Abstract
Combining spatially localized X-ray diffraction (XRD) with X-ray microtomography (XMT) enables the mapping of the micro- and nanoscale structures simultaneously. The combination of these methods results in a powerful tool when considering the structural studies of hierarchical materials, allowing one to couple the relationships and connections of the structures at various scales. In this study, XMT was used to map the anatomy and cellular structures in 3D in tension and opposite wood with 1.5 µm resolution, while XRD was used to determine the cellulose crystallite widths and microfibril orientations with 100 µm spatial resolution within the same tissues. Tension wood (TW) has an important biological function with clearly distinct properties to opposite (OW) and normal wood, e.g. differing cellular structures with a higher cellulose content. This is the first study of very young hybrid aspen saplings (1-month-old) using the combined diffraction tomography method. The TW tissues could be identified from the OW tissues based on both the XMT and XRD results: TW had a higher average size of the cellulose crystallites and smaller mean microfibril angles (mMFA) than those in OW. With the XRD data, we were able to reconstruct the images of the cross sections of the saplings using the structural parameters (cellulose crystallite width and mMFA) as contrast mechanisms. As far as the authors know, there are no previous studies with images on any TW samples using the XRD-based contrast. Home laboratory bench-top set-up offers its advantages for these studies, considering the number of samples characterized, time-dependent studies and larger field of views.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The hierarchy of structures, forming from the atomistic to the macroscopic scale, is determinative for all biomaterials. Covering the different size scales of the various structures is crucial due to the definitive nature of the structure itself: it dictates the materials’ function and properties. As a key example, the extraordinary mechanical properties of wood arise from the interplay of crystalline and amorphous order and orientation of the cellulose chains on the nanoscale, building up the elementary cellulose microfibrils and further reinforced matrix–fibril structures together with lignin and hemicellulose. These main components with the addition of other, minor compounds organize into various layered cell wall structures, which then define distinct cells and cellular tissues and finally the macroscopic stem structure. Wood structure has been extensively studied with physical, biological and chemical methods, but the structural details still offer an intriguing puzzle due to the complexity and the sensitivity of the biological living organism.
Various X-ray-based methods offer significant advantages for solutions to these questions: they are in principle non-invasive, and by using X-ray scattering and diffraction (XRD), the structures of the studied material can be revealed down to the atomistic scale. Besides the origin of the material properties, the detailed knowledge of the wood structural organization shed light on the fundamental questions on plant growth.
Reaction wood is a biologically important, special type of tissue generated in stems and branches as an adaptation to mechanical and gravitational stimulus, e.g. strong wind loads, leaning or tilting directed on the stem and branches. Deciduous trees generate tension wood (TW) on the upper side of their leaning stem, which pulls the stem upward using tension stress. The TW generation is a widely existing property of the stem growth of trees and similar fibre properties are shared with the bast fibres in, for example, flax and hemp (Mellerowicz and Gorshkova 2012; Gorshkova et al. 2018).
Tension wood xylem tissues have distinctive properties due to the specific chemical compositions, and cellular and cellulose crystallite structures compared to normal wood (NW) (Gardiner et al. 2014; Chang et al. 2015). In TW, the average width of cellulose crystallites is larger (Müller et al. 2006; Clair et al. 2011; Viljanen et al. 2020) and the cellulose content is higher (Foston et al. (2011)). In many hardwood species, these changes often originate from the formation of a cellulose-abundant gelatinous layer or the G-layer, often replacing parts of the other layers in the secondary cell wall of the wood fibres (Gardiner et al. (2014)). The wood tissue on the other side of the stem, directly opposite to the TW side, is called opposite wood (OW).
The special properties of TW make it highly interesting for various scientific purposes and technological applications. Structural studies on TW offer direct insights into the wood structure–properties relationships: by comparing the results on TW structure (cellulose crystallites, amorphous matrix, cell wall characteristics) to NW, conclusions on the structure–function relations can be made.
In addition, the differences in reaction wood formation in stems play a vital role regarding the lignocellulosic yield from the crops (Brereton et al. 2012; Ding et al. 2012) and with that reaction wood may also help in solving the recalcitrance problem (Sawada et al. 2018). Additionally, the possibilities of TW for nanocellulose production have been recently considered (Jonasson et al. 2021).
Reaction wood has been characterized intensively using microscopy and chemical methods (e.g. Gardiner et al. 2014). Microscopy methods offer results in 2D, whereas full 3D information is reached by using tomography methods. X-ray microtomography (XMT) has proven to be an efficient tool for the quantitative analysis of the anatomical structure of various plant and wood tissues (Stuppy et al. 2003; Brodersen and Roddy 2016; Van den Bulcke et al. 2019). Regarding this background, the TW formation and structures have been studied relatively little using the possibilities of XMT. To our knowledge, there exist only a few previous examples of these kinds of studies: Brereton et al. (2015) studied the structure of TW tissue in willow saplings by using microtomography. They conducted the measurements using a resolution of 9 µm, which enabled the analysis of vessel structures in more detail. In our study, we aimed to use significantly higher resolution and thus, very young hybrid aspen saplings were selected. The diameter of the studied saplings was limited to below 2 mm, which enabled the usage of high-resolution tomographic imaging with 1.5 µm resolution. The chosen imaging parameters allowed the coverage of the entire cross section of the single saplings. Another advantage of studying these juvenile saplings is the ability to cover the possible start pattern of the TW formation.
The highest available spatial and time resolution can be achieved by using synchrotron light sources. Schmelzle et al. (2021) were able to study in situ the deformations of TW and OW samples as a function of tensional stress in 3D by using synchrotron X-ray microtomography. However, regarding synchrotron techniques on the biological samples, beam damage might be a severe issue (Schmelzle et al. 2021; Storm et al. 2015). The other limiting factor, regarding the use of the synchrotron techniques, is that the beam time availability is highly limited, both considering the number of samples and time invested. Thus, it is a great advantage that the synchrotron studies can be complemented by using home laboratory bench-top devices. For example, home laboratory experiments enable a wider field of view, larger sample series and longer measurement times when considering experiments including time series or strictly scheduled sampling, e.g. the case of living plants. For these reasons, it is highly important to develop novel home laboratory-based methods for especially plant and wood studies.
In this study, young, 1-month-old hybrid aspen saplings were imaged using simultaneous X-ray diffraction and microtomography utilizing a laboratory bench-top device. The microtomography results were obtained with a resolution of 1.5 µm and the spatial resolution in the localized diffraction was 100 µm. This study represents the first structural study on reaction wood tissues in young wood saplings examined on the micro- and nanometre scales using a simultaneous combination of high-resolution tomography and XRD line scans. Structural details on TW and OW sides were determined both at the nano- and micrometre levels and the relationships of these two structural scales were mapped.
Materials and methods
Samples: growth and tilting procedure of the saplings
Four T89 hybrid aspen saplings (Populus tremula x tremuloides) were selected for the study. The trees were in vivo saplings grown at the Viikki Plant Growth Facilities, University of Helsinki. Around 1 month old, the plants were transplanted from the in vivo pots into the soil in a table-top-greenhouse (see Fig. 1). After 22 days in a greenhouse, three of the saplings were tilted to 45 degrees for 16 days: two of them using a tilted stage (samples 1 and 2) and one of the saplings using leaning (sample 3). One of the saplings (sample 4) was left to grow in a straight position, i.e. it was not exposed to physical tilting or leaning. All the samples were exposed to non-isotropic lighting conditions because they grew in a table-top greenhouse in front of a window (opening to the south direction). Thus, sample 4 can be considered as a reference sample of the possible phototropic effect on the samples. All of the saplings and their growing positions and conditions can be seen in Fig. 1. The tilting conditions lasted for 16 days, after which all the samples were cut and prepared from the saplings.
A Example of the growing and tilting conditions of the saplings (samples 1–4 are marked in the figure). The dashed line in figure B shows the height and position where the samples were cut from the stems. The width of the stem at the time of cutting was between 1.0 and 2.0 mm, allowing the tomographic data to be acquired on the highest possible magnification and resolution
Pretreatments and drying protocol of the samples
To achieve the highest possible resolution for the microtomography images, the wood samples should be studied in their dry state as the contrast between the cell wall materials and the air is much higher than that of the cell wall materials and water. Due to these reasons, the studied samples were freeze-dried prior to any XRD or µCT measurements.
Using a razor blade, the samples were cut from the stems at a height of 10 cm from the soil. The stem samples were approximately 5 mm long cylinders with a diameter of 2 mm, including all the different tissues from pith to bark within the stem. The assumed OW side of the samples was marked with a slight incision.
After the cutting procedure, the stem pieces were kept in a freezer (+ 2 °C) for 72 h immersed in a fixative of ethyl alcohol (50%), acetic acid (5%), 40%-formaldehyde (10%), and distilled water (35%). After that, the samples were treated with ascending ethanol series (50%-60%-70%-80%-90%-95%) for 30 min in each step, after which the samples were kept in 100% ethanol for 24 h at 2 °C (adapted from Zhang et al. (2021)). After that the samples were freeze-dried (Christ Alpha 1–4 LSCBasic) at − 63 °C and 94 Pa for 48 h.
Experimental
All the scattering and tomography experiments were conducted using a custom-built combined X-ray scattering-X-ray microtomography set-up of the University of Helsinki (Suuronen et al. (2014)). The combined set-up consists of two independently operated X-ray devices. The microtomography device is based on a cone-beam geometry of the X-ray scanner (Phoenix|x-ray Systems and Services GmbH, Waygate Technologies) with microfocus X-ray tube and CMOS flat-panel detector (C7942SK-25, Hamamatsu Photonics, Japan). The scattering device is based on a molybdenum (Mo) anode source (IμS, Incoatec GmbH, Germany) with a pencil beam and an area detector (Pilatus 1 M, Dectris Ltd, Switzerland).
A shared, motorized sample holder stage was used to ensure the spatial alignment of the sample between the scattering and the tomography modalities. The calibration of the tomography coordinates to the scattering coordinates was conducted by measuring a silver behenate sample. Due to this alignment, regions of interest for localized micro-diffraction could be selected directly from the tomographic reconstruction slice. In this experiment, the tomographic reconstruction was used to align the sapling cross section so that the line scans with 100 µm step size included either tension wood xylem or opposite wood xylem (see Fig. 2).
Approximative scan lines (1…13) for the localized WAXS measurements for sapling 1 after the alignment of the sample according to the tomography reconstruction so that the scanned areas include either pure TW or OW tissue as much as possible. The corresponding analysis results based on the WAXS patterns for each of these line scans are given in Table 2
Line scanning WAXS
The wide-angle X-ray scattering (WAXS) patterns were measured with 100 µm step size (i.e. the line scans), using perpendicular transmission geometry with a wavelength (\(\lambda\)) of 0.729 Å (Mo K-α). The measurement time for each WAXS measurement was 10 min and the scattering data was collected onto a Pilatus 1 M area detector. The q-scale calibration was conducted by using a silicon standard sample and the instrumental broadening was determined to be 0.03 Å−1. The data is presented as a function of the length of the scattering vector (q):
where \(\theta\) is half of the scattering angle.
The data were corrected for air scattering, and in addition, the geometrical, polarization and transmission corrections were applied. The transmission values were set for the samples to be 1.1 (data/background) based on both the validation of the air scattering correction and the previous transmission experiments on hybrid aspen samples having similar thicknesses (Svedström et al. 2012).
From the WAXS data, the average crystallite width and the microfibril orientation were determined. To obtain the radial data, the WAXS patterns were integrated from the 40° wide sectors around both maxima of 200 reflections of the cellulose Iβ as observed on the area detector, dictated by the fibre symmetry of the 2D scattering pattern. To improve the statistics of the data, these two integrated intensity curves were summed together.
The radial scattering pattern around the q range 0.75–1.95 Å−1 (corresponding 2θ range 5–13°) was modelled by using four Gaussian curves (each one for cellulose reflections \(110, 1\overline{1 }0, 102\) and \(200\), respectively). The amorphous contribution was modelled using an experimentally measured scattering curve of a lignin sample (Andersson et al. (2003)). Examples of this fitting procedure are presented in the supplementary figures S1 and S2.
The average crystallite width (B) was determined from the FWHM of the 200-reflection using the Scherrer equation with the assumption of both 200-reflection and instrumental broadening being Gaussians and by setting the shape factor K = 0.9:
where INST was the instrumental broadening (0.03 Å−1).
The WAXS patterns were integrated around the cellulose 200-reflection corresponding to the q range 1.45–1.55 Å−1 (2θ = 9.6–10.3°) to obtain the azimuthal data. The mean microfibril angle (mmfa) was analysed from these azimuthal curves by using T-parameter values determined as mmfa = 0.6 T (Cave 1966). The value of the T-parameter is simply obtained by fitting a tangent to the azimuthal integral curve of the 200-reflection and then determining the distance from the origo (zero angle) to the intersection of the tangent and the polar angle axis.
The decision to use the more robust T-parameter analysis instead of a more validated MFA analysis method (see, for example, Rüggeberg et al. (2013), Sarén et al. (2006)) was made because in these juvenile cross-sectional samples the beam position related to the cell wall and the cell shapes highly varied across all the samples. Thus, it was observed, that the differences between the samples and different locations could be compared most reliably by using the robust T-parameter analysis. It must be noted that the given mmfa values are for comparative purposes inside this data set, and they are not meant to be read as absolute values of the MFA values in these saplings.
X-ray microtomography (XMT)
X-ray microtomography measurements were conducted with a current of 240 μA and a voltage of 60 kV for the X-ray tube. A total of 10 transmission images of 500 ms exposure were averaged to produce one projection image.
Pixel sizes of 1.2–1.5 µm resulting in a field of view of 1.3–1.7 mm (with a 2 × 2-pixel binning) and a pixel size of 0.8 µm and a field of view of 1.8 mm (without binning) were used. 400–800 projection images were taken over the 360-degree. The parameters for the individual scans of the samples are given in Table 1. The reconstruction of the tomographic data was done with GE phoenix datos|x software using a back-projection algorithm.
X-ray diffraction tomography (XDT)
Sample 1 was measured by X-ray diffraction tomography. A total of 31 rotation steps were taken over 180° (step size of 6°). At each rotation step a line scan was performed with a step size of 100 µm by measuring the scattering pattern for 120 s at each step (a total of 17 line scans), which made the total duration of the whole scan to be 17.5 h.
Thus, in the XDT scan, a total of 17 × 31 XRD patterns were measured. From each of these scattering patterns, any XRD-based parameter (chosen intensity, peak position or width) can be determined and used as a contrast for the XDT image. The XDT image was then computed by MATLAB (MathWorks Inc., USA) by using inverse filtered Radon transform (with linear interpolation and Hamming filter). The computed XDT image represents the scanned sample cross section.
In this study, two different contrasts were used to construct the XDT images: 1) the intensity of cellulose 200-reflection (reflecting the cellulose content in the sample) and 2) the MFA determined based on the T-parameter value (reflecting the orientation of the cellulose microfibrils).
Results and discussion
Tomography imaging
As can be seen in Fig. 3, the full cross-sectional width of the saplings varied significantly even though all the samples were the same age and grown in the same small table-top greenhouse. The widest cross section was in the case of sample 2 (in the range of 1.4–1.5 mm) and the thinnest cross section was in sample 4 (in the range of 0.8–1.0 mm). Additionally, the differences in the cross-sectional symmetry and the width and shape of the TW zones can be observed in these figures.
The cross sections of all the samples are non-symmetrical in the way that the possible TW side (set to point up in these images) has larger dimensions compared to the OW side. The TW zones which presumably consist of G-fibres are observed in these tomographic images as brighter areas, i.e. more dense tissue areas. In these denser areas, the G-layer containing cells have collapsed which can, for example, be observed from the TW structures for samples 1–3 in Fig. 3. Presumably, even the careful drying protocol used for these samples did not eventually prohibit the collapse of the G-layer structures.
The detachment and collapsing of G-fibres is a well-known phenomenon; it has been studied by microscopy (Clair et al. 2005) but also observed in previous studies on hybrid aspen saplings by microtomography (Svedström et al. 2012).
With X-ray µCT, it is possible to obtain volumetric data of the sample: the full advantage of this tomographic data is presented in Fig. 4 with the simultaneous cross-sectional, radial-longitudinal (RL) and tangential-longitudinal (TL) sections of the sample. In the longitudinal images (see B and the left part of A), the TW zone can be observed based on the brighter areas in middle.
Using the tomographic data, the relative amount of the formed TW tissues across the whole cross-sectional area was determined for all the samples. This was done by calculating the approximate TW and cross-sectional areas per slice through the whole volume. Additionally, the spread of the TW tissues across the cross section in terms of sector angle was also approximated by calculating the angle between two fitted lines (see Fig. 3, sample 1). For sample 1, the amount of the formed TW tissue was 22% ± 5% of the cross-sectional area, spread at 80° ± 10° angle over the cross section. For sample 2, the values were 15% ± 5% and 90° ± 10°, for sample 3, 18% ± 5% and 95° ± 10° respectively. The errors were obtained using standard deviation.
By studying the X-ray microtomography images, Brereton et al. (2015) reported an increase in the attenuation of the X-rays in the lateral parts of the willow stems. The lateral part is the region formed by vascular cambium and the maturing secondary xylem, which in the work by Brereton et al., was observed to extend to the TW side of the stems. They stated that on the TW side of the stems, the delay of the programmed cell death was present, and this observation was attributed to more striking attenuation of the X-rays in the TW tissues in their study. In our very young hybrid aspen saplings containing TW, we did not detect a similar kind of feature, at least not to the same extent. It must be noted that our sapling material represented different species and younger wood material compared to the study by Brereton et al. so the difference might be explained by these factors.
Brereton et al. (2015) also analysed the number frequency of the vessels in TW, OW and NW and observed that the number of the vessels was lesser in TW whereas it was about the same in OW and NW. In addition, they determined the average vessel volumes and vessel surface area to vessel volume ratio in TW, OW and NW. They found out that the vessel volume was greater on the TW side but the vessel surface area-volume ratio was smaller in TW in comparison to the average. On the OW and NW sides, the vessel surface-volume ratio was similar to the average.
In our very young saplings, this kind of analysis was not reliably conducted due to large variations in the sizes of the individual vessels: some of the vessel diameters were of the same order as the fibre cells, thus making the differentiation of the vessels from other xylem cells futile.
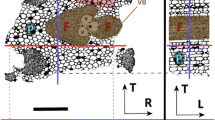
Additionally, for samples 1, 2 and 3, very dense (seemingly white voxels in absorption images) mineral particles of various shapes and sizes were observed. An example of one of the largest, observed mineral deposits is shown in Fig. 5. For sample 4, these deposits were not as prominent as for the other samples. Interestingly, the deposits in samples 1, 2 and 3 were heavily located near the pith of the sapling and within the bark layer structures. The diameters of these deposits varied a lot: for the larger conglomerates (see the upper part of Fig. 5), the diameters were approximately between 17 and 30 µm with lengths up to 55 µm. The smaller deposits (lower part of Fig. 5) ranged in size between 9 and 18 µm in diameter and length. However, all the deposits most likely consist of multiple, separate smaller units which tend to merge into one solid area in the absorption-based tomographic data.
Tangential–longitudinal cross sections of sample 1, showing the longitudinal distribution of the mineral-containing zones. A Mineral zones located near the pith of the sample, B showing the smaller deposits within the bark layers. The longitudinal and tangential directions are marked with L and T, respectively. In both images, the tangential direction is pointing towards the pith and the scale bar is the same
These crystal deposits were also visible in the X-ray scattering data. Silica, calcium-based oxalates and carbonates are very commonly found in all species of plants (Franceschi and Horner 1980), serving as nutritional storage and providing mechanical protection against herbivores (He et al. 2012). Due to the variety of the compounds and their allomorphs, tracking the exact compounds behind the peaks in the XRD data is extremely challenging. However, based on the commonness of these compounds in plants and the literature, it is highly likely that the observed compounds in the XRD patterns are compounds of silica- and calcium-based oxalates and carbonates. We have also observed similar deposits in our previous XRD imaging studies of phloem and bark layers in young silver birch saplings (unpublished work) and by XRD in bast fibres, especially in nettle and hemp (Viljanen et al. 2022).
Nanostructural results: cellulose crystallite widths and microfibril orientation
The line scan results measured with 100 µm step size are given in Table 2. The error limits have been estimated from the goodness of the fits in both mmfa and crystallite widths analysis. Sample 2 was the thickest sample; thus, there are the most locational results for that sample (from 15 locations). Based on the nanostructural parameters (size and orientation of the cellulose microfibrils), there might be TW tissue in all these four saplings studied, including sample 4 in which case the TW properties would be induced possibly only by phototropic effects. However, it is also observed that in greenhouse grown juvenile hybrid aspen, TW tissue is often generated in the straight-grown stems (Bjurhager et al. 2010; Svedström et al. 2012).
The examples of the differences in the shapes of the azimuthal profiles are presented in Fig. 6, on which the T-parameter analysis and thus the mmfa values are based. It can be observed that the profiles corresponding to locations 3, 4, and 5 are narrower than the profiles corresponding to locations 8 and 9, which corresponds to the lower and higher mmfa values, respectively.
In Fig. 7, the CT cross sections created using different contrast mechanisms are presented for sample: A) based on the absorption value of the voxel by XMT, B) the intensity of the cellulose 200-reflection by XDT and C) the mmfa value by XDT. Considering the cellulose 200-reflection intensity, the zone corresponding to the same areas as the collapsed cells can be observed. The mmfa values are more spread, so they do not point as clearly to this zone; however, the lowest mmfa values are connected to that same area.
An example of the generated XDT images of sample 1. A The cross section of the absorption tomography with brighter areas corresponds to the denser structure. B The XRD-based tomographic cross section with the intensity of the cellulose 200-reflection as a contrast. On C, The XRD-based tomographic cross section with the orientation (mmfa) of the microfibrils as a contrast. The bar on A describes the scale (length of the bar 100 um) and the bars in B and C show the colour scale of the XRD-CT results: the upper end of the colour scale corresponds to the higher intensity of the 200-reflection in B), and the lower scale to smaller mmfa value in C)
We have previously studied young T89 clone hybrid aspen samples, including 21 plants with ages between 2.5 to 3.5 months (Svedström et al. 2012). In that study, it was observed, that the mean MFA was significantly lower in juvenile T89 compared to that of juvenile birch wood (Leppänen et al. 2011; Svedström et al. 2012; Bonham and Barnett 2001). It was also observed that the low MFA values in most cases occurred alongside crystallite widths of around 3 nm, which corresponds to the average crystallite width of normal wood (see, e.g., Andersson et al. 2003; Leppänen et al. 2011; Svedström et al. 2012). The property of hybrid aspen to produce fibres with low MFA values was also observed in the current study. As such, this is an interesting property of hybrid aspen, because generally it is expected that juvenile saplings are connected to high MFAs to sustain mechanical adaptivity (Barnett and Bonham 2004).
Compared to the crystallite width results of Table 2 corresponding to the TW side, the average crystallite width has been determined to be larger in more mature TW (typically around 4–5 nm) based on the literature values for poplar, European aspen, hybrid aspen and many tropical hardwood species (Müller et al. 2006; Leppänen et al. 2011; Viljanen et al. 2020). This observation is valid also when taking into account the proportions of the TW tissues among the whole line scan area. So, it might be possible that in these very juvenile saplings (less than 2 months old), the cellulose microfibrils are not yet fully crystallized and aggregated in TW G-fibre cell walls.
Another observation, which can be made based on the XRD results is that in the samples (samples 1, 2, 3) the crystallite sizes in the positions which correspond to the OW side also are slightly smaller than those of the hybrid aspen NW (Svedström et al. 2012). This similar kind of slight difference can be also observed in the results represented by Sawada et al. (2018). However, also in their study, the difference is just within the accuracy of the results: the mean crystallite width based on the cellulose reflection 200 is in OW 3.1 and 3.2 nm, whereas in NW they obtained 3.3 nm (Sawada et al. 2018). The slightly smaller crystallite widths of OW are interesting to be compared to the similar crystallite widths (around 2.9 nm) observed for compression wood samples (Andersson et al. 2000) and compression wood-like species such as juniper (Hänninen et al. 2012).
In the future, it would be essential to repeat the experiments for a larger group of samples due to the biological variation between the individual saplings. Additionally, it would be interesting to compare the TW formation between greenhouse-, outdoor- and wild-grown saplings to see if the density and occurrence of the TW are similar between all the studied groups.
Combining the nanostructural results obtained by X-ray scattering with the cellular scale structures observed by XMT, offered intriguing views of the structural features of the juvenile hybrid aspen saplings. Overall, the combination of these methods is a powerful tool for wood characterization.
Conclusion
The combined scattering and tomography techniques reveal tissue-specific structural information both at the nano- and microscales and especially map these structural properties together. Using these methods, the average width of the cellulose crystallites in TW was 3.5 ± 0.1 nm, and in OW it was 3.1 ± 0.1 nm. Thus, a consistent difference was observed between TW and OW. The difference was further confirmed and detected also regarding the orientation, i.e. mean microfibril angle, which was determined to be below 14° in TW and above 20 degrees in OW. In previous studies, the cellulose crystallite width has been observed to be distinctly larger in the mature TW (around 4–5 nm); thus, it might be that in the juvenile saplings (less than 2-month-old and stem width smaller than 2 mm), the cellulose microfibrils are not yet fully crystallized and aggregated in TW G-fibres.
Home laboratory bench-top set-up offers its own advantages for these studies, considering the number of samples characterized, time-dependent studies and larger field of views.
References
Andersson S, Serimaa R, Torkkeli M, Saranpää P, Pesonen E (2000) Microfibril angle of Norway spruce [Picea abies (L.) Karst.] compression wood: comparison of measuring techniques. J Wood Sci 46:343–349. https://doi.org/10.1007/BF00776394
Andersson S, Serimaa R, Paakkari T, Saranpää P, Pesonen E (2003) Crystallinity of wood and the size of cellulose crystallites in Norway spruce (Picea abies). J Wood Sci 49:531–537. https://doi.org/10.1007/s10086-003-0518-x
Barnett JR, Bonham VA (2004) Cellulose microfibril angle in the cell wall of wood fibres. Biol Rev 79(2):461–472. https://doi.org/10.1017/S1464793103006377
Bjurhager I, Olsson AM, Zhang B, Gerber L, Kumar M, Berglund LA, Burgert I, Sundberg B, Salmén L (2010) Ultrastructure and mechanical properties of populus wood with reduced lignin content caused by transgenic down-regulation of Cinnamate 4-hydroxylase. Biomacromol 11:2359. https://doi.org/10.1021/bm100487e
Bonham VA and Barnett JR (2001) Fibre Length and Microfibril Angle in Silver Birch (Betula pendula Roth 55(2):159–162. https://doi.org/10.1515/HF.2001.026
Brereton NJB, Ray MJ, Shield I, Martin P, Karp A, Murphy RJ (2012) Reaction wood—A key cause of variation in cell wall recalcitrance in willow. Biotech Biofuels 5:38. https://doi.org/10.1186/1754-6834-5-83
Brereton NJB, Ahmed F, Sykes D, Ray JR, Shield I, Karp A, Murphy RJ (2015) X-ray micro-computed tomography in willow reveals tissue patterning of reaction wood and delay in programmed cell death. BMC Plant Biol 15:83. https://doi.org/10.1186/s12870-015-0438-0
Brodersen CR, Roddy AB (2016) New frontiers in the three-dimensional visualization of plant structure and function. Am J Bot 103:184–188. https://doi.org/10.3732/ajb.1500532
Cave ID (1966) Theory of X-ray measurement of microfibril angle in wood. For Prod J 16:37–42
Chang SS, Quignard F, Alméras T, Clair B (2015) Mesoporosity changes from cambium to mature tension wood: a new step toward the understanding of maturation stress generation in trees. New Phytol 205:1277. https://doi.org/10.1111/nph.13126
Clair B, Thibaut B, Sugiyama J (2005) On the detachment of the gelatinous layer in tension wood fiber. J Wood Sci 51:218–221. https://doi.org/10.1007/s10086-004-0648-9
Clair B, Alméras T, Pilate G, Jullien D, Sugiyama J, Riekel C (2011) Maturation stress generation in poplar tension wood studied by synchrotron radiation microdiffraction. Plant Phys 155:562. https://doi.org/10.1104/pp.109.149542
Ding SY, Liu YS, Zeng Y, Himmel ME, Baker JO, Bayer EA (2012) How does plant cell wall architecture correlate with enzymatic digestibility? Science 338:1055. https://doi.org/10.1126/science.1226073
Foston M, Hubbell CA, Samuel R, Jung S, Fan H, Ding SY, Zeng Y, Jawdy S, Davis M, Sykes R, Gjersing E, Tuskan GA, Kalluri U, Ragauskas AJ (2011) Chemical, ultrastructural and supramolecular analysis of tension wood in Populus tremula x alba as a model substrate for reduced recalcitrance, Energy Environ. Sci 4:1754–5692. https://doi.org/10.1039/C1EE02073K
Franceschi VR, Horner HT (1980) Calcium oxalate crystals in plants. Bot Rev 46(4):361–427. https://doi.org/10.1007/BF02860532
Gardiner B, Barnett J, Saranpää P and Gril J (2014) The biology of reaction wood, Springer Series in Wood Science. https://doi.org/10.1007/978-3-642-10814-3
Gorshkova T, Chernova T, Mokshina N, Ageeva M, Mikshina P (2018) Plant ‘muscles’: fibers with a tertiary cell wall. New Phytol 218(1):66–72. https://doi.org/10.1111/nph.14997
Hänninen T, Tukiainen P, Svedström K, Serimaa R, Saranpää P, Kontturi E, Hughes M, Vuorinen T (2012) Ultrastructural evaluation of compression wood-like properties of common juniper (Juniperus communis). Holzforschung 66(3):389–395. https://doi.org/10.1515/hf.2011.166
He H, Bleby TM, Veneklaas EJ, Lambers H, Kuo J (2012) Morphologies and elemental compositions of calcium crystals in phyllodes and branchlets of Acacia robeorum (Leguminosae: Mimosoideae). Ann Bot 109(5):887–896. https://doi.org/10.1093/aob/mcs004
Jonasson S, Bünder A, Das O, Niittylä T, Oksman K (2021) Comparison of tension wood and normal wood for oxidative nanofibrillation and network characteristics. Cellulose 28:1085–1104. https://doi.org/10.1007/s10570-020-03556-1
Leppänen K, Bjurhager I, Peura M, Kallonen A, Suuronen JP, Penttilä P, Love J, Fagerstedt K, Serimaa R (2011) X-ray scattering and microtomography study on the structural changes of never-dried silver birch, European aspen and hybrid aspen during drying. Holzforschung 65:865. https://doi.org/10.1515/HF.2011.108
Mellerowicz EJ, Gorshkova TA (2012) Tensional stress generation in gelatinous fibres: a review and possible mechanism based on cell-wall structure and composition. J Exp Bot 63(2):551–565. https://doi.org/10.1093/jxb/err339
Müller M, Burghammer M, Sugiyama J (2006) Direct investigation of the structural properties of tension wood cellulose microfibrils using microbeam x-ray fibre diffraction. Holzforschung 60(5):474–479. https://doi.org/10.1515/HF.2006.078
Rüggeberg M, Saxe F, Metzger TH, Sundberg B, Fratzl P, Burgert I (2013) Enhanced cellulose orientation analysis in complex model plant tissues. J Struct Biol 183(3):419–428. https://doi.org/10.1016/j.jsb.2013.07.001
Sarén MP, Serimaa R (2006) Determination of microfibril angle distribution by x-ray diffraction. Wood Sci Technol 40:445–460. https://doi.org/10.1007/s00226-005-0052-7
Sawada D, Kalluri UC, O’Neill H, Urban V, Langan P, Davison B, Venkatesh S (2018) Tension wood structure and morphology conducive for better enzymatic digestion. Biotechnol Biofuels 11:44. https://doi.org/10.1186/s13068-018-1043-x
Schmelzle S, Bruns S, Beckmann F, Moosmann J, Lautner S (2021) Using in situ synchrotron-radiation-based microtomography to investigate 3d structure-dependent material properties of tension wood. Adv Eng Mater 23:2100235. https://doi.org/10.1002/adem.202100235
Storm S, Ogurreck M, Laipple D, Krywka C, Burghammer M, Di Cola E, Müller M (2015) On radiation damage in FIB-prepared softwood samples measured by scanning x-ray diffraction. J Synchrotron Radiat 22(2):267–272. https://doi.org/10.1107/S1600577515001241
Stuppy WH, Maisano JA, Colbert MW, Rudall PJ, Rowe TB (2003) Three-dimensional analysis of plant structure using high-resolution x-ray computed tomography. Trends Plant Sci 8:2–6. https://doi.org/10.1016/S1360-1385(02)00004-3
Suuronen JP, Kallonen A, Hänninen V, Blomberg M, Hämäläinen K, Serimaa R (2014) Bench-top x-ray microtomography complemented with spatially localized x-ray scattering experiments. J Appl Cryst 47:471–475. https://doi.org/10.1107/S1600576713031105
Svedström K, Lucenius J, Van den Bulcke J, Van Loo D, Immerzeel P, Suuronen JP, Brabant L, Van Acker J, Saranpää P, Fagerstedt K, Mellerowicz E, Serimaa R (2012) Hierarchical structure of juvenile hybrid aspen xylem revealed using x-ray scattering and tomography. Trees 26:1793. https://doi.org/10.1007/s00468-012-0748-x
Van den Bulcke J, Boone MA, Dhaene J, Van Loo D, Van Hoorebeke L, Boone MN, Wyffels F, Beeckman H, Van Acker J, De Mil T (2019) Advanced x-ray CT scanning can boost tree-ring research for earth-system sciences. Ann Bot 124:837–847. https://doi.org/10.1093/aob/mcz126
Viljanen M, Ahvenainen P, Penttilä P, Help H, Svedström K (2020) Ultrastructural X-ray scattering studies of tropical and temperate hardwoods used as tonewoods. IAWA J 41(3):301–319. https://doi.org/10.1163/22941932-bja10010
Viljanen M, Suomela JA, Svedström K (2022) Wide-angle x-ray scattering studies on contemporary and ancient bast fibres used in textiles—ultrastructural studies on stinging nettle. Cellulose 29(4):2645–2661. https://doi.org/10.1007/s10570-021-04400-w
Zhang T, Cieslak M, Owens A, Wang F, Broholm SK, Teeri TH, Elomaa P, Prusinkiewicz P (2021) Phyllotactic patterning of gerbera flower heads. PNAS 118(13):e2016304118. https://doi.org/10.1073/pnas.2016304118
Funding
Open Access funding provided by University of Helsinki including Helsinki University Central Hospital. This work was supported by the University of Helsinki (3-year Research Grant) and by Väisälä Fund.
Author information
Authors and Affiliations
Contributions
M.V together with K.S. wrote the main manuscript text and M.V. prepared all the figures and tables presented in manuscript and in supplementary file. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Viljanen, M., Help, H., Suhonen, H. et al. Combined X-ray diffraction tomography imaging of tension and opposite wood tissues in young hybrid aspen saplings. Wood Sci Technol 57, 797–814 (2023). https://doi.org/10.1007/s00226-023-01477-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00226-023-01477-3