Abstract
Observational studies suggest a link between depression and osteoporosis, but these may be subject to confounding and reverse causality. In this two-sample Mendelian randomization analysis, we included the large meta-analysis of genome-wide association studies for depression among 807,553 individuals (246,363 cases and 561,190 controls) of European descent, the large meta-analysis to identify genetic variants associated with femoral neck bone mineral density (FN-BMD), forearm BMD (FA-BMD) and lumbar spine BMD (LS-BMD) among 53,236 individuals of European ancestry, and the GWAS summary data of heel BMD (HE-BMD) and fracture among 426,824 individuals of European ancestry. The results revealed that genetic predisposition towards depression showed no causal effect on FA-BMD (beta-estimate: 0.091, 95% confidence interval [CI] − 0.088 to 0.269, SE:0.091, P value = 0.320), FN-BMD (beta-estimate: 0.066, 95% CI − 0.016 to 0.148, SE:0.042, P value = 0.113), LS-BMD (beta-estimate: 0.074, 95% CI − 0.029 to 0.177, SE:0.052, P value = 0.159), HE-BMD (beta-estimate: 0.009, 95% CI − 0.043 to 0.061, SE:0.027, P value = 0.727), or fracture (beta-estimate: 0.008, 95% CI − 0.071 to 0.087, SE:0.041, P value = 0.844). These results were also confirmed by multiple sensitivity analyses. Contrary to the findings of observational studies, our results do not reveal a causal role of depression in osteoporosis or fracture.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The United Nations have predicted that the ratio of people aged more than 65 years to those aged 15–64 years will triple globally by 2100 [1]. Disordered musculoskeletal conditions may result in severe pain and physical disability, and their prevalence will increase as the ageing of society [2]. Among the diseases associated with musculoskeletal conditions, osteoporosis is a common, aging-related disease characterized by decreased bone mineral density (BMD) and increased risk of fracture [3,4,5,6]. The treatment of osteoporosis is still a big challenge and growing public health problem in the world [7,8,9]. Genome-wide association studies (GWASs) has demonstrated that BMD is a highly polygenic trait [10,11,12].
Depression is the leading cause of disability and one in six people is estimated to develop depression during their lifetime [13]. Depression is a chronic disease that affects 18% of men and 26% of women [14]. Several meta-analyses included cross-sectional or case–control studies to investigate the association of depression and osteoporosis, and found that depression might be a significant risk factor for low BMD and fracture, but the results were not consistent [15,16,17,18]. However, none of these studies assessed the their association in the prospective cohort design, and these studies were limited by confounding factors and reverse causality.
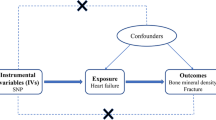
Genetic epidemiology has emerged as an important approach to unravel the determinants of diseases, because the inheritance of genetic variants at conception is random and cannot be confounded by other risk factors. Mendelian randomization (MR) study has become an effective, powerful and efficient method to establish the causal relationships between exposure phenotype and exposure phenotype through using the GWAS summary statistics [19, 20]. These genetic variants in GWAS summary statistics are randomly allocated before birth and fixed at conception, and thus serve as unconfounded proxies for modifiable risk factors, which affords an analogy to randomized controlled trials (RCTs) in a non-experimental (observational) setting [21, 22].
Two-sample MR analysis greatly increases the scope and statistical power of MR using the published summary data from GWASs [23, 24]. In this study, we use single nucleotide polymorphisms (SNPs) strongly associated with depression as instrumental variables. To our knowledge, this is the first two sample MR study to explore the causal effect of depression on forearm BMD (FA-BMD), femoral neck BMD (FN-BMD), lumbar spine BMD (LS-BMD), heel BMD (HE-BMD) and fracture.
Methods
Data on Depression
A large GWAS meta-analysis of depression involved 807,553 individuals of European ancestry (246,363 cases and 561,190 controls) from the three largest GWASs [13]. Depression was defined as a debilitating psychiatric illness that was typically associated with low mood and anhedonia. Initially, 102 independent SNPs were identified to have robust association with depression at the GWAS threshold of statistical significance (P < 5 × 10–8) after adjusting for sex and age (Supplementary Table 1). These SNPs involved both genes and gene-pathways associated with synaptic structure and neurotransmission.
In one MR study, SNPs were ideally expected to not be in linkage disequilibrium (LD), because SNPs in strong LD may produce some bias. We performed the clumping process (R2 < 0.001, window size = 10,000 kb) with the European samples from the 1000 genomes project and estimated LD between SNPs. Among the pairs of SNPs with r2 ≥ 0.001, the SNP with a larger association P value would be removed. We also excluded the SNPs that were absent from the LD reference panel. Therefore, 23 SNPs were excluded due to LD and 79 SNPs remained for the subsequent analysis. Finally, 78 SNPs for FA-BMD, 79 SNPs for FN-BMD and LS-BMD, 74 SNPs for HE-BMD and fracture were used as the instrumental variables (Table 1).
Data on BMD and Fracture
Osteoporotic fractures commonly occur in the skeletal sites including femoral neck, forearm, lumbar spine and heel [25]. One large meta-analysis reported the genetic variants associated with FN-BMD, FA-BMD and LS-BMD among 53,236 individuals of European ancestry. Each SNP was tested after adjusting for sex, age, age2 and weight [25]. In addition, the GWAS summary data for the associations with HE-BMD and fracture were obtained from 426,824 individuals of European ancestry after adjusting for age, sex and genotyping [3]. Table 1 presented the genetic associations between depression and each outcome including FN-BMD, FA-BMD, LS-BMD, HE-BMD and fracture.
Statistical Analyses
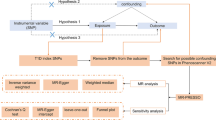
To determine MR estimates of depression for FN-BMD, FA-BMD, LS-BMD, HE-BMD and fracture, we conducted the inverse variance-weighted (IVW) meta-analysis of Wald ratio for individual SNPs. The weighted median and MR-Egger regression methods were also applied to estimate the effects. The MR method was based on the following three assumptions: (i) instrumental variables were strongly associated with depression; (ii) instrumental variables affected outcomes only through their effect on depression and not through any alternative causal pathway; and (iii) instrumental variables were independent of any confounders [26]. The strength of each instrument was measured by calculating the F-statistic using the following formula: F = R2(N−2)/(1−R2), where R2 was the proportion of the depression variability explained by each instrument and N was the sample size of the GWAS for the depression association [27].
Sensitivity Analyses
Several sensitivity analyses were used to check and correct for the presence of pleiotropy in the causal estimates. Cochran’s Q was computed to quantify heterogeneity across the individual causal effects, and the random effects IVW MR analysis was used [28, 29]. To assess the potential violation of these assumptions, we evaluated the directional pleiotropy based on the intercept obtained from the MR-Egger analysis [30]. We also assessed the presence of pleiotropy using the MR pleiotropy residual sum and outlier test (MR-PRESSO), during which outlying SNPs were excluded and the effect estimates were reassessed [31].
All tests were two-tailed, and differences with P < 0.05 were considered statistically significant. All of these analyses were conducted in R V.4.0.4 by using R packages of ‘MendelianRandomization’ [32] and ‘TwoSampleMR’ [33].
Results
Causal Effect of Depression on FA-BMD, FN-BMD and LS-BMD
We evaluated the causal effect of depression on three sites of BMD (FA-BMD, FN-BMD and LS-BMD, Table 2 and Fig. 1). In the primary IVW analyses, depression showed no MR association with FA-BMD (beta-estimate: 0.091, 95% CI − 0.088 to 0.269, SE:0.091, P value = 0.320), FN-BMD (beta-estimate: 0.066, 95% CI − 0.016 to 0.148, SE:0.042, P value = 0.113) or LS-BMD (beta-estimate: 0.074, 95% CI − 0.029 to 0.177, SE:0.052, P value = 0.159). These results were also confirmed by weighted-median analyses with regards to FA-BMD (beta-estimate: 0.144, 95% CI − 0.092 to 0.380, SE:0.120, P value = 0.231), FN-BMD (beta-estimate: 0.070, 95% CI − 0.061 to 0.180, SE:0.056, P value = 0.215) or LS-BMD (beta-estimate: 0.068, 95% CI − 0.702 to 0.197, SE:0.066, P value = 0.302).
Beta (95% CIs) for association between depression and three sites of BMD (FA-BMD, FN-BMD and LS-BMD). These effects were obtained using summary-level data from the GWASs of depression (n = 807,553) on A FA-BMD (n = 53,236), B FN-BMD (n = 53,236) and C LS-BMD (n = 53,236). Error bars represented 95% confidence intervals. All statistical tests were two-sided
Causal Effect of Depression on HE-BMD and Fracture
Depression showed null association with HE-BMD in the IVW (beta-estimate: 0.009, 95% CI − 0.043 to 0.061, SE:0.027, P value = 0.727) and weighted-median analyses (beta-estimate: − 0.001, 95% CI − 0.034 to 0.033, SE:0.017, P value = 0.976, Table 2 and Fig. 2). Consistently, there was also no relationship between depression and fracture in the results of IVW (beta-estimate: 0.008, 95% CI − 0.071 to 0.087, SE:0.041, P value = 0.844) or weighted-median analyses (beta-estimate: 0.020, 95% CI − 0.075 to 0.115, SE:0.049, P value = 0.680, Table 2 and Fig. 2).
Beta (95% CIs) for association between depression and HE-BMD/fracture. These effects were obtained using summary-level data from the GWASs of depression (n = 807,553) on A HE-BMD (n = 426,824) and B fracture (n = 426,824). Error bars represented 95% confidence intervals. All statistical tests were two-sided
Evaluation of Assumptions and Sensitivity Analyses
The strength of the genetic instruments denoted by the F-statistic was ≥ 10 for all the depression variants (Supplementary Table 1). Little evidence of directional pleiotropy was found for all models except for FA-BMD (MR-Egger intercept P value = 0.014) (Table 2). The estimates from the weighted-median approach for the SNP instrument were all consistent with those of IVW models (Table 2).
Among 79 SNP instrument variables, MR-PRESSO method identified 14 outliers (rs301799, rs10789214, rs2568958, rs1568452, rs200949, rs3823624, rs1448938, rs2509805, rs198457, rs7932640, rs1409379, rs1343605, rs12923444, and rs5995992) for HE-BMD and 2 outliers (rs1448938 and rs10789214) for fracture. After excluding these outliers, depression still revealed no causal effect on HE-BMD (beta-estimate: − 0.017, 95% CI − 0.048 to 0.014, SE:0.016, P value = 0.292) or fracture (beta-estimate: 0.007, 95% CI − 0.064 to 0.077, SE:0.036, P value = 0.855) (Table 3 and Fig. 2).
Discussion
Observational studies reported inconsistent results regarding the association between depression and osteoporosis [34,35,36,37,38]. Positive associations were supported by previous meta-analyses that suggested that depression was associated with low BMD and the increased risk of fracture [15,16,17, 39, 40]. Previous studies reported that depression may affect bone formation and bone resorption through altering the concentrations of many hormones. For instance, depression elevated the cortisol level through activating the hypothalamic–pituitary–adrenal axis, and hypercortisolemia was an important causal factor to decrease BMD [39]. The inverse regulation between depression and bone formation may be associated with gonadal hormones estrogen, testosterone and growth hormone/insulin growth factors [41, 42].
However, several limitations may result in some bias for these positive results. First, these meta-analyses only included case–control or cross-sectional studies, and it was unclear whether depression was prospectively associated with increased risk of fracture and bone loss. Second, the most of original studies employed self-report scales to define depression, which might produce some bias of misclassification. Third, some original reports lacked the data of medication use such as corticosteroid and glucocorticoid, which may affect the observed association.
This insistent association may be biased due to the methodological limitations (i.e. confounding, reverse causation and measurement error) of traditional observational study [43]. The MR study has been widely used to evaluate causal inferences between risk factors and disease outcomes using genetic variants as instrumental variables [44]. To date, our work is the first two-sample MR study to explore the causal effect of depression on BMD and fracture. Our study included the three large GWASs of depression [13], FA-BMD, FN-BMD and LS-BMD [25], HE-BMD and fracture [3]. This MR analysis revealed no causal effect of depression on four sites of BMD or fracture. We did not find the evidence of a causal link between depression and osteoporosis, which were contrast to previous observational studies. These suggested that false associations between depression and osteoporosis may be caused by confounding factors such as smoking, increased alcohol drinking and decreased physical activity [45].
Several important strengths exit in this study. This is the first two-sample MR study to investigate the causality between depression and osteoporosis, which is the closest approximation to RCT and allows the random allocation based on the genotype. This study design can prevent some limitations of conventional observational studies, including reverse causation and potential confounding factors. The large sample sizes of included studies and instrumental variables robustly associated with depression (F statistics ≥ 10) are used in our MR study. The intercepts for the MR-Egger analysis suggest that all observed causal associations are not affected by directional pleiotropy except FA-BMD. We conduct multiple sensitivity analyses to test the influence of pleiotropy on our causal estimates, and our results are robust according to these various tests.
Several limitations also should be taken into consideration. First, all the included participants are of European origin, and more studies should be conducted to confirm whether our findings are generalizable to other populations. Secondly, the broader self-declared definitions of depression are used in the GWAS meta-analysis of depression [13], although there is a strong genetic correlation between broader self-declared definitions of depression and clinically diagnosed depressive disorder [46]. Third, significant heterogeneity remains for the analysis of HE-BMD, which may be caused by different patient population and definitions of depression.
Conclusion
This two-sample MR provides strong evidence for no casual association between depression and osteoporosis, and suggests the confounding factors and reverse may cause the reported observational associations between them.
Data Availability
Data supporting the findings of this study were available within the paper.
References
Trajanoska K, Morris JA, Oei L, Zheng HF, Evans DM, Kiel DP, Ohlsson C, Richards JB, Rivadeneira F (2018) Assessment of the genetic and clinical determinants of fracture risk: genome wide association and mendelian randomisation study. BMJ. https://doi.org/10.1136/bmj.k3225
Harvey N, Dennison E, Cooper C (2010) Osteoporosis: impact on health and economics. Nat Rev Rheumatol 6(2):99–105
Morris JA, Kemp JP, Youlten SE, Laurent L, Logan JG, Chai RC, Vulpescu NA, Forgetta V, Kleinman A, Mohanty ST, Sergio CM, Quinn J, Nguyen-Yamamoto L, Luco AL, Vijay J, Simon MM, Pramatarova A, Medina-Gomez C, Trajanoska K, Ghirardello EJ, Butterfield NC, Curry KF, Leitch VD, Sparkes PC, Adoum AT, Mannan NS, Komla-Ebri DSK, Pollard AS, Dewhurst HF, Hassall TAD, Beltejar MG, Adams DJ, Vaillancourt SM, Kaptoge S, Baldock P, Cooper C, Reeve J, Ntzani EE, Evangelou E, Ohlsson C, Karasik D, Rivadeneira F, Kiel DP, Tobias JH, Gregson CL, Harvey NC, Grundberg E, Goltzman D, Adams DJ, Lelliott CJ, Hinds DA, Ackert-Bicknell CL, Hsu YH, Maurano MT, Croucher PI, Williams GR, Bassett JHD, Evans DM, Richards JB (2019) An atlas of genetic influences on osteoporosis in humans and mice. Nat Genet 51(2):258–266
Liu J, Curtis EM, Cooper C, Harvey NC (2019) State of the art in osteoporosis risk assessment and treatment. J Endocrinol Invest 42(10):1149–1164
Black DM, Geiger EJ, Eastell R, Vittinghoff E, Li BH, Ryan DS, Dell RM, Adams AL (2020) Atypical femur fracture risk versus fragility fracture prevention with bisphosphonates. N Engl J Med 383(8):743–753
Leder BZ, Mitlak B, Hu MY, Hattersley G, Bockman RS (2020) Effect of abaloparatide vs alendronate on fracture risk reduction in postmenopausal women With osteoporosis. J Clin Endocrinol Metab 105(3):938–943
Reid IR (2020) A broader strategy for osteoporosis interventions. Nat Rev Endocrinol 16(6):333–339
Khosla S, Hofbauer LC (2017) Osteoporosis treatment: recent developments and ongoing challenges. Lancet Diabetes Endocrinol 5(11):898–907
Compston JE, McClung MR, Leslie WD (2019) Osteoporosis. Lancet 393(10169):364–376
Richards JB, Zheng HF, Spector TD (2012) Genetics of osteoporosis from genome-wide association studies: advances and challenges. Nat Rev Genet 13(8):576–588
Trajanoska K, Rivadeneira F (2019) The genetic architecture of osteoporosis and fracture risk. Bone 126:2–10
Yang TL, Shen H, Liu A, Dong SS, Zhang L, Deng FY, Zhao Q, Deng HW (2020) A road map for understanding molecular and genetic determinants of osteoporosis. Nat Rev Endocrinol 16(2):91–103
Howard DM, Adams MJ, Clarke TK, Hafferty JD, Gibson J, Shirali M, Coleman JRI, Hagenaars SP, Ward J, Wigmore EM, Alloza C, Shen X, Barbu MC, Xu EY, Whalley HC, Marioni RE, Porteous DJ, Davies G, Deary IJ, Hemani G, Berger K, Teismann H, Rawal R, Arolt V, Baune BT, Dannlowski U, Domschke K, Tian C, Hinds DA, Trzaskowski M, Byrne EM, Ripke S, Smith DJ, Sullivan PF, Wray NR, Breen G, Lewis CM, McIntosh AM (2019) Genome-wide meta-analysis of depression identifies 102 independent variants and highlights the importance of the prefrontal brain regions. Nat Neurosci 22(3):343–352
Shim RS, Baltrus P, Ye J, Rust G (2011) Prevalence, treatment, and control of depressive symptoms in the United States: results from the National Health and Nutrition Examination Survey (NHANES), 2005–2008. JABFM 24(1):33–38
Schweiger JU, Schweiger U, Hüppe M, Kahl KG, Greggersen W, Fassbinder E (2016) Bone density and depressive disorder: a meta-analysis. Brain Behav 6(8):e00489
Stubbs B, Brefka S, Dallmeier D, Stubbs J, Vancampfort D, Denkinger MD (2016) Depression and reduced bone mineral density at the hip and lumbar spine: a comparative meta-analysis of studies in adults 60 years and older. Psychosom Med 78(4):492–500
Wu Q, Magnus JH, Liu J, Bencaz AF, Hentz JG (2009) Depression and low bone mineral density: a meta-analysis of epidemiologic studies. Osteoporos Int 20(8):1309–1320
Yirmiya R, Bab I (2009) Major depression is a risk factor for low bone mineral density: a meta-analysis. Biol Psychiat 66(5):423–432
Burgess S, Dudbridge F, Thompson SG (2016) Combining information on multiple instrumental variables in Mendelian randomization: comparison of allele score and summarized data methods. Stat Med 35(11):1880–1906
Dalbeth N, Topless R, Flynn T, Cadzow M, Bolland MJ, Merriman TR (2015) Mendelian randomization analysis to examine for a causal effect of urate on bone mineral density. J Bone Miner Res 30(6):985–991
Davey Smith G, Ebrahim S (2005) What can mendelian randomisation tell us about modifiable behavioural and environmental exposures? BMJ 330(7499):1076–1079
Davies NM, Holmes MV, Davey Smith G (2018) Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ. https://doi.org/10.1136/bmj.k601
Pierce BL, Burgess S (2013) Efficient design for Mendelian randomization studies: subsample and 2-sample instrumental variable estimators. Am J Epidemiol 178(7):1177–1184
Burgess S, Scott RA, Timpson NJ, Davey Smith G, Thompson SG (2015) Using published data in Mendelian randomization: a blueprint for efficient identification of causal risk factors. Eur J Epidemiol 30(7):543–552
Zheng HF, Forgetta V, Hsu YH, Estrada K, Rosello-Diez A, Leo PJ, Dahia CL, Park-Min KH, Tobias JH, Kooperberg C, Kleinman A, Styrkarsdottir U, Liu CT, Uggla C, Evans DS, Nielson CM, Walter K, Pettersson-Kymmer U, McCarthy S, Eriksson J, Kwan T, Jhamai M, Trajanoska K, Memari Y, Min J, Huang J, Danecek P, Wilmot B, Li R, Chou WC, Mokry LE, Moayyeri A, Claussnitzer M, Cheng CH, Cheung W, Medina-Gómez C, Ge B, Chen SH, Choi K, Oei L, Fraser J, Kraaij R, Hibbs MA, Gregson CL, Paquette D, Hofman A, Wibom C, Tranah GJ, Marshall M, Gardiner BB, Cremin K, Auer P, Hsu L, Ring S, Tung JY, Thorleifsson G, Enneman AW, van Schoor NM, de Groot LC, van der Velde N, Melin B, Kemp JP, Christiansen C, Sayers A, Zhou Y, Calderari S, van Rooij J, Carlson C, Peters U, Berlivet S, Dostie J, Uitterlinden AG, Williams SR, Farber C, Grinberg D, LaCroix AZ, Haessler J, Chasman DI, Giulianini F, Rose LM, Ridker PM, Eisman JA, Nguyen TV, Center JR, Nogues X, Garcia-Giralt N, Launer LL, Gudnason V, Mellström D, Vandenput L, Amin N, van Duijn CM, Karlsson MK, Ljunggren Ö, Svensson O, Hallmans G, Rousseau F, Giroux S, Bussière J, Arp PP, Koromani F, Prince RL, Lewis JR, Langdahl BL, Hermann AP, Jensen JE, Kaptoge S, Khaw KT, Reeve J, Formosa MM, Xuereb-Anastasi A, Åkesson K, McGuigan FE, Garg G, Olmos JM, Zarrabeitia MT, Riancho JA, Ralston SH, Alonso N, Jiang X, Goltzman D, Pastinen T, Grundberg E, Gauguier D, Orwoll ES, Karasik D, Davey-Smith G, Smith AV, Siggeirsdottir K, Harris TB, Zillikens MC, van Meurs JB, Thorsteinsdottir U, Maurano MT, Timpson NJ, Soranzo N, Durbin R, Wilson SG, Ntzani EE, Brown MA, Stefansson K, Hinds DA, Spector T, Cupples LA, Ohlsson C, Greenwood CM, Jackson RD, Rowe DW, Loomis CA, Evans DM, Ackert-Bicknell CL, Joyner AL, Duncan EL, Kiel DP, Rivadeneira F, Richards JB (2015) Whole-genome sequencing identifies EN1 as a determinant of bone density and fracture. Nature 526(7571):112–117
Boef AG, Dekkers OM, le Cessie S (2015) Mendelian randomization studies: a review of the approaches used and the quality of reporting. Int J Epidemiol 44(2):496–511
Burgess S, Thompson SG (2011) Avoiding bias from weak instruments in Mendelian randomization studies. Int J Epidemiol 40(3):755–764
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327(7414):557–560
Bowden J, Del Greco MF, Minelli C, Davey Smith G, Sheehan N, Thompson J (2017) A framework for the investigation of pleiotropy in two-sample summary data Mendelian randomization. Stat Med 36(11):1783–1802
Burgess S, Thompson SG (2017) Interpreting findings from Mendelian randomization using the MR-Egger method. Eur J Epidemiol 32(5):377–389
Verbanck M, Chen CY, Neale B, Do R (2018) Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet 50(5):693–698
Yavorska OO, Burgess S (2017) MendelianRandomization: an R package for performing Mendelian randomization analyses using summarized data. Int J Epidemiol 46(6):1734–1739
Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, Laurin C, Burgess S, Bowden J, Langdon R, Tan VY, Yarmolinsky J, Shihab HA, Timpson NJ, Evans DM, Relton C, Martin RM, Davey Smith G, Gaunt TR, Haycock PC (2018) The MR-Base platform supports systematic causal inference across the human phenome. Elife. https://doi.org/10.7554/eLife.34408
Milliken LA, Wilhelmy J, Martin CJ, Finkenthal N, Cussler E, Metcalfe L, Guido TA, Going SB, Lohman TG (2006) Depressive symptoms and changes in body weight exert independent and site-specific effects on bone in postmenopausal women exercising for 1 year. J Gerontol Series A Biol Sci Med Sci 61(5):488–494
Diem SJ, Blackwell TL, Stone KL, Yaffe K, Haney EM, Bliziotes MM, Ensrud KE (2007) Use of antidepressants and rates of hip bone loss in older women: the study of osteoporotic fractures. Arch Intern Med 167(12):1240–1245
Cheng BH, Chen PC, Yang YH, Lee CP, Huang KE, Chen VC (2016) Effects of depression and antidepressant medications on hip fracture: a population-based cohort study in Taiwan. Medicine 95(36):e4655
Williams LJ, Pasco JA, Jackson H, Kiropoulos L, Stuart AL, Jacka FN, Berk M (2016) Depression as a risk factor for fracture in women: a 10 year longitudinal study. J Affect Disord 192:34–40
Rauma PH, Honkanen RJ, Williams LJ, Tuppurainen MT, Kröger HP, Koivumaa-Honkanen H (2016) Effects of antidepressants on postmenopausal bone loss—a 5-year longitudinal study from the OSTPRE cohort. Bone 89:25–31
Wu Q, Liu B, Tonmoy S (2018) Depression and risk of fracture and bone loss: an updated meta-analysis of prospective studies. Osteoporos Int 29(6):1303–1312
Liu Y, Wang Z, Xiao W (2018) Risk factors for mortality in elderly patients with hip fractures: a meta-analysis of 18 studies. Aging Clin Exp Res 30(4):323–330
Khosla S, Melton LJ 3rd, Atkinson EJ, O’Fallon WM, Klee GG, Riggs BL (1998) Relationship of serum sex steroid levels and bone turnover markers with bone mineral density in men and women: a key role for bioavailable estrogen. J Clin Endocrinol Metab 83(7):2266–2274
Gkiatas I, Lykissas M, Kostas-Agnantis I, Korompilias A, Batistatou A, Beris A (2015) Factors affecting bone growth. Am J Orthop 44(2):61–67
Boyko EJ (2013) Observational research–opportunities and limitations. J Diabetes Complicat 27(6):642–648
Smith GD, Ebrahim S (2003) ‘Mendelian randomization’: can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol 32(1):1–22
Alghadir AH, Gabr SA, Al-Eisa E (2015) Physical activity and lifestyle effects on bone mineral density among young adults: sociodemographic and biochemical analysis. J Phys Ther Sci 27(7):2261–2270
Howard DM, Adams MJ, Shirali M, Clarke TK, Marioni RE, Davies G, Coleman JRI, Alloza C, Shen X, Barbu MC, Wigmore EM, Gibson J, Hagenaars SP, Lewis CM, Ward J, Smith DJ, Sullivan PF, Haley CS, Breen G, Deary IJ, McIntosh AM (2018) Genome-wide association study of depression phenotypes in UK Biobank identifies variants in excitatory synaptic pathways. Nat Commun 9(1):1470
Acknowledgements
The authors acknowledged the GEnetic Factors for OSteoporosis Consortium and the UK Biobank for contributing the data used in this work. We thank all the genetics consortiums for making the GWAS summary data publicly available.
Funding
This study was funded by Natural Science Foundation of Chongqing (cstc2019jcyj-msxmX0836), National Natural Science Foundation of China (81701382), Foundation of The First Affiliated Hospital of Chongqing Medical University (PYJJ2018-13) and Chongqing Yuzhong Nature Science Foundation of China (Grant No. 2018114).
Author information
Authors and Affiliations
Contributions
BH, QL, LFY and MZZ conducted study design, data collection, statistical analysis. BH, QL, ZXQ and YSO conducted data interpretation, manuscript preparation, literature search. BH and QL conducted funds collection.
Corresponding author
Ethics declarations
Conflict of interest
Bin He, Qiong Lyu, Lifeng Yin, Muzi Zhang, Zhengxue Quan, Yunsheng Ou declared that they had no conflicts of interest.
Ethical Approval
The ethical approval for each study included in the investigation can be found in the original publications (including informed consent from each participant).
Human and Animal Rights and Informed Consent
All participants of the original studies included in the GWASs have provided the informed consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
He, B., Lyu, Q., Yin, L. et al. Depression and Osteoporosis: A Mendelian Randomization Study. Calcif Tissue Int 109, 675–684 (2021). https://doi.org/10.1007/s00223-021-00886-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-021-00886-5