Abstract
Objective
Several different animal models are used to study disuse-induced bone loss. This systematic review aims to give a comprehensive overview of the animal models of disuse-induced bone loss and provide a detailed narrative synthesis of each unique animal model.
Methods
PubMed and Embase were systematically searched for animal models of disuse from inception to November 30, 2019. In addition, Google Scholar and personal file archives were searched for relevant publications not indexed in PubMed or Embase. Two reviewers independently reviewed titles and abstracts for full-text inclusion. Data were extracted using a predefined extraction scheme to ensure standardization.
Results
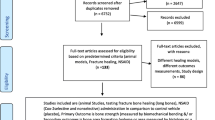
1964 titles and abstracts were screened of which 653 full-text articles were included. The most common animal species used to model disuse were rats (59%) and mice (30%). Males (53%) where used in the majority of the studies and genetically modified animals accounted for 7%. Twelve different methods to induce disuse were identified. The most frequently used methods were hindlimb unloading (44%), neurectomy (15%), bandages and orthoses (15%), and botulinum toxin (9%). The median time of disuse was 21 days (quartiles: 14 days, 36 days) and the median number of animals per group subjected to disuse was 10 (quartiles: 7, 14). Random group allocation was reported in 43% of the studies. Fewer than 5% of the studies justified the number of animals per group by a sample size calculation to ensure adequate statistical power.
Conclusion
Multiple animal models of disuse-induced bone loss exist, and several species of animals have successfully been studied. The complexity of disuse-induced bone loss warrants rigid research study designs. This systematic review emphasized the need for standardization of animal disuse research and reporting.



Similar content being viewed by others
References
Betts DC, Müller R (2014) Mechanical regulation of bone regeneration: Theories, models, and experiments. Front Endocrinol (Lausanne) 5:211
Brent MB, Brüel A, Thomsen JS (2018) PTH (1–34) and growth hormone in prevention of disuse osteopenia and sarcopenia in rats. Bone 110:244–253. https://doi.org/10.1016/j.bone.2018.02.017
Hart NH, Nimphius S, Rantalainen T et al (2017) Mechanical basis of bone strength: Influence of bone material, bone structure and muscle action. J Musculoskelet Neuronal Interact 17:114–139
Fitzpatrick LA (2002) Secondary causes of osteoporosis. Mayo Clin Proc 77:453–468
Thomsen JS, Morukov BV, Vico L et al (2005) Cancellous bone structure of iliac crest biopsies following 370 days of head-down bed rest. Aviat Sp Environ Med 76:915–922
Iolascon G, Paoletta M, Liguori S et al (2019) Neuromuscular diseases and bone. Front Endocrinol (Lausanne) 10:794. https://doi.org/10.3389/fendo.2019.00794
Ceroni D, Martin X, Delhumeau C et al (2012) Effects of cast-mediated immobilization on bone mineral mass at various sites in adolescents with lower-extremity fracture. J Bone Jt Surg Ser A 94:208–216. https://doi.org/10.2106/JBJS.K.00420
Stavnichuk M, Mikolajewicz N, Corlett T et al (2020) A systematic review and meta-analysis of bone loss in space travelers. NPJ Microgravity 6:1–9. https://doi.org/10.1038/s41526-020-0103-2
Nasse H (1880) Ueber den Einfluss der Nervendurchschneidung auf die Ernährung, insbesondere auf die Form und die Zusammensetzung der Knochen. Arch für die gesamte Physiol des Menschen und der Tiere 23:361–405
Pottarf JL, Pottorf JL (1916) An experimental study of the bone growth in the dog. Anat Rec 10:234–235
Howell JA (1917) An experimental study of the effect of stress and strain on bone development. Anat Rec 13:233–252
Allison N, Brooks B (1921) Bone atrophy. An experminetal and clinical study of the changes in bone which result from non-use. Surg Gynecol Obstet 33:250–260
Morey ER (1979) Spaceflight and bone turnover: correlation with a new rat model of weightlessness. Bioscience 29:168–172. https://doi.org/10.2307/1307797
Morey-Holton ER, Globus RK (2002) Hindlimb unloading rodent model: technical aspects. J Appl Physiol 92:1367–1377
Chappard D, Chennebault A, Moreau M et al (2001) Texture analysis of X-ray radiographs is a more reliable descriptor of bone loss than mineral content in a rat model of localized disuse induced by the Clostridium botulinum toxin. Bone 28:72–79
Wood MW, Hart LA (2007) Selecting appropriate animal models and strains: Making the best use of research, information and outreach
Moher D, Liberati A, Tetzlaff J et al (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. https://doi.org/10.1371/journal.pmed.1000097
Brent MB, Brüel A, Thomsen JS (2020) Animal models of disuse-induced bone loss: study protocol for a systematic review. Syst Rev 9:185. https://doi.org/10.1186/s13643-020-01441-3
Hooijmans CR, Tillema A, Leenaars M, Ritskes-Hoitinga M (2010) Enhancing search efficiency by means of a search filter for finding all studies on animal experimentation in PubMed. Lab Anim 44:170–175. https://doi.org/10.1258/la.2010.009117
de Vries RBM, Hooijmans CR, Tillema A et al (2011) A search filter for increasing the retrieval of animal studies in Embase. Lab Anim 45:268–270. https://doi.org/10.1258/la.2011.011056
Wallace BC, Small K, Brodley CE, et al (2012) Deploying an interactive machine learning system in an Evidence-based Practice Center: Abstrackr. In: IHI’12—Proceedings of the 2nd ACM SIGHIT International Health Informatics Symposium. ACM Press, New York. pp 819–823
Mcugh ML (2012) Interrater reliability: The kappa statistic. Biochem Med 22:276–282. https://doi.org/10.11613/bm.2012.031
Saiki H, Nakaya M, Sugita Y, Kamachi M (1976) Metabolic and hormonal mechanisms of mineral metabolic adaptation to induced hypokinetics in rats. Aviat Sp Environ Med 47:846–852
Musacchia XJ, Deavers DR, Meininger GA, Davis TP (1980) A model for hypokinesia: effects on muscle atrophy in the rat. J Appl Physiol Respir Environ Exerc Physiol 48:479–486. https://doi.org/10.1152/jappl.1980.48.3.479
Globus RK, Bikle DD, Morey-Holton E (1986) The temporal response of bone to unloading. Endocrinology 118:733–742. https://doi.org/10.1210/endo-118-2-733
Wronski TJ, Morey-Holton ER (1987) Skeletal response to simulated weightlessness: a comparison of suspension techniques. Aviat Sp Environ Med 58:63–68
Fiorentino S, Melillo G, Fedele G et al (1996) Ketoprofen lysine salt inhibits disuse-induced osteopenia in a new non-traumatic immobilization model in the rat. Pharmacol Res 33:277–281. https://doi.org/10.1006/phrs.1996.0039
Ferreira JA, Crissey JM, Brown M (2011) An alternant method to the traditional NASA hindlimb unloading model in mice. J Vis Exp. https://doi.org/10.3791/2467
Wagner EB, Granzella NP, Saito H et al (2010) Partial weight suspension: a novel murine model for investigating adaptation to reduced musculoskeletal loading. J Appl Physiol 109:350–357. https://doi.org/10.1152/japplphysiol.00014.2009
Mortreux M, Nagy JA, Ko FC et al (2018) A novel partial gravity ground-based analog for rats via quadrupedal unloading. J Appl Physiol 125:175–182. https://doi.org/10.1152/japplphysiol.01083.2017
Bogren LK, Johnston EL, Barati Z et al (2016) The effects of hibernation and forced disuse (neurectomy) on bone properties in arctic ground squirrels. Physiol Rep. https://doi.org/10.14814/phy2.12771
Murakami H, Nakamura T, Tsurukami H et al (1994) Effects of tiludronate on bone mass, structure, and turnover at the epiphyseal, primary, and secondary spongiosa in the proximal tibia of growing rats after sciatic neurectomy. J Bone Miner Res 9:1355–1364. https://doi.org/10.1002/jbmr.5650090906
Piet J, Hu D, Baron R, Shefelbine SJ (2019) Bone adaptation compensates resorption when sciatic neurectomy is followed by low magnitude induced loading. Bone 120:487–494. https://doi.org/10.1016/j.bone.2018.12.017
Cravens EM, Kirkwood JS, Wolfe LM et al (2020) The effects of neurectomy and hibernation on bone properties and the endocannabinoid system in marmots (Marmota flaviventris). Comp Biochem Physiol Part A Mol Integr Physiol. https://doi.org/10.1016/j.cbpa.2019.110621
Sugiyama H (1980) Clostridium botulinum neurotoxin. Microbiol Rev 44:419–448
Vegger JB, Brüel A, Brent MB, Thomsen JS (2018) Disuse osteopenia induced by botulinum toxin is similar in skeletally mature young and aged female C57BL/6J mice. J Bone Miner Metab 36:170–179. https://doi.org/10.1007/s00774-017-0830-y
Rauch F, Hamdy R (2006) Effect of a single botulinum toxin injection on bone development in growing rabbits. J Musculoskelet Neuronal Interact 6:264–268
Brent MBMB, Lodberg A, Thomsen JS, Brüel A (2020) Rodent model of disuse-induced bone loss by hind limb injection with botulinum toxin A. MethodsX 7:101079. https://doi.org/10.1016/j.mex.2020.101079
Lindgren JU (1976) Studies of the calcium accretion rate of bone during immobilization in intact and thyroparathyroidectomized adult rats. Calcif Tissue Res 22:41–47. https://doi.org/10.1007/BF02010345
Lindgren JU (1976) The effect of thyroparathyroidectomy development of disuse osteoporosis in adult rats. Clin Orthop Relat Res 188:251–256
Grynpas MD, Kasra M, Renlund R, Pritzker KPH (1995) The effect of pamidronate in a new model of immobilization in the dog. Bone 17:S225–S232. https://doi.org/10.1016/8756-3282(95)00296-P
Michelsson JE, Videman T, Langenskiöld A (1977) Changes in bone formation during immobilization and development of experimental osteoarthritis: a study using oxytetracycline in rabbits. Acta Orthop 48:443–449. https://doi.org/10.3109/17453677708989728
Li CY, Majeska RJ, Laudier DM et al (2005) High-dose risedronate treatment partially preserves cancellous bone mass and microarchitecture during long-term disuse. Bone 37:287–295. https://doi.org/10.1016/j.bone.2005.04.041
Damrongrungruang T, Kuroda S, Kondo H et al (2004) A simple murine model for immobilization osteopenia. Clin Orthop Relat Res. https://doi.org/10.1097/00003086-200408000-00035
Orsatti MB, Fucci LL, Valenti JL, Puche RC (1976) Effect of bicarbonate feeding on immobilization osteoporosis in the rat. Calcif Tissue Res 21:195–205. https://doi.org/10.1007/BF02547396
Verhas M, Martinello Y, Mone M et al (1980) Demineralization and pathological physiology of the skeleton in paraplegic rats. Calcif Tissue Int 30:83–90. https://doi.org/10.1007/BF02408611
Schoutens A, Verhas M, Dourov N et al (1988) Bone loss and bone blood flow in paraplegic rats treated with calcitonin, diphosphonate, and indomethacin. Calcif Tissue Int 42:136–143. https://doi.org/10.1007/BF02556346
Okumura H, Yamamuro T, Kasai R et al (1987) Effect of 1α-hydroxyvitamin D3 on osteoporosis induced by immobilization combined with ovariectomy in rats. Bone 8:351–355. https://doi.org/10.1016/8756-3282(87)90066-4
Hayashi T, Yamamuro T, Okumura H et al (1989) Effect of (Asu1,7)-eel calcitonin on the prevention of osteoporosis induced by combination of immobilization and ovariectomy in the rat. Bone 10:25–28. https://doi.org/10.1016/8756-3282(89)90143-9
Yarrow JF, Conover CF, Beggs LA et al (2014) Testosterone dose dependently prevents bone and muscle loss in rodents after spinal cord injury. J Neurotrauma 31:834–845. https://doi.org/10.1089/neu.2013.3155
Verma R, Virdi JK, Singh N, Jaggi AS (2019) Animals models of spinal cord contusion injury. Korean J Pain 32:12–21
In Lee J, Hyun Kim J, Won Kim H et al (2005) Changes in bone metabolism in a rat model of traumatic brain injury. Brain Inj 19:1207–1211. https://doi.org/10.1080/02699050500309338
Bikle DD, Harris J, Halloran BP, Morey-Holton E (1994) Altered skeletal pattern of gene expression in response to spaceflight and hindlimb elevation. Am J Physiol Endocrinol Metab. https://doi.org/10.1152/ajpendo.1994.267.6.e822
Yamada G, Sugimura K, Nakamura S et al (1997) Trace element composition and histological analysis of rat bones from the space shuttle. Life Sci 60:635–642. https://doi.org/10.1016/S0024-3205(96)00699-6
Bateman TA, Zimmerman RJ, Ayers RA et al (1998) Histomorphometric, physical, and mechanical effects of spaceflight and insulin-like growth factor-I on rat long bones. Bone 23:527–535. https://doi.org/10.1016/S8756-3282(98)00135-5
Blaber EA, Dvorochkin N, Lee C et al (2013) Microgravity induces pelvic bone loss through osteoclastic activity, osteocytic osteolysis, and osteoblastic cell cycle inhibition by CDKN1a/p21. PLoS ONE. https://doi.org/10.1371/journal.pone.0061372
Maupin KA, Childress P, Brinker A et al (2019) Skeletal adaptations in young male mice after 4 weeks aboard the International Space Station. NPJ Microgravity. https://doi.org/10.1038/s41526-019-0081-4
Hettrich CM, Gasinu S, Beamer BS et al (2013) The effect of immobilization on the native and repaired tendon-to-bone interface. J Bone Jt Surg Ser A 95:925–930. https://doi.org/10.2106/JBJS.K.01329
Gadomski BC, McGilvray KC, Easley JT et al (2018) An investigation of shock wave therapy and low-intensity pulsed ultrasound on fracture healing under reduced loading conditions in an ovine model. J Orthop Res 36:921–929. https://doi.org/10.1002/jor.23666
Tian L, Sheng Y, Huang L et al (2018) An innovative Mg/Ti hybrid fixation system developed for fracture fixation and healing enhancement at load-bearing skeletal site. Biomaterials 180:173–183. https://doi.org/10.1016/j.biomaterials.2018.07.018
Zeng QQ, Jee WSS, Ke HZ, Wechter WJ (1993) S-Ketoprofen inhibits tenotomy-induced bone loss and dynamics in weanling rats. Bone Miner 21:203–218. https://doi.org/10.1016/S0169-6009(08)80231-0
Geiser M, Trueta J (1958) Muscle action, bone rarefaction and bone formation; an experimental study. J Bone Joint Surg Br 40B:282–311. https://doi.org/10.1302/0301-620X.40B2.282
Rubin CT, Pratt GW, Porter AL et al (1988) Ultrasonic measurement of immobilization-induced osteopenia: An experimental study in sheep. Calcif Tissue Int 42:309–312. https://doi.org/10.1007/BF02556365
Welch RD, Ashman RB, Baker KJ, Browne RH (1996) Intraosseous infusion of prostaglandin E2 prevents disuse-induced bone loss in the tibia. J Orthop Res 14:303–310. https://doi.org/10.1002/jor.1100140220
Wang X, Xie L, Crane J et al (2018) Aberrant TGF-β activation in bone tendon insertion induces enthesopathy-like disease. J Clin Invest 128:846–860. https://doi.org/10.1172/JCI96186
Bollman JL (1948) A cage which limits the activity of rats. J Lab Clin Med 13:1348
Girardet R (1974) A simple and inexpensive restraining cage for rats. J Surg Res 17:131–133. https://doi.org/10.1016/0022-4804(74)90133-4
Sato T, Yamamoto H, Sawada N et al (2006) Immobilization decreases duodenal calcium absorption through a 1,25-dihydroxyvitamin D-dependent pathway. J Bone Miner Metab 24:291–299. https://doi.org/10.1007/s00774-006-0686-z
Marmonti E, Busquets S, Toledo M et al (2017) A rat immobilization model based on cage volume reduction: a physiological model for bed rest? Front Physiol 8:184. https://doi.org/10.3389/fphys.2017.00184
Aguado E, Pascaretti-Grizon F, Goyenvalle E et al (2015) Bone mass and bone quality are altered by hypoactivity in the chicken. PLoS ONE. https://doi.org/10.1371/journal.pone.0116763
Aguado E, Mabilleau G, Goyenvalle E, Chappard D (2017) Hypodynamia alters bone quality and trabecular microarchitecture. Calcif Tissue Int 100:332–340. https://doi.org/10.1007/s00223-017-0235-x
Howard WH, Parcher JW, Young DR (1971) Primate restraint system for studies of metabolic responses during recumbency. Lab Anim Sci 1:112–117
Grynpas MD, Patterson-Allen P, Simmons DJ (1986) The changes in quality of mandibular bone mineral in otherwise totally immobilized Rhesus monkeys. Calcif Tissue Int 39:57–62. https://doi.org/10.1007/BF02553291
Rubin CT, Lanyon LE (1984) Regulation of bone formation by applied dynamic loads. J Bone Jt Surg Ser A 66:397–402. https://doi.org/10.2106/00004623-198466030-00012
Rubin CT, Lanyon LE (1985) Regulation of bone mass by mechanical strain magnitude. Calcif Tissue Int 37:411–417. https://doi.org/10.1007/BF02553711
Rubin C, Sun YQ, Hadjiargyrou M, McLeod K (1999) Increased expression of matrix metalloproteinase-1 in osteocytes precedes bone resorption as stimulated by disuse: Evidence for autoregulation of the cell’s mechanical environment? J Orthop Res 17:354–361. https://doi.org/10.1002/jor.1100170309
Colton HS (1929) How bipedal habit affects the bones of the hind legs of the albino rat. J Exp Zool 53:1–11. https://doi.org/10.1002/jez.1400530102
Kim HKW, Aruwajoye O, Stetler J, Stall A (2012) Effects of non-weight-bearing on the immature femoral head following ischemic osteonecrosis: an experimental investigation in immature pigs. J Bone Jt Surg Ser A 94:2228–2237. https://doi.org/10.2106/JBJS.L.00300
Kim HKW, Aruwajoye O, Du J, Kamiya N (2014) Local administration of bone morphogenetic protein-2 and bisphosphonate during non-weight-bearing treatment of ischemic osteonecrosis of the femoral head: an experimental investigation in immature pigs. J Bone Jt Surg Am 96:1515–1524. https://doi.org/10.2106/JBJS.M.01361
Shen WW, Zhao JH (2010) Pulsed electromagnetic fields stimulation affects BMD and local factor production of rats with disuse osteoporosis. Bioelectromagnetics 31:113–119. https://doi.org/10.1002/bem.20535
Wilson CJ, Dahners LE (1988) An examination of the mechanism of ligament contracture. Clin Orthop Relat Res 227:286–291
Judex S, Garman R, Squire M et al (2004) Genetically linked site-specificity of disuse osteoporosis. J Bone Miner Res 19:607–613. https://doi.org/10.1359/JBMR.040110
Lin C, Jiang X, Dai Z et al (2009) Sclerostin mediates bone response to mechanical unloading through antagonizing Wnt/β-catenin signaling. J Bone Miner Res 24:1651–1661. https://doi.org/10.1359/jbmr.090411
Tajima T, Menuki K, Okuma KF et al (2018) Cortical bone loss due to skeletal unloading in aldehyde dehydrogenase 2 gene knockout mice is associated with decreased PTH receptor expression in osteocytes. Bone 110:254–266. https://doi.org/10.1016/j.bone.2018.02.020
Lloyd SA, Loiselle AE, Zhang Y, Donahue HJ (2013) Connexin 43 deficiency desensitizes bone to the effects of mechanical unloading through modulation of both arms of bone remodeling. Bone 57:76–83. https://doi.org/10.1016/j.bone.2013.07.022
Biewener AA, Bertram JEA (1994) Structural response of growing bone to exercise and disuse. J Appl Physiol 76:946–955. https://doi.org/10.1152/jappl.1994.76.2.946
Il SB, Ku SK, Cha EM et al (2005) Effect of Mornidae Radix extracts on experimental osteoporosis in sciatic neurectomized mice. Phyther Res 19:231–238. https://doi.org/10.1002/ptr.1683
Uchii M, Takashima M, Sugiyama T, Kosaka N (1998) Effect of KW-8232, a novel anti-osteoporotic agent, on bone loss in sciatic neurectomized rats. Jpn J Pharmacol 78:241–243. https://doi.org/10.1254/jjp.78.241
Guertin PA, Ung RV, Rouleau P, Steuer I (2011) Effects on locomotion, muscle, bone, and blood induced by a combination therapy eliciting weight-bearing stepping in nonassisted spinal cord-transected mice. Neurorehabil Neural Repair 25:234–242. https://doi.org/10.1177/1545968310378753
Suzue N, Nikawa T, Onishi Y et al (2006) Ubiquitin ligase Cbl-b downregulates bone formation through suppression of IGF-I signaling in osteoblasts during denervation. J Bone Miner Res 21:722–734. https://doi.org/10.1359/jbmr.060207
Aryal ACS, Miyai K, Hayata T et al (2013) Nck1 deficiency accelerates unloading-induced bone loss. J Cell Physiol 228:1397–1403. https://doi.org/10.1002/jcp.24317
Yamashita T, Sekiya I, Kawaguchi N et al (2001) Klotho-deficient mice are resistant to bone loss induced by unloading due to sciatic neurectomy. J Endocrinol 168:347–351. https://doi.org/10.1677/joe.0.1680347
Qin W, Zhao W, Li X et al (2016) Mice with sclerostin gene deletion are resistant to the severe sublesional bone loss induced by spinal cord injury. Osteoporos Int 27:3627–3636. https://doi.org/10.1007/s00198-016-3700-x
Wada N, Shimizu T, Takai S et al (2017) Post-injury bladder management strategy influences lower urinary tract dysfunction in the mouse model of spinal cord injury. Neurourol Urodyn 36:1301–1305. https://doi.org/10.1002/nau.23120
Kjell J, Olson L (2016) Rat models of spinal cord injury: From pathology to potential therapies. DMM Dis Model Mech 9:1125–1137
Weaver LC, Verghese P, Bruce JC et al (2001) Autonomic dysreflexia and primary afferent sprouting after clip-compression injury of the rat spinal cord. J Neurotrauma 18:1107–1119. https://doi.org/10.1089/08977150152693782
Bach-Gansmo FL, Wittig NK, Brüel A et al (2016) Immobilization and long-term recovery results in large changes in bone structure and strength but no corresponding alterations of osteocyte lacunar properties. Bone 91:139–147. https://doi.org/10.1016/j.bone.2016.07.005
Lodberg A, Vegger JB, Jensen MV et al (2015) Immobilization induced osteopenia is strain specific in mice. Bone Reports 2:59–67. https://doi.org/10.1016/j.bonr.2015.04.001
Kaneps AJ, Stover SM, Lane NE (1997) Changes in canine cortical and cancellous bone mechanical properties following immobilization and remobilization with exercise. Bone 21:419–423. https://doi.org/10.1016/S8756-3282(97)00167-1
Kazarian LE, Von Gierke HE (1981) The effects of hypokinesia in primates on bone strength. Acta Astronaut 8:1075–1082. https://doi.org/10.1016/0094-5765(81)90081-3
van Harreveld PD, Lillich JD, Kawcak CE et al (2002) Effects of immobilization followed by remobilization on mineral density, histomorphometric features, and formation of the bones of the metacarpophalangeal joint in horses. Am J Vet Res 63:276–281. https://doi.org/10.2460/ajvr.2002.63.276
Sivachelvan MN, Davies AS (1986) Induction of relative growth changes in the musculoskeletal system of the sheep by limb immobilisation. Res Vet Sci 40:173–182. https://doi.org/10.1016/s0034-5288(18)30509-5
Lindgren U, Mattsson S (1977) The reversibility of disuse osteoporosis - Studies of bone density, bone formation, and cell proliferation in bone tissue. Calcif Tissue Res 23:179–184. https://doi.org/10.1007/BF02012784
Minematsu A, Imagita H, Kanemura N, Yoshimura O (2006) The progression of bone and muscle atrophy in mice hind limb with immobilization. Hiroshima J Med Sci 55:79–83
Trudel G, Koike Y, Ramachandran N et al (2007) Mechanical alterations of rabbit achilles’ tendon after immobilization correlate with bone mineral density but not with magnetic resonance or ultrasound imaging. Arch Phys Med Rehabil 88:1720–1726. https://doi.org/10.1016/j.apmr.2007.07.034
Grindeland RE, Ballard RW, Connolly JP, Vasques MF (1992) COSMOS 2044 mission. Overview. J Appl Physiol 73:1S-3S
Ellman R, Grasso DJ, Van Vliet M et al (2014) Combined effects of botulinum toxin injection and hind limb unloading on bone and muscle. Calcif Tissue Int 94:327–337. https://doi.org/10.1007/s00223-013-9814-7
Speacht TL, Krause AR, Steiner JL et al (2018) Combination of hindlimb suspension and immobilization by casting exaggerates sarcopenia by stimulating autophagy but does not worsen osteopenia. Bone 110:29–37. https://doi.org/10.1016/j.bone.2018.01.026
Percie du Sert N, Hurst V, Ahluwalia A et al (2020) The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. PLoS Biol. https://doi.org/10.1371/journal.pbio.3000410
Nieves JW, Formica C, Ruffing J et al (2005) Males have larger skeletal size and bone mass than females, despite comparable body size. J Bone Miner Res 20:529–535. https://doi.org/10.1359/JBMR.041005
Nguyen TV, Maynard LM, Towne B et al (2001) Sex differences in bone mass acquisition during growth. J Clin Densitom 4:147–157. https://doi.org/10.1385/JCD:4:2:147
Wolfe MS, Klein L (1996) Sex differences in absolute rates of bone resorption in young rats: appendicular versus axial bones. Calcif Tissue Int 59:51–57. https://doi.org/10.1007/s002239900085
Orwoll ES, Belknap JK, Klein RF (2001) Gender specificity in the genetic determinants of peak bone mass. J Bone Miner Res 16:1962–1971. https://doi.org/10.1359/jbmr.2001.16.11.1962
David V, Lafage-Proust MH, Laroche N et al (2006) Two-week longitudinal survey of bone architecture alteration in the hindlimb-unloaded rat model of bone loss: Sex differences. Am J Physiol Endocrinol Metab 290:E440–E447. https://doi.org/10.1152/ajpendo.00293.2004
Hefferan TE, Evans GL, Lotinun S et al (2003) Effect of gender on bone turnover in adult rats during simulated weightlessness. J Appl Physiol 95:1775–1780. https://doi.org/10.1152/japplphysiol.00455.2002
Qi W, Yan YB, Lei W et al (2012) Prevention of disuse osteoporosis in rats by Cordyceps sinensis extract. Osteoporos Int 23:2347–2357. https://doi.org/10.1007/s00198-011-1842-4
Squire M, Brazin A, Keng Y, Judex S (2008) Baseline bone morphometry and cellular activity modulate the degree of bone loss in the appendicular skeleton during disuse. Bone 42:341–349. https://doi.org/10.1016/j.bone.2007.09.052
Ko CY, Seo DH, Kim HS (2011) Deterioration of bone quality in the tibia and fibula in growing mice during skeletal unloading: Gender-related differences. J Biomech Eng. https://doi.org/10.1115/1.4005350
Grimston SK, Silva MJ, Civitelli R (2007) Bone loss after temporarily induced muscle paralysis by Botox is not fully recovered after 12 weeks. Ann N Y Acad Sci 1116:444–460. https://doi.org/10.1196/annals.1402.009
Thomsen JS, Christensen LL, Vegger JB et al (2012) Loss of bone strength is dependent on skeletal site in disuse osteoporosis in rats. Calcif Tissue Int 90:294–306. https://doi.org/10.1007/s00223-012-9576-7
Colleran PN, Wilkerson MK, Bloomfield SA et al (2000) Alterations in skeletal perfusion with simulated microgravity: a possible mechanism for bone remodeling. J Appl Physiol 89:1046–1054. https://doi.org/10.1152/jappl.2000.89.3.1046
Bloomfield SA, Allen MR, Hogan HA, Delp MD (2002) Site- and compartment-specific changes in bone with hindlimb unloading in mature adult rats. Bone 31:149–157. https://doi.org/10.1016/S8756-3282(02)00785-8
Jee WSS (2005) The past, present, and future of bone morphometry: Its contribution to an improved understanding of bone biology. J Bone Miner Metab 23:1–10
Rüegsegger P, Koller B, Müller R (1996) A microtomographic system for the nondestructive evaluation of bone architecture. Calcif Tissue Int 58:24–29. https://doi.org/10.1007/BF02509542
Morey-Holton E, Globus RK, Kaplansky A, Durnova G (2005) The hindlimb unloading rat model: literature overview, technique update and comparison with space flight data. Adv Space Biol Med 10:7–40
Acknowledgements
We thank research librarian and information specialist Annette Balle Sørensen from Aarhus University for valuable insights and help in developing the search strategy. Jose Luis Ferretti, Neil Fedarko, and Piret Hussar are thanked for providing copies of their research not retrievable elsewhere.
Funding
The authors received no specific funding for this work.
Author information
Authors and Affiliations
Contributions
MBB is the project manager, guarantor, and drafted the manuscript. MBB and JST developed the search and methodological strategy and screened eligible abstracts and full-text articles. MBB, MB, and JST read, provided feedback, and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Mikkel Bo Brent, Annemarie Brüel, and Jesper Skovhus Thomsen have no relationship that might pose a conflict of interest in connection with the submitted article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Annemarie Brüel and Jesper Skovhus Thomsen are joint senior author.
Supplementary Information
Below is the link to the electronic supplementary material.
Appendix A
Appendix A
Search string for PubMed and Embase.
PubMed | Embase | |
|---|---|---|
Block 1 | (bone*[tiab] OR skeleton*[tiab]) | (bone*:ab,ti OR skeleton*:ab,ti) |
AND | ||
Block 2 | (immobilisation*[tiab] OR immobilization*[tiab] OR disuse*[tiab] OR "non ambulating"[tiab] OR restraint*[tiab] OR unloading*[tiab] OR "non weight bearing"[tiab] OR "non load bearing"[tiab] OR "non loadbearing"[tiab] OR paralyzed*[tiab] OR paralysed*[tiab] OR monoplegic*[tiab] OR paraplegic*[tiab] OR hemiplegic*[tiab] OR tetraplegic*[tiab] OR quadroplegic*[tiab]) | (immobilisation*:ab,ti OR immobilization*:ab,ti OR disuse*:ab,ti OR "non ambulating":ab,ti OR restraint*:ab,ti OR unloading*:ab,ti OR "non weight bearing":ab,ti OR "non load bearing":ab,ti OR "non loadbearing":ab,ti OR paralyzed*:ab,ti OR paralysed*:ab,ti OR monoplegic*:ab,ti OR paraplegic*:ab,ti OR hemiplegic*:ab,ti OR tetraplegic*:ab,ti OR quadroplegic*:ab,ti) |
AND | ||
Block 3 | Search filter for animal experimentation developed by Hooijmans et al. [19] | Search filter for animal experimentation developed by de Vries et al. [20] |
NOT | ||
Block 4 | ("letter"[publication type] OR "comment"[publication type] OR "editorial"[publication type] OR review[publication type]) | ('conference abstract'/it OR 'conference paper'/it OR 'conference review'/it OR 'review'/it OR 'editorial'/it OR 'letter'/it OR 'note'/it) AND [embase]/lim NOT [medline]/lim |
Rights and permissions
About this article
Cite this article
Brent, M.B., Brüel, A. & Thomsen, J.S. A Systematic Review of Animal Models of Disuse-Induced Bone Loss. Calcif Tissue Int 108, 561–575 (2021). https://doi.org/10.1007/s00223-020-00799-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-020-00799-9