Abstract
Stable isotopes have provided important insight into the trophic structure and interaction in many ecosystems, but to date have scarcely been applied to the complex food webs of coral reefs. We sampled white muscle tissues from the fish species composing 80% of the biomass in the 4–512 g body mass range at Cape Eleuthera (the Bahamas) in order to examine isotopic niches characterised by δ13C and δ15N data and explore whether fish body size is a driver of trophic position based on δ15N. We found the planktivore isotopic niche was distinct from those of the other trophic guilds suggesting the unique isotopic baseline of pelagic production sources. Other trophic guilds showed some level of overlap among them especially in the δ13C value which is attributable to source omnivory. Surprising features of the isotopic niches included the benthivore Halichoeres pictus, herbivores Acanthurus coeruleus and Coryphopterus personatus and omnivore Thalassoma bifasciatum being close to the planktivore guild, while the piscivore Aulostomus maculatus came within the omnivore and herbivore ellipses. These characterisations contradicted the simple trophic categories normally assigned to these species. δ15N tended to increase with body mass in most species, and at community level, the linear δ15N–log2 body mass relationship pointing to a mean predator–prey mass ratio of 1047:1 and a relatively long food chain compared with studies in other aquatic systems. This first demonstration of a positive δ15N–body mass relationship in a coral reef fish community suggested that the Cape Eleuthera coral reef food web was likely supported by one main pathway and bigger reef fishes tended to feed at higher trophic position. Such finding is similar to other marine ecosystems (e.g. North Sea).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In coral reef food webs, fishes are typically categorised into strict trophic guilds (e.g. Hiatt and Strasburg 1960; Jennings et al. 1995; Polunin 1996; McClanahan et al. 1999; Hughes et al. 2003; MacNeil et al. 2015; D’Agata et al. 2016; Graham et al. 2017; Stamoulis et al. 2017; Hadi et al. 2018; Moustaka et al. 2018). Yet this may overlook trophoplasticity, where many species feed across trophic boundaries (e.g. Robertson 1982; Chen 2002) and thus their trophic functions and the overall functioning of the reef (Mouillot et al. 2014). Inaccurate trophic information jeopardises comprehensive understanding of these food webs. Traditional gut contents analysis gives detailed dietary information; however, this typically has high temporal and spatial variability (Jennings et al. 2001) and it might include items accidently ingested (e.g. eDNA in gut contents DNA bar coding; Leal and Ferrier-Pagès 2016). Stable isotopes provide a time-integrated signal of what has been assimilated from the diet (Jennings et al. 2001). The stable isotope ratio of carbon (13C/12C, expressed as δ13C) is commonly used to distinguish production sources such as benthic and pelagic autotrophs (Tieszen et al. 1983; Bearhop et al. 2004), whereas the stable isotope ratio of nitrogen (15N/14N, expressed as δ15N) is used as a proxy for trophic position (TP) because it has a higher trophic enrichment factor (TEF) (DeNiro and Epstein 1978, 1981; McCutchan et al. 2003; Strieder Philippsen and Benedito 2013) and shows less variation at the baseline (Hesslein et al. 1991) than does carbon. Combining δ13C and δ15N delineates ‘isotopic niches’ (Leibold 1995; Newsome et al. 2007) which inform feeding strategies and trophic interactions (Post 2002) at species, trophic guild and community levels (France et al. 1998; Jennings et al. 2001, 2002a, b; Al-Habsi et al. 2008). Combining these levels of data can explain a species’ trophic ecology including how strict it is and what potential source(s) it is utilising with the latter being very important because trophic interactions among omnivorous species within the same community remain understudied in coral reefs.
Trophodynamics can be size based. In aquatic systems, large individuals generally feed at higher TPs (Jennings et al. 2001, 2002b; Al-Habsi et al. 2008; Romanuk et al. 2011). This is a result of ontogenetic dietary shifts, morphometric changes including increasing gape size, post-maturity factors and greater predator ability that influence foraging (Peters 1986; Jennings et al. 2001; Munday 2001; Jennings et al. 2002b; Al-Habsi et al. 2008; Newman et al. 2012; Robinson and Baum 2015; Ríos et al. 2019). However, some large fishes forage at lower TPs due to dietary shifts (e.g. Chen 2002; Layman et al. 2005), seasonality effects (Bronk and Glibert 1993; Rolff 2000) and human disturbance (Pastorok and Bilyard 1985). TPs of some species remain relatively unchanged with increasing body size (e.g. herbivores; Plass-Johnson et al. 2013; Dromard et al. 2015). Size-based feeding remains almost unstudied in coral reef ecosystems (Robinson and Baum 2015) where large herbivores contribute greatly to the biomass.
Investigating TP to body mass relationships at community level can improve understanding of predator to prey relationships and energetic pathways (Romanuk et al. 2011; Robinson and Baum 2015) and of changes in community trophic composition (Graham et al. 2017). Where a positive TP relationship with body mass exists, the predator–prey mass ratio (PPMR) can be calculated. This reflects constraints on community structure (Trebilco et al. 2013) and can be used to evaluate general food web properties such as food chain length (Jennings and Warr 2003) and food chain transfer efficiency (Jennings et al. 2002c; Barnes et al. 2010) across different aquatic systems (Jennings et al. 2001, 2002c; Bode et al. 2003, 2006; Al-Habsi et al. 2008). The great diversity of production sources and trophic partitioning by consumers on coral reefs suggest that the PPMR may be smaller and food chains longer than in many other marine ecosystems. But to date, the community-level δ15N to body mass relationship and the PPMR have not been reported for a coral reef, so important insights such as measures of food chain length cannot be gained.
Here, underwater visual census and stable isotope data were used to explore the role of body size and trophic interactions among fish species in structuring a coral reef fish community at Cape Eleuthera (the Bahamas). Specifically, the study aimed to: (1) define isotopic niches at species and trophic guild levels in order to understand the principal energy pathways supporting them; and (2) assess whether there are positive δ15N–body mass relationships at the species and community levels and if so, derive an average predator–prey biomass ratio (PPMR).
Materials and methods
Study site
Four accessible and conservation-protected reef sites (Fig. 1) on the Exuma side of Cape Eleuthera (the Bahamas) with relatively high structural complexity and a diverse fish community were selected for visual surveys and fish sampling. These sites were close to each other and to shore and represented both bommies and patch reefs. In spite of the conservation status of the study sites, the reefs have been subject to chronic overfishing and otherwise impacted by cyclones and coral bleaching episodes.
Fish survey
Underwater visual census (UVC) was conducted by two divers using eight single-sweep 30 m × 5 m transects at each site to record fish species, individual total length (L, to nearest cm) and numbers of individuals. Surveyors’ length estimation precision was repeatedly measured by conducting underwater fish-shaped object length estimation training (Bell et al. 1985) to minimise error (± 5%). Transects ran parallel to each other to avoid intersection. Transects were carried out in the morning (9:30–11:30) or afternoon (14:00–16:00), while swimming at a steady speed for 30 min. Highly mobile transient individuals (e.g. sharks and jacks) were excluded since they had large home range and were not necessarily reef associated, and also large schools of fishes were excluded since they were sighted only sporadically (Ferreira et al. 2001).
Sampling for stable isotope analysis
Abundant species were selected by their contribution to the total biomass (B). From UVC data, individual body mass values (M, g) were calculated from L (cm) using:
with published length to weight conversion factors a and b (“Appendix A” in supplementary materials) from fishbase.org (Froese and Pauly 2017). Where conversion factors were linked with standard or fork length rather than L, or L was in units other than centimetres, length was converted into appropriate units or length types using the equations in fishbase.org. All body mass data were log2 transformed to remove any effects of relationship between body size and phylogeny (Freckleton 2000). For each M class (2–512 g), B was calculated by summing individual M values. Species were ranked in order of their contribution to the B of each log2M interval, and those making up 80% of the B were selected for the community trophic structure analysis.
Samples of selected species (Table 1) were collected through the length range recorded in the UVC to adequately describe species δ15N–log2M relationships (Galván et al. 2010). The size range cover ratio (rL = LSIA sample range/LUVC range) was used to check whether the sampling objective was met. Fish were collected using a variety of techniques depending on their behaviour towards divers, feeding habits and swimming patterns. Hand net, gill net (mesh size: 1 cm × 1 cm, net size: 2 m × 1 m), BINCKE net (Anderson and Carr 1998), underwater fishing hook and line, static hook and line, spearfishing (local fishermen only) and hook and line surface trolling were all used in the sampling in the survey sites and adjacent areas (Table 2). Fish were killed by spine dislocation and stored in an ice chest on board. Samples were all collected within a 1-month period and from nearby sites to reduce spatial and temporal isotopic variation (Bronk and Glibert 1993; Jennings et al. 1997; Rolff 2000; McCutchan et al. 2003).
After landing, approximately 2 g of dorsal white muscle tissue near the dorsal fin were dissected, rinsed with water and stored in individual whirlpack bags in a − 20 °C freezer, and algal samples were only rinsed and stored in a freezer. All samples were dried in individual tin trays in an oven at 40 °C for ~ 12 h until fully dried and then in individual sealed Eppendorf tubes in zip-lock bags.
Stable isotope analysis preparation
All dried samples were imported to Newcastle University, freeze dried and then manually ground with mortar and pestle. Fish samples were weighed to 1.0 ± 0.1 mg in tin capsules with a Mettler MT5 microbalance. The prepared samples were analysed by Iso-Analytical Ltd (Crewe, UK) by Elemental Analysis-Isotope Ratio Mass Spectrometry (EA-IRMS). The 15N/14N ratio (δ15N) was expressed relative to N2 in air for nitrogen, while that of 13C/12C (δ13C) was relative to Pee Dee Belemnite (PDB) of CO2. Reference material used for this analysis was IA-R042 (δ13C = − 21.6 ± 0.1‰, δ15N = 7.6 ± 0.1‰), with quality control check samples IA-R042, IA-R038 (δ13C = − 25.0 ± 0.1‰, δ15N = − 0.4 ± 0.1‰), a mixture of IA-R006 (δ13C = − 11.7 ± 0.0‰) and IA-R046 (δ15N = 21.9 ± 0.2‰). IAR042 and IA-R038 were calibrated against and traceable to IAEA-CH-6 (δ13C = − 10.4‰) and IAEA-N-1 (δ15N = 0.4‰), IA-R006 to IAEA-CH-6 and IA-R046 to IAEA-N-1. External standards (fish white muscle tissue, δ13C = − 18.9 ± 0.0‰, δ15N = 12.9 ± 0.1‰) were also used for future reference. The precision of analysis for δ13C, δ15N, %C and %N was ± 0.1‰, ± 0.2‰, ± 4% and ± 1%, respectively. For individual samples, no lipid extraction was needed because their C/N ratios were less than 3.7 (Fry et al. 2003; Sweeting et al. 2006).
Data analysis
All data were tested for normality and homogeneity of variance prior to analysis and analysed in R 3.24 (R Core Team 2016) using the package siar (Parnell and Jackson 2013) between δ13C and δ15N data, linear regression (Wilkinson and Rogers 1973; Bates et al. 1992) between δ15N and log2 body mass and mvtnorm to simulate values from a bivariate normal distribution. Results were visualised using ggplot2 (Wickham and Chang 2016). The assumptions of the ordinary least squares linear regression analyses were assessed with QQ plots, histograms of standardised residuals and plots of standardised residuals versus fitted values. Pearson’s correlation coefficient was used to test correlations between variables. Significance was set at p = 0.05 in all cases. All errors are reported as ± 1SE unless otherwise stated.
Species and trophic guild stable isotope analyses
The bulk δ13C and δ15N data were used to interpret δ15N–δ13C relationships in each species and trophic guild (Froese and Pauly 2017). Isotopic niches of five trophic guilds (four benthic: benthivore, herbivore, omnivore and piscivore and one pelagic: planktivore) were investigated using SIBER in the siar package. This was achieved by investigating standard ellipse parameters: eccentricity (E) and the angle in degrees (θ, 0°–180°) between semi-major axis and the x axis, the sample size corrected standard ellipse areas (SEAC) and Bayesian standard ellipse areas (SEAB) (Jackson et al. 2011). θ and E values potentially distinguish among isotopic niches where different trophic guilds have similar sized SEAC but different relationships between δ13C and δ15N (Reid et al. 2016). θ values close to 0° or 90° suggest dispersion in only one axis: θ values close to 0° represent relative dispersion along the x axis (δ13C), indicating multiple production sources, while θ values close to 90° highlight relative dispersion along the y axis (δ15N), indicating feeding across multiple trophic positions from a uniform basal source. E explains the variance on the x and y axes: low E refers to similar variance on both axes with a more circular shape, while high E indicates that the isotopic niche is stretched along either x or y axis. The overlap of the standard ellipses between guilds was calculated using “sea.overlap” using SIBER. In order to compare isotopic niche areas among trophic guilds, a Bayesian approach was used that calculated 20,000 posterior estimates of SEAB based on the data set. The mode and 95% credible intervals (CI) were reported. A significant difference among SEAB was interpreted graphically whereby if the 95% CI did not overlap, then the SEAB were deemed to be significantly different (Parnell and Jackson 2013).
δ15N–body size relationship
Cross-species relationships between stable isotope data and M were analysed using linear regression between mean bulk δ13C and δ15N of each species and their maximum body mass (Mmax) recorded by Humann and DeLoach (1989). Comparing fishes at a fixed proportion of maximum size (here ≥ 55% of Lmax) reduced the risk of comparing different life stages (Charnov 1993; Jennings et al. 2001; Galván et al. 2010).
For each species, the δ15N–log2M relationship was generated using linear regression, the slope and intercept values being used to calculate δ15N values of individuals of the same species with other body mass values. To derive the community relationship between δ15N and log2B (B was used instead of M to differentiate the community-level analysis from the species-level analysis), the mean weighted δ15N value of each log2B class was derived by calculating (1) the mass ratio (r) of each individual (i) in each log2B class, using \(r_{i} = M_{i} /\sum\nolimits_{i = 1}^{n} {M_{i} }\), where n is the total number of individuals in the log2B class; and (2) mean weighted δ15N for each log2B class j of the whole community as \(\updelta^{15} {\text{N}}_{j} = \sum\nolimits_{i = 1}^{n} {\updelta^{15} } {\text{N}}_{i} \times r_{i}\) (Al-Habsi et al. 2008). The δ15N of each individual (δ15Ni) was either calculated using the species-level linear δ15N–log2M relationship if it is significant or equivalent to the mean δ15N of the species if not.
Because of the species richness of these reefs and limitation of fishing techniques, not all species could be sampled. To obtain δ15N values of such species, several methods were used (full details in “Appendix B” supplementary materials): (1) using samples from similar species within the same genus if possible (order of priority: same genus, family, site, trophic position, diet and feeding habit from fishbase.org) by comparing 3 criteria: (a) length to weight conversion factors, (b) dietary similarities and (c) their feeding behaviours in this area; and (2) using existing data in the literature from nearby locations or elsewhere in the Caribbean with baseline adjustment (D. cavernosa to D. cavernosa if possible, otherwise D. cavernosa to turf algae) to reduce temporal and/or spatial baseline variations (Cabana and Rasmussen 1996).
Predator–prey mass ratio
The mean PPMR was calculated using the slope (b) of the regression line of the TP–log2B relationship as \({\text{PPMR}} = 2^{1/b}\). TP was calculated using the additive framework as
where the Δδ15N is assumed constant and equal to 3.4‰ (DeNiro and Epstein 1981; Minagawa and Wada 1984). TPbase was indicated by the striped parrotfish (S. iserti, δ15Nbase = 4.2 ± 0.1‰; TPbase = 2.0) due to its low isotopic variation across sizes, time integration of seasonality from producers and adequate sample size (n = 10). The slope (b) of TP–log2B relationship was obtained from the slope (s) of δ15N–log2B relationship where \(b = s/3.4\). Thus, the PPMR is calculated as
The uncertainty of PPMR was estimated by (1) simulating 10,000 times of independent variables s (the mean and standard deviation from the linear regression statistics) and Δδ15N values (mean and standard deviation of 3.4 ± 1.0‰; Post 2002) from a bivariate normal distribution (ρ = 0) and (2) calculating PPMR estimates using Eq. 3. The median, 25th and 75th quantiles were reported. The same method was applied to existing studies for comparison.
Results
Isotopic niches at species and trophic guild levels
In total 9055 individuals (L from 1 to 120 cm, M from 0.01 to 2742 g) were recorded in 32 UVCs over 4800 m2 of reef. Of 41 fish species collected and analysed for δ15N, 11 had sample sizes under three, and three (Coryphopterus glaucofraenum, Balistes vetula, Holocentrus adscensionis) were sampled to represent certain uncollected species. The size range cover ratio (rL) ranged from 0 to 100% (mean = 31.0 ± 5.0%). Mean species δ13C ranged from − 17.4 ± 0.2‰ (Halichoeres pictus) to − 9.5 ± 0.0‰ (Lutjanus griseus), while mean δ15N ranged from 3.9 ± 0.8‰ (S. aurofrenatum) to 9.9 ± 1.5‰ (Sphyraena barracuda). δ15N values were significantly but weakly correlated with δ13C (p < 0.05, \(r_{\text{adjusted}}^{2} = 0.29\)) at the species level (Fig. 2). Some species had large SE values in δ13C (≥ 1‰) (e.g. S. barracuda) or δ15N (≥ 0.5‰) (e.g. Aulostomus maculatus), or both δ13C and δ15N (e.g. Elacatinus genie). Mean δ13C and δ15N values of strict pelagic planktivores (e.g. Chromis cyanea, TP = 3.7, δ13C = − 17.0 ± 0.1‰, δ15N = 5.2 ± 0.1‰) and strict benthivores of similar TP (e.g. B. vetula, TP = 3.8, δ13C = − 12.4 ± 0.3‰, δ15N = 7.7 ± 0.1‰) were significantly different.
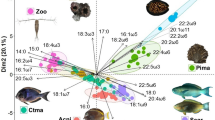
Plot of bulk δ15N against δ13C (mean ± SE) of all sampled fish species (for codes see Table 1) and standard ellipses (solid line-ellipses) for five trophic guilds (and one species of parasitivore, ELGE) of fish at Cape Eleuthera (the Bahamas)
The herbivore and benthivore guilds had the largest isotopic niches, followed by the piscivore guild, and then the omnivore guild; the planktivore guild had an isotopic niche significantly smaller than others (Table 3). The planktivore guild had a lower δ13C than others and was separated from the herbivore, benthivore and piscivore trophic guilds (Fig. 2, Table 4). The isotopic niche of the omnivores overlapped with those of the herbivores and planktivores as did those of the benthivore and piscivore (Table 4). The isotopic niche of the herbivores was vertically separated from those of the benthivores and piscivores. E and θ values differed among trophic guilds (Table 3); the herbivore guild had the lowest E (0.77), while the omnivores had the highest (0.98). The benthivore (0.94) and planktivore (0.79) trophic guilds had E values very similar to those of the omnivore and herbivore trophic guilds, respectively. The θ values of the δ15N versus δ13C relationships of all the trophic guilds were positive; the herbivores had the lowest θ (6°), while the planktivores had the highest (76°). Among the benthivore, omnivore, piscivore and herbivore trophic guilds, the isotopic niche was spread along the x axis (θ < 45°), while that of the planktivores was more vertically spread (θ > 45°). Some species (the benthivore H. pictus, herbivores Acanthurus coeruleus and Coryphopterus personatus and omnivore Thalassoma bifasciatum) had isotopic coordinates close to the ellipse of the planktivore trophic guild, and one piscivore (A. maculatus) had an isotopic coordinate within the standard ellipse of the herbivore trophic guild.
δ15N–body mass relationships at species and community levels and PPMR
There were significant but weak relationships across species between log2Mmax (maximum body mass) and both δ15N (\(r_{\text{adjusted}}^{2} = 0.12\), p < 0.05; Fig. 3a) and δ13C data (\(r_{\text{adjusted}}^{2} = 0.17\), p < 0.05; Fig. 3b). The δ15N values of several species did not scale positively with log2Mmax (e.g. E. genie, Sparisoma viride, Scarus iserti and S. aurofrenatum). The SE values of δ13C were generally higher than those of δ15N regardless of Mmax. δ15N tended to vary with log2M for 29 species (Fig. 4) with n ≥ 3; of these 24 relationships were positive (significantly so, e.g. C. cyanea), while five were negative (significantly so, e.g. Pomacanthus arcuatus, “Appendix C” supplementary materials). There was considerable variability around the regression line for nine species (\(r_{\text{adjusted}}^{2} < 0.5\), e.g. S. barracuda), whereas this was not the case for others (e.g. Clepticus parrae, Calamus pennatula, Halichoeres garnoti). At the community level, the combined isotope data demonstrated a strong positive linear relationship between mean δ15N and log2B, the regression equation being δ15N = 0.34 ± 0.04log2B + 4.03 ± 0.24 (\(r_{\text{adjusted}}^{2} = 0.64\), p < 0.05, Fig. 5). The slope value of TP–log2B was b = 0.10 (\(r_{\text{adjusted}}^{2} = 0.80\), p < 0.05), and the PPMR estimates were 1047:1 (Table 5).
Plots of bulk δ15N (a) and bulk δ13C (b) (mean ± SE) versus log2 maximum body mass for all collected fish species (for codes see Table 1) at Cape Eleuthera with mean isotope values of individuals bigger than 55% of their Lmax. Solid line: linear regression line (p < 0.05)
Plot of δ15N versus log2 body mass (linear regression) of all sampled species at Cape Eleuthera (the Bahamas). Solid line: significant relationship (p < 0.05), dashed line: non-significant relationship. For codes see Table 1
Discussion
Species and trophic guild trophodynamics
Stable isotope data at both species and trophic guild levels indicated that at the Cape Eleuthera site there were large differences in trophic ecology within and among species, and species utilising a range of production source types were common.
High within-species variability in δ13C and δ15N values for some species suggested the existence of individual specialisation (Matthews and Mazumder 2004) in the food web where different individuals of the same species were consistently sampling different production sources. For example, similarly sized individuals of the apex predator S. barracuda had similar δ15N values but differed greatly in δ13C values. The δ13C value of approximately − 16‰ was close to that within the planktivore trophic guild, while the δ13C value of − 10‰ was more consistent with the piscivore trophic guild. Trophic position omnivory indicated by differences in δ15N also occurs, for example, in the parasitivore E. genie which feeds on parasites from fish at different trophic positions.
The SIBER analysis indicated at least two types of production sources, namely benthic (e.g. algae) and pelagic (e.g. plankton), and mixed-feeding patterns for some species that are typically regarded as relying solely on single types of source materials (e.g. herbivorous fish; Plass-Johnson et al. 2013; Dromard et al. 2015). In this study, isotopic niche areas of the planktivores were significantly smaller than other guilds even though plankton can have highly variable isotopic signatures (McClelland and Montoya 2002; Kürten et al. 2013), which indicated a level of dietary strictness or consistency. High θ, low E and low SEA values of the planktivore guild nevertheless suggested TP omnivory, with these fish feeding at different TPs albeit from the same type of pelagic source (e.g. phytoplankton and zooplankton). The omnivore guild had high E, low θ and SEA values, the δ13C data indicating that the two species are supported by plankton and benthic algae with similar δ15N baselines. Although based on only two species, the omnivores may be connecting these two pathways to some extent (McMeans et al. 2016). The benthivore, piscivore and herbivore trophic guilds, which share mostly benthic production sources, had similar isotopic niche areas which were much greater than those of the planktivores and omnivores, with their isotopic niches spread along the x axis as indicated by E and θ values, suggesting source omnivory within the benthic producer category. Overlapping isotopic niches among the trophic guilds (e.g. piscivore and benthivore) suggested that they might share dietary resources to some extent; for example, some lutjanids are both piscivorous and feed on zoobenthos (Allen 1985; Kulbicki et al. 2005; Layman and Allgeier 2012). The vertical distribution in the isotopic niches for the four benthic trophic guilds reflected the herbivorous fish feeding at low trophic positions, while the omnivores, benthivores and piscivores utilised a wider range of energy sources from different TPs. There were species with stable isotope values outside the isotopic niches of their assumed trophic guilds, which suggested feeding on different food sources than previously known or those derived from snapshot diet studies. For example, the four benthic feeders (H. pictus, A. coeruleus, C. personatus and T. bifasciatum) were likely relying on plankton sources, and the piscivore A. maculatus might be predating on smaller herbivores. Some herbivores came partly within the isotopic niches of other trophic guilds, indicating feeding on food sources in addition to algae such as invertebrates or planktivore faeces (Robertson 1982; Wulff 1997; Dunlap and Pawlik 1998; Chen 2002; Plass-Johnson et al. 2013). This can only be confirmed with detailed dietary analysis including baseline variation (i.e. during the 3–6 months isotopic turn over period).
δ15N–body mass relationship
The majority of species had a positive trend between δ15N and log2M indicating that they tend to feed at higher TPs as size increases. This could be a result of increasing gape size, predatory skill and fitness level allowing individuals to feed on higher TP prey as they grow (Peters 1986; Munday 2001; Newman et al. 2012). Those with negative or highly variable stable isotope-size relationships potentially have dietary shifts from isotopically high value production sources to low or multi-pathway (e.g. exploitation of short food chain) feeding patterns in their sampled size ranges, or otherwise assimilating significantly different isotopic baselines across the population (Jennings et al. 2002a; Layman et al. 2005). Unlike Robinson and Baum (2015) where δ15N–log2M relationships of all individuals in two separate trophic pathways (herbivore and carnivore) were investigated, species-level and whole-community-level analyses were conducted in this study. The variation among species is attributable to differences in the trophic pathways supporting them, but to understand better how trophic pathways affect such relationships, more data are clearly needed. At community level, a positive linear relationship between δ15N and log2B across the combined sites was found, indicating that TP tended to increase with body mass regardless of taxonomy and larger coral reef fish in this Cape Eleuthera community on average fed at higher TPs.
The weak cross-species relationship between isotopic signatures and log2Mmax suggested that maximum body mass could scarcely constrain species’ trophic capabilities in this food web in which there were small-bodied benthivores and planktivores and large-bodied herbivores. The body-size structuring is similar to that of North Sea and Western Arabian Sea community data (Jennings et al. 2001; Al-Habsi et al. 2008). Here, the small size class biomass data were dominated by herbivores rather than higher TP omnivores such as Labridae (Graham et al. 2017), while the large size classes were dominated by piscivores and omnivores rather than large-bodied herbivores such as Scarinae (Hughes et al. 2007; Zhu 2019). Although the surveyed Cape Eleuthera sites are now legally protected, they were previously fished and are structurally degraded. The linearity of the δ15N–log2B relationship at Cape Eleuthera may not be generic; it could be influenced by the loss of habitat structural complexity and aspects of past overfishing (e.g. removal of large herbivores).
The present study also had limitations. All individuals were treated as if they had the same isotopic baseline, yet significant isotopic differences between benthic and pelagic sources are expected (McConnaughey and McRoy 1979; Polunin and Pinnegar 2002), whereas other baselines would have been taken into consideration when estimating the trophic positions of consumers with significantly mixed diets. Also, for some species, sample sizes failed to adequately fulfil requirements for confidently describing stable isotope changes as a function of body mass (Galván et al. 2010), yet linear regression was still applied to these species to explore their δ15N–log2M relationships. Low sample sizes and/or size ranges (rL) meant that for some species, stable isotope data could not be derived across whole UVC size ranges; for these stable isotope data were assumed to be size invariant. For missing species, the methods used to infer stable isotope values had limitations including species within the same genus or family having ontogenetic and/or dietary differences; some not meeting all three criteria and using published data from the same species could be subject to feeding strategies varying ontogenetically (Plass-Johnson et al. 2013) or spatially (Jennings et al. 1997; Matthews and Mazumder 2004). Unlike studies using combined baselines (Mill et al. 2007), here the benthic alga D. cavernosa was the sole baseline and this might not adequately represent the whole benthic assemblage, which includes turf algae, cyanobacteria and other potential production sources.
Predator–prey mass ratio
The mean PPMR at Cape Eleuthera indicates a relatively long food chain in this coral reef system compared with other aquatic systems (Table 5), suggesting potentially greater ecosystem size and stability (Jennings and Warr 2003). However, surveys at adjacent non-protected sites failed to show the presence of large predators there; thus, the PPMR data we report seem to be specific to the protected sites.
Conclusions
Stable isotope data indicate more than one production source and mixed reliance on them by some coral reef fishes suggesting evidence of reef fish crossing trophic boundaries described by their trophic guilds and that current categorisations are often simplistic. Combining visual census and stable isotope data indicated that the Cape Eleuthera coral reef fish community was size structured. The relationship at this site points to body size as a driver of predator–prey relationships and trophic pathways at community level, with the isotope data suggesting that trophic position plasticity is common at species level. This is the first indication of a positive linear δ15N–log2 body mass relationship in a coral reef system, but this may not pertain to all coral reefs.
References
Al-Habsi SH, Sweeting CJ, Polunin NVC, Graham NAJ (2008) δ15N and δ13C elucidation of size structured food webs in a Western Arabian Sea demersal trawl assemblage. Mar Ecol Prog Ser 353:55–63
Allen GR (1985) FAO species catalogue. Vol. 6. Snappers of the world: an annotated and illustrated catalogue of lutjanid species known to date. Food and Agriculture Organization of the United Nations (FAO). Fisheries Synopsis
Anderson TW, Carr MH (1998) BINCKE: a highly efficient net for collecting reef fishes. Environ Biol Fishes 51:111–115
Barnes C, Maxwell D, Reuman DC, Jennings S (2010) Global patterns in predator–prey size relationships reveal size dependency of trophic transfer efficiency. Ecology 91:222–232
Bates DM, Chambers JM, Hastie TJ (1992) Statistical models in S Comp Sci and Stat. In: Proceedings of the 19th symposium on the interface. Wadsworth & Brooks California
Bearhop S, Adams CE, Waldron S, Fuller RA, MacLeod H (2004) Determining trophic niche width: a novel approach using stable isotope analysis. J Anim Ecol 73:1007–1012
Bell JD, Craik GJS, Pollard DA, Russell BC (1985) Estimating length frequency distributions of large reef fish underwater. Coral Reefs 4:41–44
Bode A, Carrera P, Lens S (2003) The pelagic foodweb in the upwelling ecosystem of Galicia (NW Spain) during spring: natural abundance of stable carbon and nitrogen isotopes. ICES J Mar Sci J Cons 60:11–22
Bode A, Carrera P, Porteiro C (2006) Stable nitrogen isotopes reveal weak dependence of trophic position of planktivorous fish on individual size: a consequence of omnivorism and mobility. Radioact Environ 8:281–293
Bronk DA, Glibert PM (1993) Application of a 15 N tracer method to the study of dissolved organic nitrogen uptake during spring and summer in Chesapeake Bay. Mar Biol 115:501–508
Cabana G, Rasmussen JB (1996) Comparison of aquatic food chains using nitrogen isotopes. Proc Natl Acad Sci 93:10844–10847
Charnov EL (1993) Life history invariants. Oxford University Press, Oxford
Chen L-S (2002) Post-settlement diet shift of Chlorurus sordidus and Scarus schlegeli (Pisces: Scardiae). Zool Stud 41:47–58
D’Agata S, Vigliola L, Graham NAJ, Wantiez L, Parravicini V, Villéger S, Mou-Tham G, Frolla P, Friedlander AM, Kulbicki M (2016) Unexpected high vulnerability of functions in wilderness areas: evidence from coral reef fishes. Proc R Soc B 1844:20160128
DeNiro MJ, Epstein S (1978) Influence of diet on the distribution of carbon isotopes in animals. Geochim Cosmochim Acta 42:495–506
DeNiro MJ, Epstein S (1981) Influence of diet on the distribution of nitrogen isotopes in animals. Geochim Cosmochim Acta 45:341–351
Dromard CR, Bouchon-Navaro Y, Harmelin-Vivien M, Bouchon C (2015) Diversity of trophic niches among herbivorous fishes on a Caribbean reef (Guadeloupe, Lesser Antilles), evidenced by stable isotope and gut content analyses. J Sea Res 95:124–131
Dunlap M, Pawlik JR (1998) Spongivory by parrotfish in Florida mangrove and reef habitats. Mar Ecol 19:325–337
Ferreira CEL, Goncçalves JEA, Coutinho R (2001) Community structure of fishes and habitat complexity on a tropical rocky shore. Environ Biol Fishes 61:353–369
France R, Chandler M, Peters R (1998) Mapping trophic continua of benthic food-webs: body size–δ15N relationships. Mar Ecol Prog Ser 174:301–306
Freckleton RP (2000) Phylogenetic tests of ecological and evolutionary hypotheses: checking for phylogenetic independence. Funct Ecol 14:129–134
Froese R, Pauly D (2017) FishBase 2017, version (March, 2017). World Wide Web electronic publication
Fry B, Baltz DM, Benfield MC, Fleeger JW, Gace A, Haas HL, Quiñones-Rivera ZJ (2003) Stable isotope indicators of movement and residency for brown shrimp (Farfantepenaeus aztecus) in coastal Louisiana marshscapes. Estuaries 26:82–97
Galván DE, Sweeting CJ, Reid WDK (2010) Power of stable isotope techniques to detect size-based feeding in marine fishes. Mar Ecol Prog Ser 407:271–278
Graham NAJ, McClanahan TR, MacNeil MA, Wilson SK, Cinner JE, Huchery C, Holmes TH (2017) Human disruption of coral reef trophic structure. Curr Biol 27:231–236
Hadi TA, Tuti Y, Hadiyanto Abrar M, Suharti SR, Suharsono Gardiner NM (2018) The dynamics of coral reef benthic and reef fish communities in Batam and Natuna Islands, Indonesia. Biodiversity 19:1–14
Hesslein RH, Capel MJ, Fox DE, Hallard KA (1991) Stable isotopes of sulfur, carbon, and nitrogen as indicators of trophic level and fish migration in the lower Mackenzie River basin, Canada. Can J Fish Aquat Sci 48:2258–2265
Hiatt RW, Strasburg DW (1960) Ecological relationships of the fish fauna on coral reefs of the Marshall Islands. Ecol Monogr 30:65–127
Hughes TP, Baird AH, Bellwood DR, Card M, Connolly SR, Folke C, Grosberg R, Hoegh-Guldberg O, Jackson JBC, Kleypas J (2003) Climate change, human impacts, and the resilience of coral reefs. Science 301:929–933
Hughes TP, Rodrigues MJ, Bellwood DR, Ceccarelli D, Hoegh-Guldberg O, McCook L, Moltschaniwskyj N, Pratchett MS, Steneck RS, Willis B (2007) Phase shifts, herbivory, and the resilience of coral reefs to climate change. Curr Biol 17:360–365
Humann P, DeLoach N (1989) Reef fish identification: Florida. Caribbean, Bahamas
Jackson AL, Inger R, Parnell AC, Bearhop S (2011) Comparing isotopic niche widths among and within communities: SIBER–stable isotope Bayesian ellipses in R. J Anim Ecol 80:595–602
Jennings S, Warr KJ (2003) Smaller predator–prey body size ratios in longer food chains. Proc R Soc Lond B Biol Sci 270:1413–1417
Jennings S, Grandcourt EM, Polunin NVC (1995) The effects of fishing on the diversity, biomass and trophic structure of Seychelles’ reef fish communities. Coral Reefs 14:225–235
Jennings S, Reñones O, Morales-Nin B, Polunin NVC, Moranta J, Coll J (1997) Spatial variation in the 15N and 13C stable isotope composition of plants, invertebrates and fishes on Mediterranean reefs: implications for the study of trophic pathways. Mar Ecol Prog Ser 146:109–116
Jennings S, Pinnegar JK, Polunin NVC, Boon TW (2001) Weak cross-species relationships between body size and trophic level belie powerful size-based trophic structuring in fish communities. J Anim Ecol 70:934–944
Jennings S, Greenstreet S, Hill L, Piet G, Pinnegar J, Warr KJ (2002a) Long-term trends in the trophic structure of the North Sea fish community: evidence from stable-isotope analysis, size-spectra and community metrics. Mar Biol 141:1085–1097
Jennings S, Pinnegar JK, Polunin NVC, Warr KJ (2002b) Linking size-based and trophic analyses of benthic community structure. Mar Ecol Prog Ser 226:77–85
Jennings S, Warr KJ, Mackinson S (2002c) Use of size-based production and stable isotope analyses to predict trophic transfer efficiencies and predator–prey body mass ratios in food webs. Mar Ecol 240:11–20
Kulbicki M, Bozec Y-M, Labrosse P, Letourneur Y, Mou-Tham G, Wantiez L (2005) Diet composition of carnivorous fishes from coral reef lagoons of New Caledonia. Aquat Living Resour 18:231–250
Kürten B, Painting SJ, Struck U, Polunin NVC, Middelburg JJ (2013) Tracking seasonal changes in North Sea zooplankton trophic dynamics using stable isotopes. Biogeochemistry 113:167–187
Layman CA, Allgeier JE (2012) Characterizing trophic ecology of generalist consumers: a case study of the invasive lionfish in The Bahamas. Mar Ecol Prog Ser 448:131–141
Layman CA, Winemiller KO, Arrington DA, Jepsen DB (2005) Body size and trophic position in a diverse tropical food web. Ecology 86(9):2530–2535
Leal MC, Ferrier-Pagès C (2016) Molecular trophic markers in marine food webs and their potential use for coral ecology. Mar Genom 29:1–7
Leibold MA (1995) The niche concept revisited: mechanistic models and community context. Ecology 76:1371–1382
MacNeil MA, Graham NAJ, Cinner JE, Wilson SK, Williams ID, Maina J, Newman S, Friedlander AM, Jupiter S, Polunin NVC (2015) Recovery potential of the world’s coral reef fishes. Nature 520:341–344
Matthews B, Mazumder A (2004) A critical evaluation of intrapopulation variation of δ13C and isotopic evidence of individual specialization. Oecologia 140:361–371
McClanahan TR, Hendrick V, Rodrigues MJ, Polunin NVC (1999) Varying responses of herbivorous and invertebrate-feeding fishes to macroalgal reduction on a coral reef. Coral Reefs 18:195–203
McClelland JW, Montoya JP (2002) Trophic relationships and the nitrogen isotopic composition of amino acids in plankton. Ecology 83:2173–2180
McConnaughey T, McRoy CP (1979) Food-web structure and the fractionation of carbon isotopes in the Bering Sea. Mar Biol 53:257–262
McCutchan JH, Lewis WM, Kendall C, McGrath CC (2003) Variation in trophic shift for stable isotope ratios of carbon, nitrogen, and sulfur. Oikos 102:378–390
McMeans BC, McCann KS, Tunney TD, Fisk AT, Muir AM, Lester N, Shuter B, Rooney N (2016) The adaptive capacity of lake food webs: from individuals to ecosystems. Ecol Monogr 86:4–19
Mill AC, Pinnegar J, Polunin NVC (2007) Explaining isotope trophic-step fractionation: why herbivorous fish are different. Funct Ecol 21:1137–1145
Minagawa M, Wada E (1984) Stepwise enrichment of 15 N along food chains: further evidence and the relation between δ15N and animal age. Geochim Cosmochim Acta 48:1135–1140
Mouillot D, Villéger S, Parravicini V, Kulbicki M, Arias-González JE, Bender M, Chabanet P, Floeter SR, Friedlander A, Vigliola L (2014) Functional over-redundancy and high functional vulnerability in global fish faunas on tropical reefs. Proc Natl Acad Sci 111:13757–13762
Moustaka M, Langlois TJ, McLean D, Bond T, Fisher R, Fearns P, Dorji P, Evans RD (2018) The effects of suspended sediment on coral reef fish assemblages and feeding guilds of north-west Australia. Coral Reefs 37:1–15
Munday PL (2001) Fitness consequences of habitat use and competition among coral-dwelling fishes. Oecologia 128:585–593
Newman SP, Handy RD, Gruber SH (2012) Ontogenetic diet shifts and prey selection in nursery bound lemon sharks, Negaprion brevirostris, indicate a flexible foraging tactic. Environ Biol Fishes 95:115–126
Newsome SD, Martinez del Rio C, Bearhop S, Phillips DL (2007) A niche for isotopic ecology. Front Ecol Environ 5:429–436
Parnell A, Jackson A (2013) siar: Stable isotope analysis in R. R package version 4.2.2
Pastorok RA, Bilyard GR (1985) Effects of sewage pollution on coral-reef communities. Mar Ecol Prog Ser 21:175–189
Peters RH (1986) The ecological implications of body size. Cambridge University Press, Cambridge
Plass-Johnson JG, McQuaid CD, Hill JM (2013) Stable isotope analysis indicates a lack of inter-and intra-specific dietary redundancy among ecologically important coral reef fishes. Coral Reefs 32:429–440
Polunin NVC (1996) Trophodynamics of reef fisheries productivity reef fisheries. Springer, Berlin, pp 113–135
Polunin NVC, Pinnegar JK (2002) Trophic ecology and the structure of marine food webs. Handb Fish Biol Fish Fish Biol 1:301–320
Post DM (2002) Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology 83:703–718
R Core Team (2016) R: a language and environment for statistical computing. In: Computing RFfS (ed)
Reid WDK, Sweeting CJ, Wigham BD, McGill RAR, Polunin NVC (2016) Isotopic niche variability in macroconsumers of the East Scotia Ridge (Southern Ocean) hydrothermal vents: What more can we learn from an ellipse? Mar Ecol Prog Ser 542:13–24
Reum JCP, Jennings S, Hunsicker ME (2015) Implications of scaled δ15N fractionation for community predator–prey body mass ratio estimates in size-structured food webs. J Anim Ecol 84(6):1618–1627
Ríos MF, Venerus LA, Karachle PK, Reid WDK, Erzini K, Stergiou KI, Galván DE (2019) Linking size-based trophodynamics and morphological traits in marine fishes. Fish Fish 20:355–367
Robertson DR (1982) Fish feces as fish food on a Pacific coral reef. Mar Ecol Prog Ser 7:253–265
Robinson JPW, Baum JK (2015) Trophic roles determine coral reef fish community size structure. Can J Fish Aquat Sci 73:496–505
Rolff C (2000) Seasonal variation in δ13C and δ15N of size-fractionated plankton at a coastal station in the northern Baltic proper. Mar Ecol Prog Ser 203:47–65
Romanuk TN, Hayward A, Hutchings JA (2011) Trophic level scales positively with body size in fishes. Glob Ecol Biogeogr 20(2):231–240
Stamoulis KA, Friedlander AM, Meyer CG, Fernandez-Silva I, Toonen RJ (2017) Coral reef grazer-benthos dynamics complicated by invasive algae in a small marine reserve. Sci Rep 7:43819
Strieder Philippsen J, Benedito E (2013) Discrimination factor in the trophic ecology of fishes: a review about sources of variation and methods to obtain it. Oecol Aust 17:205–2016
Sweeting CJ, Polunin NVC, Jennings S (2006) Effects of chemical lipid extraction and arithmetic lipid correction on stable isotope ratios of fish tissues. Rapid Commun Mass Spectrom 20:595–601
Tieszen LL, Boutton TW, Tesdahl KG, Slade NA (1983) Fractionation and turnover of stable carbon isotopes in animal tissues: implications for δ13C analysis of diet. Oecologia 57:32–37
Trebilco R, Baum JK, Salomon AK, Dulvy NK (2013) Ecosystem ecology: size-based constraints on the pyramids of life. Trends Ecol Evol 28:423–431
Wickham H, Chang W (2016) ggplot2: Create elegant data visualisations using the grammar of graphics. R package version 2.2. 1. https://CRAN.R-project.org/package=ggplot2
Wilkinson GN, Rogers CE (1973) Symbolic description of factorial models for analysis of variance. J R Stat Soc Ser C (Appl Stat) 22:392–399
Wulff JL (1997) Parrotfish predation on cryptic sponges of Caribbean coral reefs. Mar Biol 129:41–52
Zhu Y (2019) Studies in the stable isotope ecology of coral reef fish food webs. PhD thesis, Newcastle University, Newcastle upon Tyne (under review)
Acknowledgements
We thank Cape Eleuthera Institute (CEI) for providing logistic support, Jordan Atherton for underwater visual surveys, CEI interns involved in fish sampling, and local fishermen for providing fish samples.
Funding
No external funding.
Author information
Authors and Affiliations
Contributions
This study was designed by YZ and NVCP, the field work was carried out by YZ, the data analysis was conducted by YZ, SPN and WDKR, and the paper was written by YZ, NVCP, WDKR and SPN.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethic approval
All applicable international, national and/or institutional guidelines for the care and use of animals were followed. The research was approved by the Newcastle University Ethics Committee and the Bahamian Department of Marine Resources under permits MAMR/FIS/17 and MAMR/FIS/34A. No coral habitat was disturbed during this research. All fish were killed in accordance with the UK Home Office Scientific Procedures (Animal) Act. All samples were imported to UK under DEFRA permit TARP/2015/210.
Additional information
Responsible Editor: C. Trueman.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Reviewed by undisclosed experts.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Zhu, Y., Newman, S.P., Reid, W.D.K. et al. Fish stable isotope community structure of a Bahamian coral reef. Mar Biol 166, 160 (2019). https://doi.org/10.1007/s00227-019-3599-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-019-3599-9