 5). The percentage of juvenile Tripterygion sp. was higher in the artificial habitat and among the three locations was the highest in Arachovitika (Supplementary Figure S18). The higher ratio of juveniles in the artificial habitat was evident in the predictive model (Fig. 4E).
Generalized Additive Model predictions of the percentage of juveniles of the fish taxa analyzed based on the partial effects of Habitat, Location and Date and their interactions. Shaded lines indicate 95% confidence intervals. Day number = 0: 25/9/2020 (first sampling day). A Chromis chromis, B Coris julis, C Diplodus sargus, D Diplodus vulgaris, E Trypterygion sp.
Apart from the differences documented in an inferential manner by GAMs, there are indications of patterns in the ratio of juveniles of taxa not observed as frequently as the five taxa analyzed with GAMs (Fig. 5): higher proportions of the small-sized/juvenile abundances recorded were documented in the anthropogenic habitats for other taxa such as S. salpa, B. boops, Diplodus puntazzo, Mugilidae and Symphodus tinca. On the contrary, higher small-sized/juvenile proportions of Gobiidae, Lithognathus mormyrus, Mullus barbatus and S. scriba were recorded over the natural habitats.
Discussion
The present work investigates the impact of one of the various anthropogenic effects on the fish community of the shallow littoral and on individual species distribution and abundance: the structural modification of the coastal zone. This impact has been studied concerning marine plant and invertebrate animal communities (Chapman 2003), fish communities (Guidetti 2004) and individual fish populations (Pastor et al. 2013) and has even been demonstrated for fish in habitats such as lagoons (Perez-Ruzafa et al. 2006) and lakes (Scheuerell and Schindler 2004). Our first hypothesis examined whether the fish community composition is different between modified (artificial) and unmodified (natural) habitats. In our study the fish community does not vary between habitats (natural and anthropogenic), but changes across different areas/geographic locations (placed at a distance of ~ 5 km) and across the interaction of location with habitat. This is an indication that the type of structural modification or other factors (e.g., inclination, exposure, orientation, currents—which may also be indirectly affected by artificial habitats, see, e.g., Rodrigues and Vieira 2012) not investigated here might also play a role. The significance of the interaction of location with habitat and the temporal component in shaping community composition indicates that the type of habitat modification may be the crucial factor in shaping communities. This is further supported by the fact that Arachovitika, the location where the anthropogenic modification has created the most complex artificial habitat, was found to host significant differences between natural and artificial habitat in most (10 out of 12) months, while the other two locations had far less differences across months.
We also investigated whether total abundance, diversity and species richness vary across habitat type. Both total fish abundance and species richness (but not Shannon diversity index) have significant differences between the two habitat types, showing that the artificial habitat is capable of hosting more fish individuals and a higher number of species, even though the Shannon index did not indicate a different pattern in diversity weighted by abundance. Higher total fish abundances in structured or hard substrates have previously been reported (Guidetti 2000; Giakoumi and Kokkoris 2013) agreeing with the pattern in total abundance documented here. The case might also be that the two habitat types may have been situated too close to each other to show dissimilarities. Perry et al. (2018) have documented similarity in species richness between adjacent stations, despite the stations having different substrates. Total abundance, diversity and species richness also vary in time and space (diversity also across the habitat type interacting with date and species richness across location interacting with date). These findings not only demonstrate the intense spatiotemporal heterogeneity in the shallow littoral, but also indicate that different types of artificial habitat may have variable effects on fish communities (as a result of the location-habitat interaction) and also that the two habitat types may have different roles across the year cycle for example being used as a refuge from predation by juveniles or through seasonal supply and succession of food items (e.g., by growth of algae or settlement of prey on the artificial habitat). It is important to note, however, that, in some cases, univariate diversity metrics can be misleading and it is essential to complement them with community analyses (as, e.g., Shannon’s H or species richness can be found to be not significantly different, even if community composition is actually different—however, this was not the case in our work). The highly dynamic character is also indicated by the generally low similarities documented by the SIMPER analyses. The complex interactions shaping fish communities in the littoral zone (Valesini et al. 2004) call for a more mechanistic investigation of the dynamics of this ecosystem.
Another hypothesis examined in our work regards whether there are differences in the abundance of the most important species between the natural and the artificial habitat. Five out of the ten taxa whose abundance was analyzed showed higher abundance in a specific habitat, with four (C. chromis, D. annularis, D. sargus and Tripterygion sp.) being more abundant over the artificial habitat, while one (C. julis) is more abundant in the natural habitat. At the species level, C. chromis has also been documented to use structured habitats such as rocky substrates or underwater phanerogam meadows (Harmelin 1987). For D. annularis our findings are contrary to those by Sanchez-Jerez et al. (2002) who found lower abundance over artificial substrates than natural ones (however, in that work, the natural habitats were Posidonia beds that are known to be nurseries of this species). Tripterygion sp. is also more abundant in the anthropogenic habitat. Tripterygiidae, together with Gobiidae and Bleniidae, include cryptobenthic species that are often underestimated in visual census surveys (La Mesa et al. 2006), but still constitute an important part of coastal fish biomass (Tiralongo et al. 2016). D. sargus is also more abundant in the anthropogenic habitat, a finding possibly linked to this habitat functioning as a potential nursery for this species (Pastor et al. 2013).
The last hypothesis examined in our work is whether the juvenile ratio of a selection of species is different between habitat types. The juvenile ratios of D. sargus and Tripterygion sp. are also higher in the anthropogenic habitat, indicating that the artificial hard habitat may play an important role as their nursery, as high abundances of fish juveniles have been documented over artificial structures (Clynick 2006). This may be relevant to artificial habitat structural complexity and its function as refuge for predation avoidance or the provision of hard substrate for colonization by algae or invertebrates that may serve as potential food items (Mercader et al. 2018). Contrary to these species, C. julis juveniles (and likely other species not modeled, like M. barbatus, see Fig. 5) are more abundant in the natural habitat. Overall, species that are benthopelagic, form shoals or have generally low affinity to the substrate such as Atherina sp., B. boops, O. melanura, and S. salpa do not have contrasting abundance patterns between natural and artificial habitats (C. chromis is an exception to this, as despite forming large shoals it is highly attached to the substrate during spawning, Picciulin et al. 2004), contrary to others that are more attached to the seabed like C. julis, the representatives of the genus Diplodus and Tripterygion sp. The former also tend to form small or large shoals (while the latter live in small groups or are solitary), thus dominating the community and resulting in non-significant differences in community composition or diversity index between the two habitats.
Percentage of abundance of the different size classes (small, medium, large) recorded in the artificial and natural habitats for the taxa observed. Total abundance (N) of each taxon is denoted in parenthesis. The five taxa whose juvenile abundance fluctuations were modeled with GAMs are indicated by an asterisk (Chromis chromis, Coris julis, Diplodus sargus, Diplodus vulgaris, Tripterygion sp.)
Community composition, diversity, species richness, total abundance, the abundance of three species and of the juveniles of another three species were found to fluctuate across the three locations. Arachovitika was the location with the highest diversity, species richness and total abundance and also hosted the highest abundances of two taxa. Naturally, differences in abundance can be attributed to local productivity, but there are also differences in the type of structural complexity provided by the actual structural modification of the littoral: in Arachovitika, boulders and rocks of various sizes have been added to reinforce the pier with a riprap that forms the artificial station, while in the two other locations, the structural modification mostly consisted of the addition of the vertical wall-like surface. This is supported by the finding that in all six cases (five in species abundance and another in juvenile ratio) where a significant location–habitat interaction exists, the highest abundances are recorded in the artificial habitat of Arachovitika. Indeed, even though the hard substrate and the vertical inclination added can lead to more larvae selecting these locations to settle for food and shelter and increased fish abundance (Bulleri and Chapman 2010), the featureless surface of artificial substrates may fail to provide these functions (Mercader et al. 2018; 2019). Consequently, habitat complexity, combined with other elements like connectivity to other habitats, may be the main reason for increased fish productivity and abundance (Bouchoucha et al. 2016; Perry et al. 2018).
Total fish abundance varied with time in the temperate littoral zone confirming the findings of Hyndes et al. (1999). Diversity and species richness also changed, and the temporal component played a significant role in determining the abundance of eight of the taxa examined individually. This also applies to juvenile percentage fluctuations of three taxa which in some cases showed similar or partially similar patterns with overall taxon abundance. Indeed, in D. vulgaris and D. sargus the juvenile percentage fluctuations were the driver of overall abundance dynamics. Juvenile percentage maxima occur some months after the respective species’ spawning periods (D. sargus: spawning in March–April, juvenile maximum in July, D. vulgaris: spawning in November–February, juvenile maximum in May),—for spawning period timing, see Tsikliras et al. (2010) and references therein and could be attributed to the recruitment pattern of juveniles (however, it has to be noted that C. chromis spawns in June–July, and maximum juvenile abundance has been recorded in March). The significance of the temporal component at community and species level documented here calls for further investigation of the dynamics of fish littoral communities; it is essential to note that to draw conclusions about seasonal patterns, multi-annual sampling schemes would need to be performed to ensure replication of seasons in different years. Another important aspect regards the interactions of the temporal component with location (that has a significant effect on species richness, the abundance of three taxa and the percentage of the juveniles of three taxa) and its interaction with habitat (significant for the abundance of four taxa and the juvenile percentage of another one). As the interaction of time with habitat was not harmonized between juvenile percentage and total abundance of any species, there is a possibility that the reasons for this interaction may be more relevant to prey/food availability. Temporal abundance fluctuations indicating higher occupancy of the artificial littoral in certain months such as March–April for Tripterygion sp. or July–August for D. annularis, D. sargus and D. vulgaris should be investigated in multi-annual works to confirm whether they correspond to actual seasonal patterns and may be relevant to benthic prey settlement and resulting food availability. As the interactions shaping the abundance of individual species are complex (Valesini et al. 2004, but also confirmed in our work), it is essential that species-specific hypothesis-driven research is needed to comprehend the temporal dynamics of populations in artificial habitats.
The findings of the present study have obvious management implications since the addition of artificial habitats can alter the coastal fish communities as documented here. Relevant research on management strategies is mainly oriented in either mitigating the negative impacts of anthropogenic habitats (e.g., by reducing the level of habitat fragmentation they cause) or the construction of coastal structures whose characteristics will render them suitable for colonization (Chapman and Blockley 2009; Bulleri and Chapman 2010; Pastor et al. 2013; Mercader et al. 2019). Interchanging artificial substrate with natural substrate where possible or constructing milder substrate inclinations or water passages can be useful solutions. Habitat complexity is an important aspect (as also indicated by our findings that show that the type and complexity of artificial habitat can affect both community composition and total and species-specific abundance) and it could be introduced by creating rugged, rugose or cavitatious submarine surfaces, instead of smooth ones. However, the relationship between habitat complexity and fish abundance is neither straightforward, nor linear, as higher fish densities could just be the result of attraction-redistribution and resulting concentration effects (Brickhill et al. 2005). Furthermore, it should be investigated whether the addition of artificial substrate does not function as an ecological trap, i.e., does not create a habitat that is preferred by fish, but where their overall fitness is relatively lower than in available natural habitats, as the creation of such traps can be an unintended consequence of management measures (Robertson and Hutto 2006; Hale et al. 2015). Overall, it must be noted that the taxa recorded here have significant ecological importance (some are also commercially important) and should be conserved, as components of the fish diversity of the littoral zone, in an attempt to preserve ecosystem stability and functioning (Danet et al. 2021) and this could possibly be achieved by the introduction of purposefully designed artificial structures.
Visual census techniques are increasingly used to study shallow littoral fish communities (e.g., Ordines et al. 2005; Tuya et al. 2009). However, they may result in underestimation of the abundance of cryptobenthic species (La Mesa et al. 2006). Other approaches, such as underwater photographic contests and the data they can provide may provide useful data of a complementary value to scientific surveys (Tiralongo et al. 2021). In wavy or turbid conditions, visual census may also fail to record all individuals present in the transect (Harmelin-Vivien et al. 1985). Still, visual census was preferred rather than other approaches, like experimental fishing, as it is suitable for hard substrates (de Girolamo and Mazzoldi 2001). The possible bias introduced by the presence and movement of the divers during the visual census transect (possibly leading to modification of fish behavior) could possibly be mitigated using remote sensing techniques. On the same note, the time interval between the delineation of the transect and the actual sampling might not be enough for all individual fish of the area to resume previous behavior and return to their initial location, even though preliminary sampling trials had indicated relatively similar species composition and abundances between laying the transects and actual sampling. Finally, the close proximity between the natural and anthropogenic habitat in each location, may be a factor minimizing the differences between habitats (as mobile species can easily move from one station to the other), but this approach was preferred under our experimental design in order to keep the other environmental factors (e.g., productivity, exposure) as similar as possible, since other factors such as food availability, shading or wave action may affect the shallow littoral community (Franzitta and Airoldi 2019).
The perspectives of the present work are across various possible orientations: on the species level, the possible role of the artificial/anthropogenic habitat could be further investigated complementing fieldwork monitoring with specific experimental designs under controlled conditions (in water tanks). A very interesting aspect of such an experimental approach could be the settlement process and the level of success in evading predation by juvenile stages. On the community level, it should be investigated whether the communities emergent over artificial substrates may affect ecosystem functioning (e.g., by altering competition or predation relationships in the shallow littoral). Some of the most intriguing questions arise with regard to the applied management implications of the current work. These could, e.g., focus on the structural complexity of the artificial substrates and the studies relevant to the introduction of three-dimensional structures that can act as actual fish refuges, or the planning of the installation of artificial structures either interchanging them with other (natural) habitat types ensuring habitat connectivity within the broader seascape to allow the transition of ontogenetic stages from one habitat to the other or modifying their characteristics where they might operate as ecological traps (e.g., creating underwater passages allowing some sort of current flow where needed). As human presence and activity has been and will be increasing, it is essential to investigate the impacts of shoreline modification in the rapidly changing coastal zone.
Data availability
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
References
Able KW, Grothues TM, Kemp IM (2013) Fine-scale distribution of pelagic fishes relative to a large urban pier. Mar Ecol Prog Ser 476:185–198. https://doi.org/10.3354/meps10151
Airoldi L, Beck MW (2007) Loss, status and trends for coastal marine habitats of Europe. Oceanogr Mar Biol 45:345–405. https://doi.org/10.1201/9781420050943.ch7
Anderson MJ, Gorley RN, Clarke KR (2008) PERMANOVA + for PRIMER Guide to Software and StatisticalMethods. PRIMER-E, Plymounth
Balouskus RG, Targett TE (2012) Egg deposition by Atlantic silverside, Menidia menidia: substrate utilization and comparison of natural and altered shoreline type. Estuaries Coasts 35:1100–1109. https://doi.org/10.1007/s12237-012-9495-x
Beck MW, Heck KL Jr, Able KW, Childers DL, Eggleston DB, Gillanders BM, Halpern B, Hayes CG, Hoshino K, Minello TJ, Orth RJ, Sheridan PF, Weinstein MP (2001) The identification, conservation, and management of estuarine and marine nurseries for fish and invertebrates. Bioscience 51:633–641. https://doi.org/10.1641/0006-3568(2001)051[0633:TICAMO]2.0.CO;2
Biagi F, Gambaccini S, Zazzetta M (1998) Settlement and recruitment in fishes: the role of coastal areas. Ital J Zool 65(S1):269–274. https://doi.org/10.1080/11250009809386831
Bilkovic DM, Roggero MM (2008) Effects of coastal development on nearshore estuarine nekton communities. Mar Ecol Prog Ser 358:27–39. https://doi.org/10.3354/meps07279
Bouchoucha M, Darnaude AM, Gudefin A, Neveu R, Verdoit-Jarraya M, Boissery P, Lenfant P (2016) Potential use of marinas as nursery grounds by rocky fishes: insights from four Diplodus species in the Mediterranean. Mar Ecol Prog Ser 547:193–209. https://doi.org/10.3354/meps11641
Brickhill MJ, Lee SY, Connolly RM (2005) Fishes associated with artificial reefs: attributing changes to attraction or production using novel approaches. J Fish Biol 67:53–71. https://doi.org/10.1111/j.0022-1112.2005.00915.x
Bulleri F (2005) The of artificial structures on marine soft- and hard-bottoms: ecological implications of epibiota. Environ Conserv 32:101–102. https://doi.org/10.1017/S0376892905002183
Bulleri F, Chapman MG (2010) The introduction of coastal infrastructure as a driver of change in marine environments. J Appl Ecol 47:26–35. https://doi.org/10.1111/j.1365-2664.2009.01751.x
Chapman MG (2003) Paucity of mobile species on constructed seawalls: effects of urbanization on biodiversity. Mar Ecol Prog Ser 264:21–29. https://doi.org/10.3354/meps264021
Chapman MG, Blockley DJ (2009) Engineering novel habitats on urban infrastructure to increase intertidal biodiversity. Oecologia 161:625–635. https://doi.org/10.1007/s00442-009-1393-y
Cheminée A, Le Direach L, Rouanet E et al (2021) All shallow coastal habitats matter as nurseries for Mediterranean juvenile fish. Sci Rep 11:14631. https://doi.org/10.1038/s41598-021-93557-2
Clarke KR, Gorley RN (2006) PRIMER v6: User Manual/Tutorial. PRIMER-E, 192 pp., Plymouth
Clynick BG (2006) Assemblages of fish associated with coastal marinas in North-Western Italy. J Mar Biol Assoc UK 86:847–852. https://doi.org/10.1017/S0025315406013786
Clynick BG, Chapman MG, Underwood AJ (2008) Fish assemblages associated with urban structures and natural reefs in Sydney, Australia. Austral Ecol 33:140–150. https://doi.org/10.1111/j.1442-9993.2007.01802.x
Costanza R, Darge R, Degroot R, Farber S, Grasso M, Hannon B, Limburg K, Naeem S, O’ Neill RV, Paruelo J, Raskin RG, Sutton P, Vandenbelt M (1997) The value of the world’s ecosystem services and natural capital. Nature 387:253–260. https://doi.org/10.1038/387253a0
Crain CM, Halpern BS, Beck MW, Kappel CV (2009) Understanding and managing human threats to the coastal marine environment. Ann N Y Acad Sci 1162:39–62. https://doi.org/10.1111/j.1749-6632.2009.04496.x
Danet A, Mouchet M, Bonnaffé W, Thébault E, Fontaine C (2021) Species richness and food-web structure jointly drive community biomass and its temporal stability in fish communities. Ecol Lett 24:2364–2377. https://doi.org/10.1111/ele.13857
De Girolamo M, Mazzoldi C (2001) The application of visual census on Mediterranean rocky habitats. Mar Environ Res 51:1–16. https://doi.org/10.1016/s0141-1136(00)00028-3
Duffy-Anderson JT, Able KW (1999) Effects of municipal piers on the growth of juvenile fishes in the Hudson river estuary: a study across a pier edge. Mar Biol 133:409–418. https://doi.org/10.1007/s002270050479
Duffy-Anderson JT, Able KW (2001) An assessment of the feeding success of young-of-the-year winter flounder (Pseudopleuronectes americanus) near a municipal pier in the Hudson River estuary, USA. Estuaries 24:430–440. https://doi.org/10.2307/1353244
Duffy-Anderson JT, Able MJP, KW, (2003) A characterization of juvenile fish assemblages around man-made structures in the New York-New Jersey Harbor Estuary, USA. Bull Mar Sci 72:877–889
EEA (2019). The European environment —state and outlook 2020: Knowledge for transition to a sustainable Europe. European Environment Agency, Publications Office of the European Union, Luxembourg. https://data.europa.eu/doi/10.2800/085135
Franzitta G, Airoldi L (2019) Fish assemblages associated with coastal defence structures: does the surrounding habitat matter? Reg Stud Mar Sci 31:100743. https://doi.org/10.1016/j.rsma.2019.100743
Fraschetti S, Bianchi CN, Terlizzi A, Fanelli G, Morri C, Boero F (2001) Spatial variability and human disturbance in shallow subtidal hard substrate assemblages: a regional approach. Mar Ecol Prog Ser 212:1–12. https://doi.org/10.3354/meps212001
Georgiadis M, Mavraki N, Koutsikopoulos C, Tzanatos E (2014) Spatio-temporal dynamics and management implications of the nightly appearance of Boops boops (Acanthopterygii, Perciformes) juvenile shoals in the anthropogenically modified Mediterranean littoral zone. Hydrobiologia 734:81–96. https://doi.org/10.1007/s10750-014-1871-z
Giakoumi S, Kokkoris GD (2013) Effects of habitat and substrate complexity on shallow sublittoral fish assemblages in the Cyclades Archipelago, North-astern Mediterranean Sea. Mediterr Mar Sci 14:58–68. https://doi.org/10.12681/mms.318
Grothues TM, Rackovan JL, Able KW (2016) Modification of nektonic fish distribution by piers and pile fields in an urban estuary. J Exp Mar Bio Ecol 485:47–56. https://doi.org/10.1016/j.jembe.2016.08.004
Guidetti P (2000) Differences among fish assemblages associated with nearshore Posidonia oceanica seagrass beds, rocky-algal reefs and unvegetated sand habitats in the Adriatic Sea. Estuar Coast Shelf Sci 50:515–529. https://doi.org/10.1006/ecss.1999.0584
Guidetti P (2004) Fish assemblages associated with coastal defence structures in south-western Italy (Mediterranean Sea). J Mar Biol Assoc UK 84:669–670. https://doi.org/10.1017/S0025315404009725h
Hale R, Coleman R, Pettigrove V, Swearer SE (2015) Identifying, preventing and mitigating ecological traps to improve the management of urban aquatic ecosystems. J Appl Ecol 52:928–939. https://doi.org/10.1111/1365-2664.12458
Halpern BS, Walbridge S, Selkoe KA, Kappel CV, Micheli F, D’Agrosa C, Bruno JF, Casey KS, Ebert C, Fox HE, Fujita R, Heinemann D, Lenihan HS, Madin EMP, Perry MT, Selig ER, Spalding M, Steneck R, Watson R (2008) A global map of human impact on marine ecosystems. Science 319:948–952. https://doi.org/10.1126/science.1149345
Harmelin JG (1987) Structure and variability of the Ichthyo-fauna in a Mediterranean protected rocky area (National Park of Port-Cros, France). Mar Ecol 8:263–284
Harmelin-Vivien M, Harmelin J, Chauvet C et al (1985) Evaluation visuelle des peuplements et populations de poissons: méthodes et problèmes. Rev Ecol 40:467–539
Hastie TJ, Tibshirani RJ (1990) Generalized additive models. Chapman and Hall, London, p 352
Hendon JR, Peterson MS, Comyns BH (2000) Spatio-temporal distribution of larval Gobiosoma bosc in waters adjacent to natural and altered marsh-edge habitats of Mississippi coastal waters. Bull Mar Sci 66:143–156
Hyndes GA, Platell ME, Potter IC, Lenanton RCJ (1999) Does the composition of the demersal fish assemblages in temperate coastal waters change with depth and undergo consistent seasonal changes? Mar Biol 134:335–352. https://doi.org/10.1007/s002270050551
Islam MS, Tanaka M (2004) Impacts of pollution on coastal and marine ecosystems including coastal and marine fisheries and approach for management: a review and synthesis. Mar Pollut Bull 48:624–649. https://doi.org/10.1016/j.marpolbul.2003.12.004
Kiparissis S, Tserpes G, Somarakis S, Economidis PS, Koutsikopoulos C (2008) Site-attachment behaviour of Oblada melanura (Linnaeus, 1758) (Osteichthyes: Sparidae) benthic larvae: a quantitative approach. Sci Mar 72:429–436. https://doi.org/10.3989/scimar.2008.72n3429
La Mesa G, Di Muccio S, Vacchi M (2006) Structure of a Mediterranean cryptobenthic fish community and its relationships with habitat characteristics. Mar Biol 149:149–167. https://doi.org/10.1007/s00227-005-0194-z
Marra G, Wood SN (2011) Practical variable selection for generalized additive models. Comput Stat Data Anal 55:2372–2387. https://doi.org/10.1016/j.csda.2011.02.004
Mavraki N, Georgiadis M, Koutsikopoulos C, Tzanatos E (2016) Unravelling the nocturnal appearance of Bogue Boops boops shoals in the anthropogenically modified shallow littoral. J Fish Biol 88:2060–2066. https://doi.org/10.1111/jfb.12942
Mercader M, Rider M, Cheminée A, Pastor J, Zawadzki A, Mercière A, Crec’hriou R, Verdoit-Jarraya M, Lenfant P (2018) Spatial distribution of juvenile fish along an artificialized seascape, insights from common coastal species in the Northwestern Mediterranean Sea. Mar Environ Res 137:60–72. https://doi.org/10.1016/j.marenvres.2018.02.030
Mercader M, Blazy C, Di Pane J, Devissi C, Mercière A, Cheminée A, Thiriet P, Pastor J, Crec’hriou R, Verdoit-Jarraya M, Lenfant P (2019) Is artificial habitat diversity a key to restoring nurseries for juvenile coastal fish? Ex situ experiments on habitat selection and survival of juvenile seabreams. Restor Ecol 27:1155–1165. https://doi.org/10.1111/rec.12948
Morley SA, Toft JD, Hanson KM (2012) Ecological effects of shoreline armoring on intertidal habitats of a puget sound urban estuary. Estuaries Coasts 35:774–784. https://doi.org/10.1007/s12237-012-9481-3
Munsch SH, Cordell JR, Toft JD, Morgan EE (2014) Effects of seawalls and piers on fish assemblages and juvenile salmon feeding behavior. N Am J Fish Manag 34:814–827. https://doi.org/10.1080/02755947.2014.910579
Munsch SH, Cordell JR, Toft JD (2015) Effects of seawall armoring on juvenile Pacific salmon diets in an urban estuarine embayment. Mar Ecol Prog Ser 535:213–229. https://doi.org/10.3354/meps11403
Munsch SH, Cordell JR, Toft JD (2016) Fine-scale habitat use and behavior of a nearshore fish community: nursery functions, predation avoidance, and spatiotemporal habitat partitioning. Mar Ecol Prog Ser 557:1–15. https://doi.org/10.3354/meps11862
Munsch SH, Cordell JR, Toft JD (2017) Effects of shoreline armouring and overwater structures on coastal and estuarine fish: opportunities for habitat improvement. J Appl Ecol 54:1373–1384. https://doi.org/10.1111/1365-2664.12906
Nagelkerken I, Sheaves M, Baker R, Connolly RM (2015) The seascape nursery: a novel spatial approach to identify and manage nurseries for coastal marine fauna. Fish Fish 16:362–371. https://doi.org/10.1111/faf.12057
Ordines F, Moranta J, Palmer M, Lerycke A, Suau A, Morales-Nin B, Grau AM (2005) Variations in a shallow rocky reef fish community at different spatial scales in the western Mediterranean Sea. Mar Ecol Prog Ser 304:221–233. https://doi.org/10.3354/meps304221
Pastor J, Koeck B, Astruch P, Lenfant P (2013) Coastal man-made habitats: potential nurseries for an exploited fish species, Diplodus sargus (Linnaeus, 1758). Fish Res 148:74–80. https://doi.org/10.1016/j.fishres.2013.08.014
Pérez-Ruzafa A, Garcı́a-Charton JA, Barcala E, Marcos C (2006) Changes in benthic fish assemblages as a consequence of coastal works in a coastal lagoon: the Mar Menor (Spain, Western Mediterranean). Mar Pollut Bull 53:107–120. https://doi.org/10.1016/j.marpolbul.2005.09.014
Perry D, Staveley TAB, Gullström M (2018) Habitat connectivity of fish in temperate shallow-water seascapes. Front Mar Sci 4:1–12. https://doi.org/10.3389/fmars.2017.00440
Picciulin M, Verginella L, Spoto M, Ferrero EA (2004) Colonial nesting and the importance of the brood size in male parasitic reproduction of the Mediterranean Damselfish Chromis chromis (Pisces: Pomacentridae). Environ Biol Fishes 70:23–30. https://doi.org/10.1023/B:EBFI.0000022851.49302.df
Polte P, Kotterba P, Moll D, von Nordheim L (2017) Ontogenetic loops in habitat use highlight the importance of littoral habitats for early life-stages of oceanic fishes in temperate waters. Sci Rep 7:42709. https://doi.org/10.1038/srep42709
R Core Team (2021). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Ray GC (1991) Coastal-zone biodiversity patterns – Principles of landscape ecology may help explain the processes underlying coastal diversity. Bioscience 41:490–498. https://doi.org/10.2307/1311807
Rice CA (2006) Effects of shoreline modification on a Northern Puget Sound beach: microclimate and embryo mortality in surf smelt (Hypomesus pretiosus). Estuaries Coast 29:63–71. https://doi.org/10.1007/BF02784699
Riofrío-Lazo M, Zetina-Rejón MJ, Vaca-Pita L, Murillo-Posada JC, Páez-Rosas D (2022) Fish diversity patterns along coastal habitats of the southeastern Galapagos archipelago and their relationship with environmental variables. Sci Rep 12:3604. https://doi.org/10.1038/s41598-022-07601-w
Robertson BA, Hutto RL (2006) A framework for understanding ecological traps and an evaluation of existing evidence. Ecology 87:1075–1085. https://doi.org/10.1890/0012-9658(2006)87[1075:affuet]2.0.co;2
Rodrigues FL, Vieira JP (2012) Surf zone fish abundance and diversity at two sandy beaches separated by long rocky jetties. J Mar Biol Assoc UK 93(04):867–875. https://doi.org/10.1017/s0025315412001531
Samourdani A, Tzanatos E (2022) Fish distribution and behaviour with regard to the time of day and the anthropogenic structural modification of the shallow littoral. J Fish Biol 100:820–830. https://doi.org/10.1111/jfb.15001
Sánchez-Jerez P, Gillanders BM, Rodríguez-Ruiz S, Ramos-Esplá AA (2002) Effect of an artificial reef in Posidonia meadows on fish assemblage and diet of Diplodus annularis. ICES J Mar Sci 59:S59–S68. https://doi.org/10.1006/jmsc.2002.1213
Scheuerell MD, Schindler DE (2004) Changes in the spatial distribution of fishes in lakes along a residential development gradient. Ecosystems 7:98–106. https://doi.org/10.1007/s10750-013-1746-8
Scyphers SB, Gouhier TC, Grabowski JH, Beck MW, Mareska J, Powers SP (2015) Natural shorelines promote the stability of fish communities in an urbanized coastal system. PLoS One 10:e0118580. https://doi.org/10.1371/journal.pone.0118580
Seitz RD, Wennhage H, Bergstrom U, Lipcius RN, Ysebaert T (2014) Ecological value of coastal habitats for commercially and ecologically important species. ICES J Mar Sci 71:648–665. https://doi.org/10.1093/icesjms/fst152
Somarakis S, Machias A (2002) Age, growth and bathymetric distribution of red Pandora (Pagellus erythrinus) on the Cretan Shelf (eastern Mediterranean). J Mar Biol Assoc UK 82:149–160. https://doi.org/10.1017/S002531540200526X
Tiralongo F, Tibullo D, Brundo MV, Paladini De Mendoza F, Melchiorri C, Marcelli M (2016) Habitat preference of combtooth blennies (Actinopterygii: Perciformes: Blenniidae) in very shallow waters of the Ionian Sea, south-eastern sicily, Italy. Acta Ichthyol Piscat 46:65–75. https://doi.org/10.3750/AIP2016.46.2.02
Tiralongo F, La Mesa G, De Mendoza FP, Massari F, Azzurro E (2021) Underwater photo contests to complement coastal fish inventories: results from two marine protected areas in the Mediterranean. Medit Mar Sci 22(2):436–445. https://doi.org/10.12681/mms.26176
Toft JD, Cordell JR, Simenstad CA, Stamatiou LA (2007) Fish distribution, abundance, and behavior along city shoreline types in Puget Sound. N Am J Fish Manag 27:465–480. https://doi.org/10.1577/M05-158.1
Torre MP, Targett TE (2016) Nekton assemblages along riprap-altered shorelines in Delaware Bay, USA: comparisons with adjacent beach. Mar Ecol Prog Ser 548:209–218. https://doi.org/10.3354/meps11685
Tsikliras AC, Antonopoulou E, Stergiou KI (2010) Spawning period of Mediterranean marine fishes. Rev Fish Biol Fisheries 20:499–538. https://doi.org/10.1007/s11160-010-9158-6
Tuya F, Wernberg T, Thomsen MS (2009) Habitat structure affect abundances of labrid fishes across temperate reefs in South-Western Australia. Environ Biol Fishes 86:311–319. https://doi.org/10.1007/s10641-009-9520-5
Valesini FJ, Potter IC, Clarke KR (2004) To what extent are the fish compositions at nearshore sites along a heterogeneous coast related to habitat type? Estuar Coast Shelf Sci 60:737–754. https://doi.org/10.1016/j.ecss.2004.03.012
Willis TJ, Badalamenti F, Milazzo M (2006) Diel variability in counts of reef fishes and its implications for monitoring. J Exp Mar Biol Ecol 331:108–120. https://doi.org/10.1016/j.jembe.2005.10.003
Wood SN (2006) Generalized additive models: an introduction with R. Chapman and Hall/CRC, London, p 392
Wood SN (2013) On p-values for smooth components of an extended generalized additive model. Biometrika 100:221–228. https://doi.org/10.1093/biomet/ass048
Worm B, Barbier EB, Beaumont N, Duffy JE, Folke C, Halpern BS, Jackson JBC, Lotze HK, Micheli F, Palumbi SR, Sala E, Selkoe KA, Stachowicz JJ, Watson R (2006) Impacts of biodiversity loss on ocean ecosystem services. Science 314:787–790. https://doi.org/10.1126/science.1132294
Zucchetta M, Franco A, Torricelli P, Franzoi P (2010) Habitat distribution model for European flounder juveniles in the Venice lagoon. J Sea Res 64:133–144. https://doi.org/10.1016/j.seares.2009.12.003
Funding
Open access funding provided by HEAL-Link Greece. The authors declare that no relevant funds, grants or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
ET conceived the work. ET and MMN designed the work. MMN did the visual census with the assistance of ET. AL led the statistical analyses with the participation of ET and MMN. All the authors wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
No ethical approvals were required as no animals were handled during this work. All field activities were carried out in compliance with national laws.
Additional information
Responsible Editor: W. Figueira.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ntouni, MM., Lazaris, A. & Tzanatos, E. Patterns of fish occupancy of artificial habitats in the eastern Mediterranean shallow littoral. Mar Biol 170, 105 (2023). https://doi.org/10.1007/s00227-023-04253-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-023-04253-w