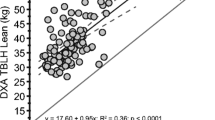
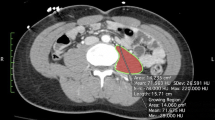
Abstract
Initial definitions of sarcopenia included the age-associated loss of skeletal muscle mass that was presumed to be associated with late-life reduced functional capacity, disability and loss of independence. Because no method for determination of muscle mass was available for large cohort studies of aging men and women, lean body mass determined by dual X-ray absorptiometry or bioelectrical impedance was used as a surrogate measure of muscle mass. The data from these studies showed either no or a poor relationship between LBM and functional capacity and health related outcomes, leading to the conclusion of many that the amount of muscle may not be associated with these age-associated outcomes. It was assumed that some undefined index of muscle quality is the critical contributor. These studies also consistently showed that muscle strength is lost more quickly than lean mass. Total body muscle mass can now be measured directly, accurately and non-invasively using the D3creatine (D3Cr) dilution method. D3Cr muscle mass, but not DXA derived LBM, is strongly associated with functional capacity, falls and insulin resistance in older men and women. In addition, D3Cr muscle mass is associated with risk of disability, hip fracture and mortality. New and emerging data demonstrate that low muscle mass may serve as a diagnostic criterion for sarcopenia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Changing body composition is, perhaps, the most prominent and obvious feature of advancing age. Body fatness increases, even in men and women who remain weight stable as they grow older, the density of bones decrease, and the amount of skeletal muscle declines. More specifically, the geriatric syndrome, sarcopenia, was originally defined as the age-associated loss of skeletal muscle mass [1], that was hypothesized to increase the risk of functional decline and disability. Subsequent to the initial definition of sarcopenia, several (> 15) definitions have been published, many of which include lean body mass (LBM) rather than muscle mass [2,3,4,5,6]. However, an accurate assessment of whole-body muscle mass has proved to be elusive, and most investigators have used dual X-ray absorptiometry (DXA) or bioelectric impedance (BIA) assessments of lean body mass (LBM) as a surrogate measurement in large cohort studies. However, muscle mass is only one component of LBM that also includes body water, viscera, fibrotic and connective tissue. As a result, several cross-sectional and longitudinal aging cohort studies have reported little or no relationship between low LBM and increased risk of health-related outcomes including functional capacity, disability, and mortality [7,8,9]. A meta-analysis [10] of longitudinal observation studies in older people (≥ 65 year), conducted between 1976 and 2012 examined body composition (BIA, DXA, CT) and physical functional capacity. In the studies that examined lean mass (incorrectly described as “muscle mass”) the authors concluded that “low muscle mass was not significantly associated with functional decline”. They also concluded that the role of muscle mass in the development of functional decline was unclear but was “much smaller than the role of fat mass and muscle strength”. These conclusions that the loss of skeletal muscle mass, per se, is only weakly associated with functional outcomes in older people may be the result of using the measurement of lean mass as a proxy for muscle mass rather than using a direct measurement of muscle mass. Because LBM is so poorly associated with health-related outcomes in aging cohort studies, investigators have used DXA derived appendicular lean mass (ALM) to, perhaps, better approximate muscle mass. ALM is the sum of lean tissue in the arms and legs, typically expressed as ALM/ht2. While there is no disagreement that skeletal muscle is diminished with advancing age, the degree to which this reduction is associated with loss of functional capacity and risk of disability has not been established. In an effort to examine the existing data on the relationship between LBM and/or ALM and outcomes and publish a consensus definition of sarcopenia, a project was supported by the Foundation for the National Institutes of Health Biomarkers Consortium Sarcopenia (FINH). The group determined that strength (grip or quadriceps) but not lean mass was associated with mortality [11]. Although muscle mass was not measured in this study (only lean mass and thigh cross-sectional area from CT were assessed), the authors concluded that “Low muscle mass did not explain the strong association of strength with mortality, demonstrating that muscle strength as a marker of muscle quality is more important than quantity in estimating mortality risk”. Reductions in strength, but not lean mass, were associated with outcomes with advancing age which has led to the conclusion by several groups attempting to define sarcopenia to conclude that loss of muscle mass is poorly or unrelated to health-related outcomes and that the continuous decline in strength and functional capacity with advancing age must result from some undefined decrease in muscle quality.
D3Creatine Dilution Method
Estimates of LBM do not provide an accurate measure of muscle mass for large cohort studies or randomized controlled trials. A suitable determination, particularly for cohort studies has proven to be elusive, until recently. Whole body magnetic resonance imaging is used to estimate total muscle volume; however, it is expensive, cumbersome, and impossible to use for some patient populations with contraindications (e.g., claustrophobia, non-MR compatible implanted metal or device). The D3creatine (D3Cr) dilution method measures the total body creatine pool size directly and takes advantage of well described features of creatine metabolism. Heymsfield et al. [12] estimated that about 98% (taken from Hunter [13]) of the body creatine pool is sequestered in the sarcomere; muscle has no capacity for creatine synthesis. Creatine is synthesized in the liver and kidney and transported against a huge concentration gradient into the sarcomere. The phosphorylation of creatine to high energy phosphocreatine represents an immediately available source of ATP for rapid muscle contraction. Because of its unique metabolic role for ATP synthesis, creatine and creatine phosphate are co-located with the contractile apparatus of muscle [14]. The method uses an oral tracer dose (30, 15 and 2 mg for adults, children, or infants) of deuterated creatine. It is 100% bioavailable and after entering the circulation, D3Cr is actively transported into all skeletal muscle cells. An additional aspect of creatine metabolism is that it is turned over through irreversible (in vivo) isomerization to creatinine which is rapidly excreted at a constant rate of about 1.7%/d. The enrichment of urine D3creatinine achieves isotopic steady state about 48 h and remains at steady state up to 96 h after ingestion of the tracer [15]. During this period of isotopic steady state, the enrichment of urine D3creatinine is the same as that of the intracellular enrichment of D3creatine, thus allowing a completely non-invasive sampling of the creatine pool. The method requires a fasting urine sample as consumption of foods with creatinine (mainly meat and meat products) results in increased urine creatinine excretion and dilution of the D3creatinine enrichment. Thus, the method requires knowledge of the tracer dose of D3Cr, ingestion of the dose, and collection of a single fasting urine sample 48 to 96 h later.
Clinical Studies Using D3Cr Dilution
The method has been validated in rats and in adult humans using whole body MRI [15] and was further validated in premature infants cared for in a neonatal intensive care unit. In that study [16], longitudinal measurements of D3Cr muscle mass were made biweekly and revealed an astonishing accretion of muscle of about 90 g/wk (15%/wk). In boys with Duchenne muscular dystrophy (DMD), D3Cr muscle mass decreased with advancing age (ages 7–17 year) compared to age-matched, healthy controls who demonstrated growth related increases with advancing age. Remarkably, 3 DMD non-ambulant boys age 14–17 year had 13, 3 and 5% muscle mass relative to body weight [17]. These data demonstrate that muscle mass and changes in muscle mass can now be accurately and directly measured in populations and patients for which such assessments have not previously been available. D3Cr dilution method has been directly compared to DXA LBM in separate studies. In the initial validation study [15] in men and women between the ages of 19–84 year, DXA LBM vs D3Cr muscle mass were strongly related (r = 0.745); In 112 older men and women (70–95 year) the correlation was r = 0.6 [18]; in 74 older women (82.3 ± 5.4 year), r = 0.50 [19]; in 1382 older men (84.2), r = 0.66 [20]. Importantly, in each of these comparisons, the estimate of LBM by DXA is significantly and substantially higher than that of D3Cr muscle mass, often by more than 100%.
A criticism of the D3Cr dilution method is that its calculation of muscle mass relies on the assumption that the concentration of creatine is set at 4.3 g/kg wet weight of muscle, as the value of creatine pool size (in grams) that is estimated by the enrichment of D3Creatinine is divided by 4.3 to obtain total muscle mass in kg. The 4.3 g/kg wet weight is derived from rodent and humans [21, 22] and is based on an average estimated muscle fiber composition of 50% type I and II fibers. We note that type II, fast, fibers have a higher amount of creatine than type I, slow, fibers. Recently, Sagayama [23] et al. showed a strong relationship between total body MRI estimates of muscle mass vs D3Creatine muscle mass in young athletes. However, the use of 5.0 g/kg conversion factor for this population provided a better fit to estimate muscle mass in this population. These data demonstrate that creatine pool size may differ by muscle fiber composition, particularly in athletic populations. Some have called for use of creatine pool size estimated by D3Cr creatine dilution as the primary variable of interest to be used in analyses, rather than D3Cr muscle mass. We note that since D3Cr muscle mass (calculated using the set 4.3 g creatine/kg of wet weight muscle) is a monotonic transformation of the creatine pool size, effect estimates for standardized variables would be identical for either creatine pool size or D3Cr muscle mass (analyzed with or without adjustment for body size). For example, in the MrOS study, each standard deviation decrement in D3Cr muscle mass/body mass was associated with a 1.9-fold increased risk (hazard ratio, 1.9 (95%CI 1.2, 3.1) of incident ADL disability 2.2 years later; the same size association seen when this expressed as risk per SD decrement in creatine pool size/body mass (hazard ratio, 1.9 (95%CI 1.2, 3.1) [24].
The method requires that collection of a fasting urine sample during isotopic steady state (between 48 and 96 h after ingestion of the dose). Creatinine found in meat (and meat products) and fish can dilute the enrichment of D3Creatinine leading to an overestimation of creatine pool size and thus muscle mass. Typically, an overnight fast (after 10:00 PM) and use of the second voided urine sample prior to consumption of food greatly reduces to variability in the measurement due to dietary factors. Shankaran et al. [25] reported a CV of 3.1% for measurement of creatinine enrichment in urine sample collected three consecutive days. The method determines the dilution of the D3Cr dose in the body creatine pool. This requires absolute knowledge of the amount of the dose and no loss of the label before it is completely distributed throughout the body, including active transport into sarcomeres. However, some individuals “spill” a small amount of the D3Cr dose into urine [15]. This urine loss of tracer indicates that in some, the rate of endogenous production of creatine is greater than the rate of storage in muscle and, thus, a small amount of creatine is filtered in the kidneys and excreted. This small loss of label must be accounted for in the calculation of creatine pool size. Shankaran et al. [25] developed a correction algorithm based on the ratio of fasting urine creatine/creatinine in the sample collected for determination of D3creatinine enrichment. They also reported that most subjects are non-spillers and that the magnitude of correction for spillage of label is quite small.
Osteoporotic Fractures in Men Cohort
The osteoporotic fractures in men (MrOS) Study is the largest study to date that has employed the D3Cr dilution method to assess muscle mass. In the study of ~ 1400 older men, low D3Cr muscle mass (but not low DXA lean mass or appendicular lean mass) was associated cross-sectionally with worse short physical performance battery (SPPB) score, slower walking speed, decreased lower extremity power, worse chair stand ability and greater likelihood of prevalent mobility limitations and disability [20]. The only muscle function related metric that was associated with both DXA appendicular lean mass and D3Cr muscle mass was grip strength. This population of older men (mean age of 84.2 year) reported multiple chronic diseases. Men in the higher quartiles of D3Cr muscle mass had significantly lower prevalence of type 2 diabetes, myocardial infarction, chronic heart failure and COPD. In addition, men in higher quartiles of D3Cr muscle mass/body mass generally had better cognitive function, had higher levels of physical activity, greater Life-Space and had markedly lower levels of exhaustion and fatigue. In longitudinal analyses over 3–5 years of follow-up, low D3Cr muscle mass was also associated with increased risk of hip fractures [26], injurious falls [20], incident disability and mortality [24]. Generally, the association of low D3Cr muscle mass with these outcomes was not explained by weakness or slow walking speed and there were few associations between low lean mass and these outcomes. In an accompanying commentary, Schaap [27] wrote, “In contrast to DXA, deuterated creatine (D3Creatine) assesses muscle mass directly” and “By isolating contractile muscle mass from noncontractile components including fat, the D3Creatine assessment is not only an accurate method to assess muscle mass but is less biased by obesity and aging than DXA ALM.”
The association of sarcopenic obesity with outcomes was also examined in this cohort of older men [28]. Sarcopenic obesity is the combination of obesity with sarcopenia, a change in body composition typical of aging and the consensus of published literature has been that sarcopenic obesity poses even greater risks for poor health-related outcomes and disability than either obesity or sarcopenia alone [29]. However, when an accurate assessment of muscle mass using D3Cr dilution (rather than lean mass) was used, reduced muscle mass was strongly associated with important health-associated outcomes and the negative effects of adiposity were minimal, suggesting that obesity has little relevance for the understanding of important outcomes of sarcopenia and that sarcopenic obesity may be an anachronistic term that has few implications for physical function and risk of disability for older men. Figure 1 describes the body composition of the individual older men from this study by combining DXA with D3Cr muscle mass assessments [28]. Muscle mass is displayed from highest to lowest value and represents only about 50% of LBM. The light blue represents ‘residual’ non-muscle LBM consisting of body water, fibrotic, connective, visceral tissue, and smooth muscle.
From Orwoll et al. [28]. Body composition using DXA and D3creatine dilution in 1376 older men. DXA provides an assessment of lean body mass (LBM, light blue + dark blue), fat mass, and bone mass while D3Cr is a measurement of muscle mass (dark blue). The men are ranked from highest muscle mass to lowest D3Cr muscle mass. Superimposed on the graph is DXA derived appendicular lean mass (ALM) in yellow. In this cohort, muscle mass was about 50% of LBM and was strongly associated with health-related outcomes including mortality, while LBM, ALM, or fat mass were not [20, 24, 26]
However, before D3Cr muscle mass can be fully considered as a biomarker of sarcopenia, several limitations must be addressed. The MrOS cohort is not representative as it is comprised of very old men who mostly identify as Non-Hispanic White. However, two published studies from older women (from the Women’s Health Initiative cohort) show similar patterns in women [19, 30]. In community dwelling older women (mean age, 82.3 ± 5.4 year) D3Cr muscle mass was moderately related to DXA LM and ALM (r = 0.50 for both) with a significantly greater relationship between muscle mass and SPPB score compared to DXA LM or ALM [19]. In the same group of women, there was a significant inverse relationship between D3Cr muscle mass, and impaired insulin-glucose homeostasis [30].
Several new studies are underway to collect data from younger individuals, women and those who report other race and ethnic backgrounds, including projects in the Women’s Health Initiative life and longevity after cancer (LILAC) study, the Framingham Offspring Cohort, the Tobago Longitudinal Aging Study, the study of muscle mobility and aging (SOMMA), and others. In addition, there are limited data describing change in D3Cr muscle mass over time in adults. A study from MrOS in 40 participants (mean age 83.3 year) [31] found that the change in D3Cr muscle mass over 1.6 years is similar in magnitude to the change in grip strength and walking speed over the same period (with a mean loss of each of about 5%). Further, change in grip strength was significantly correlated with change in D3Cr muscle mass. During this time, there was no significant change in DXA lean mass, suggesting that the disconnect between declines in lean mass and declines in strength results from the poor measurement of muscle quantity by lean mass. While previous studies [32] have concluded that the age-associated decrease in strength is greater than the loss of muscle (using LBM measurements), these data strongly suggest that the loss of strength with age is closely linked to the loss of muscle mass. Additional data about change in D3Cr muscle mass from these ongoing studies will help characterize the change in muscle mass with aging and under other conditions.
Recent randomized controlled trials have demonstrated that the D3Cr is quite responsive to interventions that result in changes in muscle mass. Balachandran et al. [33] demonstrated that the D3Cr dilution method was sufficiently sensitive to detect changes in muscle mass in frail older subjects undergoing a resistance exercise training program compared to a non-intervention education program.
Conclusion
The accurate determination of muscle mass in older men in the MrOS cohort and (so far) a limited number of older women from the WHI has provided insights into the importance of muscle rather than lean mass and its measurement. Although, these data are limited to largely white men and women at the present time, they reveal the previously unrecognized association between the amount of muscle and important health related outcomes and functional capacity in aging populations. In addition to its essential role for movement, skeletal muscle is the primary site of insulin stimulated glucose disposal, provides force to maintain bone density, principal component of age-associated decreased basal metabolic rate, and secretes myokines that exert autocrine and endocrine-like metabolic effects. The D3Cr dilution method can provide opportunities to examine how changing muscle mass may contribute to the increased risk of several age-associated diseases and syndromes. The strong and independent effects of D3Cr muscle mass to increased risk of instrumental activities of daily living (IADL) and mortality in the MrOS cohort suggest a more central role in changing cognitive function than previously thought. The use of lean body mass has resulted in a type 2 error for aging research that will continue to be propagated if LBM is considered a surrogate measurement of muscle mass. Zanker et al. [34, 35] used factor analysis of components of body composition in the MrOS cohort that were linked to poor mobility and determined that only D3Cr muscle mass factors were associated with negative outcomes. They concluded “these data support efforts to evaluate the D3Cr dilution method in clinical applications including in future definitions of sarcopenia”. In total, emerging data suggest that D3Cr muscle mass, perhaps even without assessment of strength or physical performance, could serve as a biomarker of sarcopenia in clinical or research settings that predicts adverse events.
References
Evans W (1995) What is sarcopenia? J Gerontol 50A:5–8
Bhasin S, Travison TG, Manini TM, Patel S, Pencina KM, Fielding RA et al (2020) Sarcopenia definition: the position statements of the sarcopenia definition and outcomes consortium. J Am Geriatr Soc 68(7):1410–1418
Carvalho do Nascimento PR, Bilodeau M, Poitras S (2021) How do we define and measure sarcopenia? A meta-analysis of observational studies. Age Ageing 50(6):1906–1913
Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyere O, Cederholm T et al (2019) Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 48(1):16–31
Newman AB, Kupelian V, Visser M, Simonsick E, Goodpaster B, Nevitt M et al (2003) Sarcopenia: alternative definitions and associations with lower extremity function. J Am Geriatr Soc 51(11):1602–1609
Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB et al (2011) Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology and consequences. International working group on sarcopenia. J Am Med Dir Assoc 12(4):249–256
Cawthon PM, Fox KM, Gandra SR, Delmonico MJ, Chiou CF, Anthony MS et al (2009) Do muscle mass, muscle density, strength and physical function similarly influence risk of hospitalization in older adults? J Am Geriatr Soc 57(8):1411–1419
Cawthon PM, Blackwell TL, Cauley J, Kado DM, Barrett-Connor E, Lee CG et al (2015) Evaluation of the usefulness of consensus definitions of sarcopenia in older men: results from the observational osteoporotic fractures in men cohort study. J Am Geriatr Soc 63(11):2247–2259
Janssen I (2006) Influence of sarcopenia on the development of physical disability: the cardiovascular health study. J Am Geriatr Soc 54(1):56–62
Schaap LA, Koster A, Visser M (2013) Adiposity, muscle mass and muscle strength in relation to functional decline in older persons. Epidemiol Rev 35:51–65
Newman AB, Kupelian V, Visser M, Simonsick EM, Goodpaster BH, Kritchevsky SB et al (2006) Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J Gerontol A Biol Sci Med Sci 61(1):72–77
Heymsfield SB, Arteaga C, McManus C, Smith J, Moffitt S (1983) Measurement of muscle mass in humans: validity of the 24-hour urinary creatinine method. Am J Clin Nutr 37(3):478–494
Hunter A (1928) The biological distribution of creatine and creatinine. In: Hunter A (ed) Creatine and creatinine. Longmans, Green, London, pp 73–113
Hill DK (1962) The location of creatine phosphate in frog’s striated muscle. J Physiol 164:31–50
Clark RV, Walker AC, O’Connor-Semmes RL, Leonard MS, Miller RR, Stimpson SA et al (2014) Total body skeletal muscle mass: estimation by creatine (methyl-d3) dilution in humans. J Appl Physiol 116(12):1605–1613
Evans WJ, Scottoline B, Imam F, Hellerstein M, Garton K, Czerwieniec G et al (2020) D3-creatine dilution for the noninvasive measurement of skeletal muscle mass in premature infants. Pediatr Res. https://doi.org/10.1038/s41390-020-01122-w
Evans WJ, Shankaran M, Smith EC, Morris C, Nyangau E, Bizieff A et al (2021) Profoundly lower muscle mass and rate of contractile protein synthesis in boys with Duchenne muscular dystrophy. J Physiol. https://doi.org/10.1113/JP282227
Buehring B, Siglinsky E, Krueger D, Evans W, Hellerstein M, Yamada Y et al (2018) Comparison of muscle/lean mass measurement methods: correlation with functional and biochemical testing. Osteoporos Int 29(3):675–683
Zhu K, Wactawski-Wende J, Ochs-Balcom HM, LaMonte MJ, Hovey KM, Evans W et al (2021) The association of muscle mass measured by D3-Creatine dilution method with dual energy X-ray absorptiometry and physical function in postmenopausal women. J Gerontol A Biol Sci Med Sci. https://doi.org/10.1093/gerona/glab020
Cawthon PM, Orwoll ES, Peters KE, Ensrud KE, Cauley JA, Kado DM et al (2019) Strong relation between muscle mass determined by D3-creatine dilution, physical performance, and incidence of falls and mobility limitations in a prospective cohort of older men. J Gerontol A Biol Sci Med Sci 74(6):844–852
Meador CK, Kreisberg RA, Friday JP Jr, Bowdoin B, Coan P, Armstrong J et al (1968) Muscle mass determination by isotopic dilution of creatine-14C. Metabolism 17(12):1104–1108
Kreisberg RA, Bowdoin B, Meador CK (1970) Measurement of muscle mass in humans by isotopic dilution of creatine-14C. J Appl Physiol 28(3):264–267
Sagayama H, Yamada Y, Kondo E, Tanabe Y, Uchizawa A, Shankaran M et al (2022) Skeletal muscle mass can be estimated by creatine (methyl-d(3)) dilution and is correlated with fat-free mass in active young males. Eur J Clin Nutr. https://doi.org/10.1038/s41430-022-01237-9
Cawthon PM, Blackwell T, Cummings SR, Orwoll ES, Duchowny KA, Kado DM et al (2020) Muscle mass assessed by D3-Creatine dilution method and incident self-reported disability and mortality in a prospective observational study of community dwelling older men. J Gerontol A Biol Sci Med Sci. https://doi.org/10.1093/gerona/glaa111
Shankaran M, Czerwieniec G, Fessler C, Wong PA, Killion S, Turner SM et al (2018) Dilution of oral D3 -Creatine to measure creatine pool size and estimate skeletal muscle mass: development of a correction algorithm. J Cachexia Sarcopenia Muscle 9:540–546
Cawthon PM, Peters KE, Cummings SR, Orwoll ES, Hoffman AR, Ensrud KE et al (2022) Association between muscle mass determined by D3 -Creatine dilution and incident fractures in a prospective cohort study of older men. J Bone Miner Res 2022:10
Schaap LA (2018) D3-Creatine dilution to assess muscle mass. J Gerontol A Biol Sci Med Sci. https://doi.org/10.1093/gerona/gly180
Orwoll ES, Peters KE, Hellerstein M, Cummings SR, Evans WJ, Cawthon PM (2020) The importance of muscle versus fat mass in sarcopenic obesity: a re-evaluation using D3-Creatine muscle mass versus DXA lean mass measurements. J Gerontol A Biol Sci Med Sci. https://doi.org/10.2139/ssrn.3377531
Zamboni M, Mazzali G, Fantin F, Rossi A, Di Francesco V (2008) Sarcopenic obesity: a new category of obesity in the elderly. Nutr Metab Cardiovasc Dis 18(5):388–395
Banack HR, LaMonte MJ, Manson JE, Zhu K, Evans WJ, Shankaran M et al (2022) Association of muscle mass measured by D3-Creatine (D3Cr), sarcopenic obesity and insulin-glucose homeostasis in postmenopausal women. PLoS ONE 17(12):e0278723
Duchowny KA, Peters KE, Cummings SR, Orwoll ES, Hoffman AR, Ensrud KE et al (2020) Association of change in muscle mass assessed by D3-Creatine dilution with changes in grip strength and walking speed. J Cachexia Sarcopenia Muscle 11(1):55–61
Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, Schwartz AV et al (2006) The loss of skeletal muscle strength, mass and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci 61(10):1059–1064
Balachandran A, Cawthon P, Evans W, Wang Y, Shankaran M, Hellerstein M et al (2023) Comparing D3-Creatine dilution and DXA muscle mass responses to strength training in low functioniong older adults. J Gerontol Med Sci 8:glad07
Zanker J, Blackwell T, Patel S, Duchowny K, Brennan-Olsen S, Cummings SR et al (2022) Factor analysis to determine relative contributions of strength, physical performance, body composition and muscle mass to disability and mobility disability outcomes in older men. Exp Gerontol 161:111714
Zanker J, Patel S, Blackwell T, Duchowny K, Brennan-Olsen S, Cummings SR et al (2020) Walking speed and muscle mass estimated by the D3-Creatine dilution method are important components of sarcopenia associated with incident mobility disability in older men: a classification and regression tree analysis. J Am Med Dir Assoc. https://doi.org/10.1016/j.jamda.2020.03.017
Acknowledgements
William Evans receives support from NIH grants R01CA246695, R01AG065265, R01AG074956-01, and R37CA258761-01A1 as well as the Muscular Dystrophy Association, Duchenne UK, and Parent Project Muscular Dystrophy.
Funding
Funding was provided by National Institutes of Health (Grant Nos. R01CA246695, R01AG065265, R01AG074956-01, R37CA258761-01A1).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Dr. Evans is listed as an inventor and has licensed the granted patents for the D3Creatine dilution method. Dr. Cawthon has no competing interests to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Evans, W.J., Cawthon, P.M. D3Creatine Dilution as a Direct, Non-invasive and Accurate Measurement of Muscle Mass for Aging Research. Calcif Tissue Int 114, 3–8 (2024). https://doi.org/10.1007/s00223-023-01124-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-023-01124-w