Abstract
Pathogenic variants in the LRP5, PLS3, or WNT1 genes can significantly affect bone mineral density, causing monogenic osteoporosis. Much remains to be discovered about the phenotype and medical care needs of these patients. The purpose of this study was to examine the use of medical care among Dutch individuals identified between 2014 and 2021 with a pathogenic or suspicious rare variant in LRP5, PLS3, or WNT1. In addition, the aim was to compare their medical care utilization to both the overall Dutch population and the Dutch Osteogenesis Imperfecta (OI) population. The Amsterdam UMC Genome Database was used to match 92 patients with the Statistics Netherlands (CBS) cohort. Patients were categorized based on their harbored variants: LRP5, PLS3, or WNT1. Hospital admissions, outpatient visits, medication data, and diagnosis treatment combinations (DTCs) were compared between the variant groups and, when possible, to the total population and OI population. Compared to the total population, patients with an LRP5, PLS3, or WNT1 variant had 1.63 times more hospital admissions, 2.0 times more opened DTCs, and a greater proportion using medication. Compared to OI patients, they had 0.62 times fewer admissions. Dutch patients with an LRP5, PLS3, or WNT1 variant appear to require on average more medical care than the total population. As expected, they made higher use of care at the surgical and orthopedic departments. Additionally, they used more care at the audiological centers and the otorhinolaryngology (ENT) department, suggesting a higher risk of hearing-related problems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis is a common multifactorial disorder that affects approximately 18.3% of the world’s population [1]. However, in rare cases, osteoporosis can be hereditary due to mutations in a single gene (monogenic osteoporosis). In contrast to osteoporosis found in postmenopausal women and elderly males, patients with monogenic osteoporosis present fractures associated with decreased bone mineral density (BMD) at a younger age [2]. Due to the wide implementation of next generation sequencing, several monogenic defects causing early-onset osteoporosis have been identified in recent years [3, 4]. This study focuses primarily on pathogenic or rare suspicious variants in low-density lipoprotein receptor-related protein 5 (LRP5), plastin 3 (PLS3), or proto-oncogene Wnt-1 (WNT1) as monogenic causes of osteoporosis [5].
When addressing monogenic osteoporosis, osteogenesis imperfecta (OI) cannot be omitted. Compared to the widely investigated phenotype of OI patients, little is known about the phenotype of LRP5, PLS3, or WNT1 individuals [6]. OI is a syndromic form of monogenic osteoporosis mainly caused by pathogenic variants in collagen type I encoding genes or in genes responsible for collagen type I posttranslational processing [7, 8]. Although bone fragility is the predominant clinical manifestation of OI, it is typically accompanied by extraskeletal manifestations such as blue sclerae, dentinogenesis imperfecta, impaired hearing, and increased joint laxity. It is unknown if and to what extent patients with LRP5, PLS3, or WNT1 pathogenic variants exhibit comparable extraskeletal manifestations. The LRP5, PLS3, and WNT1 genes are not directly engaged in collagen type I production but are involved in other bone formation-related pathways [9]. In particular, both WNT1 and LRP5 are involved in the Wnt signaling pathway. This pathway is activated by the interaction of WNT1 with the Frizzled receptor and an LRP5/6 co-receptor. This binding triggers an intracellular signaling cascade that stimulates preosteoblastic replication, induces osteoblastogenesis, and inhibits osteoblastic and osteocytic apoptosis [9]. The pathophysiology of PLS3 is still unclear, but it probably influences bone homeostasis by regulating osteoclast activity [10].
The LRP5, PLS3, and WNT1 genes all encode proteins in which monoallelic pathogenic variants can significantly affect the BMD. Biallelic pathogenic variants in WNT1 are known to cause a severe form of OI (MIM#615,220), while heterozygous pathogenic variants cause juvenile osteoporosis (MIM#615,221) [11]. In LRP5, loss-of-function pathogenic variants as well as gain-of-function pathogenic variants have been described [12]. Biallelic loss-of-function pathogenic variants have been shown to cause osteoporosis pseudoglioma syndrome (MIM#259,770), a disorder characterized by early-onset osteoporosis and complications in eye development [13] or exudative vitreoretinopathy (MIM#601,813), a milder eye disorder. A heterozygous loss-of-function LRP5 pathogenic variant can predispose to juvenile osteoporosis or exudative vitreoretinopathy [12, 14, 15]. Both disorders show variability in expression and incomplete penetrance and may represent different manifestations of the same disorder. The heterozygous gain-of-function LRP5 pathogenic variant causes increased bone mineral density (MIM#607,634, MIM#144,750) [16]. The gene is in addition associated with polycystic liver disease 4 (MIM#617,875), lacking bone phenotype. A PLS3 pathogenic variant causes X-linked osteoporosis [10]. Males with PLS3 pathogenic variants experience severe osteoporosis and fracture at a young age (MIM#300,910), while in females with heterozygous pathogenic variants clinical presentation can vary in severity [17, 18]. It appears that people with LRP5 and heterozygous WNT1 pathogenic variants do not have extraskeletal characteristics similar to those found in patients with OI [6]. With the exception of reported blue or gray sclerae, individuals with PLS3 pathogenic variants do not display noticeable extra-skeletal characteristics; nevertheless, this has been studied only sparingly [19, 20].
Given that the role of LRP5, PLS3, and WNT1 in the development of osteoporosis was only discovered 10–15 years ago, and the rarity of these pathogenic variants, their associated morbidity and prognosis are still largely undetermined. It remains unclear which level of multidisciplinary care is needed for these patients [21]. The aim of this study was to describe the use of medical care by patients with an LRP5, PLS3, or WNT1 pathogenic or suspicious rare variant in the Netherlands. Additionally, the goal was to compare the medical data of these patients with the medical data of the overall Dutch population and the Dutch OI population [22].
Methods
Study Participants
All patients diagnosed with either a pathogenic or a rare suspicious variant in LRP5, PLS3, or WNT1 in the national reference center for the molecular diagnosis of OI in the Amsterdam UMC (Amsterdam UMC Genome Database) between 2014 and 2021 were eligible for inclusion in this study. We used the term “rare suspicious variants” to refer to genetic variations that, according to the ACMG classification, cannot be classified as likely pathogenic or pathogenic as yet due to their rarity and recent discovery [23, 24]. However, these variants of unknown significance (VUS) are highly suspicious of being pathogenic based on molecular data, population data, and patient and family data. To ensure that only patients with an osteoporotic phenotype were included, patients with LRP5 gain-of-function pathogenic variants were excluded from the study cohort. As biallelic pathogenic variants in WNT1 are known to cause a severe form of OI, individuals with these variants were excluded. The Dutch OI population, which has been previously described and also comes from the Amsterdam UMC genome database, was used to compare individuals with LRP5, PLS3, or WNT1 mutations to OI patients [22, 25].
Data Extraction
From the Amsterdam UMC Genome Database, a cohort of 92 individuals with a pathogenic or rare suspicious variant in LRP5, PLS3, or WNT1 was extracted. Patients were matched to the cohort of Netherlands Statistics (CBS). The CBS has anonymized Dutch population health care information. Upon request, healthcare authorities and academic institutions can access CBS data for research purposes. Patients’ ages and, if applicable, their ages at death were retrieved from the CBS database (available between 1995 and 2019). Additionally, data regarding hospital admissions, diagnosis treatment combinations (DTCs), outpatient clinic consultations, and drug use was extracted. Information on admission duration, admission type, hospital type, admission date, and medical specialty was extracted from hospitalization data (available between 2013 and 2019). In the Dutch healthcare system, DTCs are used for billing purposes [26]. A DTC care product is the entirety of consultations, examinations, and treatments that a patient receives on average at a hospital for a particular diagnosis. Included in the extracted data were the number of opened DTCs (available between 2013 and 2017) and the registered medical specialty. Information regarding the date, type and number of outpatient clinic consultations was retrieved (available between 2013 and 2019). Moreover, data concerning medicine use (in 2017) was extracted. This included data on the main anatomical drug group to which the prescribed medication belonged according to the Anatomical Therapeutic Chemical (ATC) classification [27]. The extracted data included first-line medication, which could have been prescribed by a general practitioner or a specialist; data on the duration of the prescription was not available.
Data Analysis
Data is presented for the LRP5, PLS3, WNT1 cohort and, where group sizes allow, categorized by the affected gene. Patients were divided into four age groups: 0 to 24, 25 to 44, 45 to 64, and 65 years and older. Subgroup age distributions were presented as median with 10th and 90th percentiles. Data on admissions, DTCs, outpatient clinic visits, and medication prescriptions were analyzed descriptively and reported as absolute numbers and percentages, or as mean, median, and standard deviation.
Using incidence rate ratios (IRRs), the number of hospital admissions (including both day-case and inpatient admissions) and DTCs for patients with an LRP5, PLS3, or WNT1 variant was compared to that of the whole population [28]. The CBS website provides free public access to information on the number of hospital admissions and DTCs in the Dutch population each year [29, 30]. In addition, the number of hospital admissions was compared to the number of admissions in the Dutch OI population [25]. The numerator of the IRRs was the mean number of admissions or DTCs per year in the LRP5, PLS3, and WNT1 cohort, while the denominator was the mean found in the total population or the OI population. Since the age groups in this study were not the same as those in our previous study on how often OI patients used medical care [22], hospitalization rates for the OI population were calculated for the aforementioned age groups.
The percentage of patients who use medication was calculated for different age groups and compared to the percentage of people who use medication in the total population. Information on the percentage of the total Dutch population that uses medication is available to the general public [31]. The percentage of patients using medication was also investigated per anatomical main group. Medication usage for the following main groups was investigated and categorized per age group: ‘alimentary tract and metabolism’, ‘blood and blood forming organs’, ‘cardiovascular system’, ‘musculo-skeletal system’, ‘nervous system’, ‘respiratory system’ and ‘sensory organs’.
To ensure patient confidentiality, information regarding patient groups lower than 10 are generally not shown. Groups with fewer than 10 patients are only shown when they cannot be directly traced back to a person. Only descriptive statistical analyses were conducted. Since only the publicly available medical data on the total population could be used (which did not include the actual data of all individuals in the Netherlands), it was not possible to utilize inferential statistics to examine possible differences between patients with an LRP5, PLS3, or WNT1 variant and the total population. Statistical analyses were performed by authors SJEV and SS using IBM SPSS Statistics for Windows version 25 (IBM corporation, Armonk, NY, USA).
Ethical Consideration
The Medical Ethics Review Committee (MERC) of the Amsterdam UMC (Amsterdam, The Netherlands) waived the need for ethics approval and the need to acquire consent for the analysis and publishing of the retrospectively obtained and anonymized data for this non-intervention study (MERC study number 2021.0085).
Results
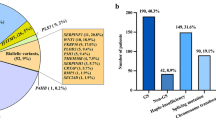
The Amsterdam UMC Genome Database comprised 92 individuals genetically identified with an LRP5, PLS3, or WNT1 pathogenic or suspicious rare variant. Figure 1 shows the variant types and the age distribution as of January 2019. Of the 92 patients, 90 (97.8%) were matched with the CBS cohort. 52 patients had an LRP5 variant, 15 had a PLS3 variant, and 23 had a WNT1 variant. In 2019, the median age and 10th and 90th percentiles were 40.2 years [13.0–68.4 years] for the entire group, 37.5 years [13.4–69.8 years] for patients with an LRP5 variant, 47.0 years [10.7–63.6 years] for patients with a PLS3 variant, and 45.5 years [20.2–75.07 years] for those with a WNT1 variant. The median age at the time of genetic testing was 40.4 years [11.5–69.4 years].
Between 2013 and 2019, a total of 200 admissions took place, including both day-case and inpatient admissions. Patients with an LRP5, PLS3, or WNT1 variant averaged 0.32 hospitalizations per person per year (median 0.14, SD ± 0.45). Table 1 presents the mean yearly admission rates for various age groups and the IRRs comparing patients with an LRP5, PLS3, or WNT1 variant to the Dutch total population and the OI population. Patients with an LRP5, PLS3, or WNT1 variant had, on average, 1.63 times more admissions than the total population and 0.62 times fewer admissions than the OI population. 40.0% (n = 36) of all patients had no admissions, 16.7% (n = 15) had one, 28.9% (n = 26) had 2 to 4, and 14.4% (n = 13) had five or more. The average length of hospital stay was 1.3 days (median 0.0; SD ± 3.1). 163 (81.5%) of the total 200 admissions were for non-acute care, while 37 (18.5%) were for acute care. There were 170 admissions to university medical centers or top clinical centers, compared to 30 admissions to general hospitals (respectively 85.0% and 15%). Of the 200 admissions, 80 concerned patients between 0 and 24 years old, 29 patients between 25 and 44 years old, 52 patients between 45 and 64 years old, and 39 patients 65 and older (respectively 40.0%, 14.5%, 26.0%, and 19.5%). Pediatrics accounted for 54 admissions (27%) while surgery and orthopedics accounted for 77 admissions (38.5%) combined.
The total number of DTCs opened for our patient cohort between 2013 and 2017 was 1002. 86.6% (n = 868) were opened in outpatient clinic settings, 7.0% (n = 70) during day-case admissions, and 5.6% (n = 56) during inpatient admissions. Table 2 presents the IRRs for the number of DTCs opened in patients with a pathogenic or a rare suspicious variant in LRP5, PLS3, or WNT1 relative to the total Dutch population. The IRRs for the number of DTCs for OI patients relative to the total Dutch population are also provided in Table 2. Patients with an LRP5, PLS3, or WNT1 variant had, on average, two times as many DTCs opened as the total Dutch population. Patients with an LRP5, PLS3, or WNT1 variant had more DTCs opened for clinical genetics (IRR: 17.00), medical revalidation (IRR 7.68), pediatrics (IRR 4.78), orthopedics (IRR 4.37), rheumatology (IRR 3.86), the audiology center (IRR 3.58), internal medicine (IRR 2.63), and surgery (IRR 2.38) than the total Dutch population. Patients with an LRP5, PLS3, or WNT1 variant had slightly more otorhinolaryngology DTCs opened than the entire Dutch population (IRR 1.85).
In 2017, the average number of prescriptions per patient was 3.8 (median 3.0; SD ± 3.6). For patients with an LRP5 variant, the mean number of drugs was 3.5 (median 2.5; SD ± 3.7); for patients with a PLS3 variant, it was 4.1 (median 4.0; SD ± 3.6); and for patients with a WNT1 variant, it was 4.1. (median 4.0; SD ± 3.4). Figure 2 displays the percentages of patients and the Dutch population as a whole who use medication, as well as for various ATC categories. For the age categories 0 to 24 years, 25 to 44 years, and 45 to 64 years, the proportion of patients with a variant in LRP5, PLS3, or WNT1 using medication was higher compared to the total Dutch population (0–24 years: 69.6% vs. 51.0%; 25–44 years: 81.3% vs. 58.6%; 45–64 years: > 88% vs. 73.3%).
The proportion of people using medication according to ATC main groups; comparing patients with a pathogenic or a rare suspicious variant in LRP5, PLS3, or WNT1 to the total Dutch population. Due to patient privacy, absolute numbers for certain categories cannot be shown; this is indicated by capped lines. For patients 0–24 years old, values ≤14% are not shown; for patients 45–64 years old, values ≥88% are not shown; for patients 65 years and older, values ≥79% are not shown. Y, years. For more information on ATC main groups the ATC/DDD index of the WHO Collaborating Centre for Drug Statistics Methodology can be consulted [27]. ATC, Anatomical Therapeutic Chemical; DDD, defined daily dose; WHO, World Health Organization.
Discussion
The aim of the current study was to investigate the use of medical care among 92 Dutch patients genetically diagnosed with a pathogenic or a rare suspicious variant in LRP5, PLS3, or WNT1 between 2014 and 2021. Their use of medical care was compared to that of the entire population and of OI patients in the Netherlands.
In our cohort, patients with variants in LRP5, PLS3, or WNT1 appeared to use more medical care compared to the total population (1.63 times more hospital admissions, 2.0 times more DTCs opened, and a larger proportion of people using medication, as shown in Fig. 2). Compared to the same age group in the total population, patients aged 0 to 24 experienced 4.81 times as many hospital admissions, and DTCs were opened 4.78 times more often for the pediatrics department. This is in agreement with the known onset of osteoporosis at childhood by LRP5, PLS3, and WNT1 pathogenic variants [32]. Patients of all ages had more surgery and orthopedics DTCs than the total population (IRR: 2.38 and 4.37, respectively). Since LRP5, PLS3, and WNT1 pathogenic variants cause osteoporosis, this was expected. This most likely also explains the relatively greater use of medication for ATC categories (‘alimentary tract and metabolism’ and ‘musculoskeletal system’) involving medications such as vitamin D, calcium, and bisphosphonates (Fig. 2).
When compared to the total population, patients with an LRP5 variant appeared to have a similar or lower number of DTCs opened for the ophthalmology department (IRR 0.67). Since patients with loss-of-function LRP5 variants may develop exudative vitreoretinopathy, a disease with a highly variability in phenotypic expression, we had anticipated that those with an LRP5 variant would have more DTCs opened for ophthalmology [14]. The results indicate that specialists are not automatically triggered to refer these patients for an ophthalmologic screening. Interestingly, patients with an LRP5, PLS3, or WNT1 variant had more audiological DTCs and slightly more otorhinolaryngology DTCs than the total population (IRR 3.58 and 1.85, respectively). This suggests that they may be at a higher risk of developing hearing issues. While it is possible that some of these DTCs could be attributed to patients being examined more often due to a suspicious diagnosis of OI, it is worth noting that routine screening for hearing issues in OI patients without hearing complaints is being proposed but is not yet commonplace as no guideline for hearing testing in OI currently exists [33]. Hearing loss, whether conductive, mixed, or sensorineural, is common in OI patients [34, 35]. Causes of hearing loss in patients with OI include footplate fixation, fractures of the ossicles, and otosclerosis. Patients with LRP5, PLS3, or WNT1 variants may have similar issues. A disruption in the Wnt signaling pathway may also directly influence the development of the inner ear [36, 37]. As hearing loss can induce social isolation and is often detected late, in patients with LRP5, PLS3, or WNT1 variants hearing screening should be considered [38]. In patients with variants in LRP5, PLS3, or WNT1, there was a slightly higher incidence of patients using respiratory system drugs (Fig. 2). Although the exact pathophysiology is not clear, patients with OI may have respiratory problems [39]. Due to skeletal thoracic problems, patients with LRP5, PLS3, or WNT1 variants may have a greater risk of respiratory complications [40]; however, drawing this conclusion solely based on medication prescription data is premature as it needs more investigation.
Despite the small sample size, patients with a variant in PLS3 or WNT1 appeared to use more medical care than patients with a variant in LRP5 (yearly admission rates: 0.40, 0.44, and 0.24, respectively; IRR for DTCs 2.22, 2.31, and 1.82, respectively). To our knowledge, no study exists comparing patients with LRP5, PLS3, or WNT1 variants, and only limited research on the characteristics of these patients is available [11,12,13, 18, 32, 41]. Despite the inclusion of heterozygote PLS3 females, the apparently increased need for hospital care reported in PLS3 patients appears to indicate that in this cohort, males with a PLS3 variant have a more severe phenotype than patients with an LRP5 variant. Additional information on the comorbidities of individuals with LRP5, PLS3, or WNT1 pathogenic variants may aid in providing more information on the prognosis and, as a result, clinical management advice.
Patients with an LRP5, PLS3, or WNT1 variant used medical care less frequently than OI patients (0.61 times fewer admissions). Relative to those with genetic variants that cause mild forms of OI, which our group has previously reported on, the admission rates appear similar [25]. Because the LRP5, PLS3, or WNT1 patients in this study included heterozygous PLS3 females, it is possible that the amount of hospital care used by hemizygous PLS3 males is greater than what is reported. Experts are divided on whether osteoporotic pathogenic variants in the LRP5, PLS3, or WNT1 genes should be classified as OI [2, 12]. Patients with OI present syndromic symptoms in addition to brittle bones, which are not present in osteoporotic patients. Nevertheless, apart from a potentially greater frequency of hearing-related disorders, the hospital data evaluated in our analyses revealed no other relevant syndromic traits.
This study cannot avoid a few limitations. Firstly, due to low patient numbers, all pathogenic and rare suspicious variants in LRP5, PLS3, or WNT1 have been grouped. Subdividing into smaller groups was not possible as it could not ensure patient confidentiality. Although the medical care data used is quite elaborate, we did not possess any additional deep phenotyping data that could be linked to the medical care data. As a result, this limited our investigation to the presence (or absence) of extraskeletal characteristics. Due to the fact that pathogenic variants of LRP5, PLS3, or WNT1 were not included in the routine genetic testing of our center until 2014, shortly after the discovery of these genes, it was not possible to estimate the prevalence of these variants in the Netherlands.
In conclusion, it appears that patients with a pathogenic or rare suspicious mutation in LRP5, PLS3, or WNT1 use more medical care than the general population. This is the first study to present medical care data for a large cohort of individuals with an LRP5, PLS3, or WNT1 variant. As anticipated, patients with an LRP5, PLS3, or WNT1 variant were more likely to require surgical and orthopedic hospital treatment. Additionally, treatment by audiological centers and otorhinolaryngology departments was increased. This may be indicative of a higher prevalence of hearing-related issues; hearing tests should thus be considered as part of the clinical management. Further research is needed to determine whether people with LRP5, PLS3, or WNT1 variants have similar medical care requirements as those with OI.
Data Availability
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding author.
Abbreviations
- ATC:
-
Anatomical Therapeutic Chemical
- BMD:
-
Bone mineral density
- CBS:
-
Statistics Netherlands
- DTC:
-
Diagnosis treatment combination
- ENT:
-
Ear, nose, throat
- IRR:
-
Incidence rate ratio
- LRP5:
-
Low-density lipoprotein receptor-related protein 5
- MERC:
-
Medical Ethics Review Committee
- MIM:
-
Mendelian Inheritance in Man
- OI:
-
Osteogenesis imperfect
- PLS3:
-
Plastin 3
- VUS:
-
Variant of uncertain significance
- WNT1:
-
Proto-oncogene Wnt-1
References
Salari N et al (2021) The global prevalence of osteoporosis in the world: a comprehensive systematic review and meta-analysis. J Orthop Surg Res. https://doi.org/10.1186/s13018-021-02772-0
Mäkitie O, Zillikens MC (2021) Early-onset osteoporosis. Calcif Tissue Int 110(5):546–561. https://doi.org/10.1007/s00223-021-00885-6
Koromani F, Trajanoska K, Rivadeneira F, Oei L (2019) Recent advances in the genetics of fractures in osteoporosis. Front Endocrinol (Lausanne) 10:1–10. https://doi.org/10.3389/fendo.2019.00337
Collet C et al (2018) Primary osteoporosis in young adults: genetic basis and identification of novel variants in causal genes. JBMR Plus 2(1):12–21. https://doi.org/10.1002/jbm4.10020
Ghatan S et al (2021) The polygenic and monogenic basis of paediatric fractures. Curr Osteoporos Rep 19(5):481–493. https://doi.org/10.1007/s11914-021-00680-0
Mäkitie RE, Costantini A, Kämpe A, Alm JJ, Mäkitie O (2019) New insights into monogenic causes of osteoporosis. Front Endocrinol (Lausanne) 10:1–15. https://doi.org/10.3389/fendo.2019.00070
Van Dijk FS, Sillence DO (2014) Osteogenesis imperfecta: clinical diagnosis, nomenclature and severity assessment. Am J Med Genet Part A 164(6):1470–1481. https://doi.org/10.1002/ajmg.a.36545
Lim J, Grafe I, Alexander S, Lee B (2017) Genetic causes and mechanisms of osteogenesis imperfecta. Bone 102:40–49. https://doi.org/10.1016/j.bone.2017.02.004
Boudin E, Van Hul W (2017) Genetics of human bone formation. Eur J Endocrinol 177(2):R69–R83. https://doi.org/10.1530/EJE-16-0990
Neugebauer J et al (2018) Plastin 3 influences bone homeostasis through regulation of osteoclast activity. Hum Mol Genet 27(24):4249–4262. https://doi.org/10.1093/hmg/ddy318
Pyott SM et al (2013) WNT1 mutations in families affected by moderately severe and progressive recessive osteogenesis imperfecta. Am J Hum Genet 92(4):590–597. https://doi.org/10.1016/j.ajhg.2013.02.009
Korvala J et al (2012) Mutations in LRP5 cause primary osteoporosis without features of OI by reducing Wnt signaling activity. BMC Med Genet 13(1):26. https://doi.org/10.1186/1471-2350-13-26
Gong Y et al (2001) LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell 107(4):513–523
Toomes C et al (2004) Mutations in LRP5 or FZD4 underlie the common familial exudative vitreoretinopathy locus on chromosome 11q. Am J Hum Genet 74(4):721–730. https://doi.org/10.1086/383202
Tang M et al (2017) Mutation spectrum of the LRP5, NDP, and TSPAN12 genes in Chinese patients with familial exudative vitreoretinopathy. Investig Ophthalmol Vis Sci 58(13):5949–5957. https://doi.org/10.1167/iovs.17-22577
Little RD et al (2002) A mutation in the LDL receptor-related protein 5 gene results in the autosomal dominant high-bone-mass trait. Am J Hum Genet 70(1):11–19. https://doi.org/10.1086/338450
Laine CM et al (2015) A novel splice mutation in PLS3 causes X-linked early onset low-turnover osteoporosis. J Bone Miner Res 30(3):437–445. https://doi.org/10.1002/jbmr.2355
van Dijk FS et al (2013) PLS3 mutations in X-linked osteoporosis with fractures. N Engl J Med 369(16):1529–1536. https://doi.org/10.1056/nejmoa1308223
Fahiminiya S et al (2014) Osteoporosis caused by mutations in PLS3: clinical and bone tissue characteristics. J Bone Miner Res 29(8):1805–1814. https://doi.org/10.1002/jbmr.2208
Hu J et al (2020) A novel mutation in PLS3 causes extremely rare X-linked osteogenesis imperfecta. Mol Genet Genomic Med 8(12):1–12. https://doi.org/10.1002/mgg3.1525
Costantini A et al (2022) Early-onset osteoporosis: rare monogenic forms elucidate the complexity of disease pathogenesis beyond type I collagen. J Bone Miner Res 37(9):1623–1641. https://doi.org/10.1002/jbmr.4668
Storoni S et al (2022) Prevalence and hospital admissions in patients with osteogenesis imperfecta in The Netherlands: a nationwide registry study. Front Endocrinol (Lausanne) 13:1–7. https://doi.org/10.3389/fendo.2022.869604
Morales A, Hershberger RE (2018) Variants of Uncertain significance: should we revisit how they are evaluated and disclosed? Circ Genom Precis Med 11:e002169. https://doi.org/10.1161/CIRCGEN.118.002169
van der Crabben SN et al (2022) Should variants of unknown significance (VUS) be disclosed to patients in cardiogenetics or not; only in case of high suspicion of pathogenicity? Eur J Hum Genet 30(11):1208–1210. https://doi.org/10.1038/s41431-022-01173-z
Storoni S et al (2023) From genetics to clinical implications : a study of 675 dutch osteogenesis imperfecta patients. Biomolecules 13(2):281
K. Folmer and E. Mot, “Diagnosis and treatment combinations in Dutch hospitals,” no. November, 2000
N. I. of P. Health, “ATC/DDD Index 2022,” WHO Collaborating Centre for Drug Statistics Methodology. Available: https://www.whocc.no/atc_ddd_index/. Accessed 25 Nov 2022
Centraal Bureau voor Statistiek, “Regionale kerncijfers Nederland.” Available: https://opendata.cbs.nl/statline/#/CBS/nl/dataset/70072ned/table?ts=1666173173774. Accessed 23 Nov 2022
Centraal Bureau voor Statistiek, “Ziekenhuisopnamen en -patiënten; diagnose-indeling ISHMT.” Available: https://opendata.cbs.nl/statline/#/CBS/nl/dataset/84068NED/table?ts=1665574711598. Accessed 23 Nov 2022
Centraal Bureau voor Statistiek, “Medisch Specialistische Zorg; DBC’s naar diagnose, zorgkenmerken.” Available: https://opendata.cbs.nl/statline/#/CBS/nl/dataset/82471NED/table?ts=1669976067576. Accessed 23 Nov 2022
Centraal Bureau voor Statistiek, “Personen met verstrekte geneesmiddelen; leeftijd en geslacht.” Available: https://opendata.cbs.nl/statline/#/CBS/nl/dataset/81071ned/table?ts=1669976019439. Accessed 23 Nov 2022
Kämpe AJ, Mäkitie RE, Mäkitie O (2015) New genetic forms of childhood-onset primary osteoporosis. Horm Res Paediatr 84(6):361–369. https://doi.org/10.1159/000439566
Goderie TPM et al (2023) The international standard set of outcome measures for the assessment of hearing in people with osteogenesis imperfecta. Otol Neurotol. https://doi.org/10.1097/MAO.0000000000003921
Pillion JP, Vernick D, Shapiro J (2011) Hearing loss in osteogenesis imperfecta: characteristics and treatment considerations. Genet Res Int 2011(1):1–6. https://doi.org/10.4061/2011/983942
Carré F, Achard S, Rouillon I, Parodi M, Loundon N (2019) Hearing impairment and osteogenesis imperfecta: literature review. Eur Ann Otorhinolaryngol Head Neck Dis 136(5):379–383. https://doi.org/10.1016/j.anorl.2019.05.004
Jansson L, Kim GS, Cheng AG (2015) Making sense of Wnt signaling—linking hair cell regeneration to development. Front Cell Neurosci 9:1–12. https://doi.org/10.3389/fncel.2015.00066
Xia W et al (2017) New role of LRP5, associated with nonsyndromic autosomal-recessive hereditary hearing loss. Hum Mutat 38(10):1421–1431. https://doi.org/10.1002/humu.23285
Shukla A et al (2020) Hearing Loss, loneliness, and social isolation: a systematic review. Otolaryngol: Head Neck Surg (United States) 162(5):622–633. https://doi.org/10.1177/0194599820910377
Bronheim R, Khan S, Carter E, Sandhaus RA, Raggio C (2019) Scoliosis and cardiopulmonary outcomes in osteogenesis imperfecta patients. Spine (Phila. Pa. 1976) 44:1057–1063. https://doi.org/10.1097/BRS.0000000000003012
Pehrsson K, Bake B, Larsson S, Nachemson A (1991) Lung function in adult idiopathic scoliosis: a 20 year follow up. Thorax 46(7):474–478. https://doi.org/10.1136/thx.46.7.474
Keupp K et al (2013) Mutations in WNT1 cause different forms of bone fragility. Am J Hum Genet 92(4):565–574. https://doi.org/10.1016/j.ajhg.2013.02.010
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of Statistics Netherlands (CBS).
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
Conceptualization, SV, SS, LZ, GP, EE and DM; Data curation, LZ, GP, AM and DM; Formal analysis, SV and SS; and Methodology, SV, SS, LZ and GP; Supervision, EE and DM; Validation, SS, GP, EE and DM; Writing—original draft, SV; Writing—review & editing, SV, SS, LZ, WZ, GP, BR, ME, AM, EE and DM. All authors listed have made a substantial, direct, and intellectual contribution to the work and have approved it for publication. All authors have read and agreed to the published version of the manuscript. Several authors of this publication are members of the European Reference Network for rare BONeDiseases - project ID No 101085766.
Corresponding author
Ethics declarations
Conflict of interest
S.J.E. Verdonk, S. Storoni, L. Zhytnik, W. Zhong, G. Pals, B.J. van Royen, M.W. Elting, A. Maugeri, E.M.W. Eekhoff and D. Micha declare that they have no conflict of interest.
Ethical Approval
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of The Medical Ethics Review Committee (MERC) of the Amsterdam UMC (Amsterdam, The Netherlands) (MERC study number 2021.0085). Patient consent was waived due to the retrospectively obtained and anonymized data for a non-intervention study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Verdonk, S.J.E., Storoni, S., Zhytnik, L. et al. Medical Care Use Among Patients with Monogenic Osteoporosis Due to Rare Variants in LRP5, PLS3, or WNT1. Calcif Tissue Int 113, 186–194 (2023). https://doi.org/10.1007/s00223-023-01101-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-023-01101-3