Abstract
Summary
Cordyceps sinensis has been known as a traditional medicine in China, and C. sinensis plus strontium could prevent osteoporosis in ovariectomized rats. The present study shows that daily oral administration of C. sinensis at higher doses in adult hind limb suspension rats can prevent disuse-induced bone loss and deterioration of trabecular microarchitecture.
Introduction
Cordyceps sinensis induces estradiol production and prevents osteoporosis in ovariectomized rats. This study was to examine whether C. sinensis can prevent disuse-induced osteoporosis.
Methods
Rats were randomly divided into six groups, and five groups were treated with hind limb suspension (HLS). One HLS group received alendronate (2.0 mg/kg/day) orally, and to the three other HLS groups to each group, a different amount of C. sinensis (100, 300, and 500 mg/kg/day) was orally administered for 8 weeks before and after HLS. The remaining HLS group was set as a control without treatment. Each group consisted of 10 males and females. The body weights, biochemical parameters in serum and urine, bone mineral density (BMD), bone mineral content (BMC), mechanical testing, and bone microarchitecture were examined.
Results
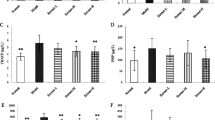
Treatments with higher C. sinensis dosage (300 and 500 mg/kg/day) or alendronate had a positive effect on body weights, mechanical strength, BMD, and BMC compared to the other HLS groups. C. sinensis decreased markers of bone turnover dose dependently and increased the osteocalcin levels in HLS rats. The result of micro-CT analysis from the L4 vertebra showed that C. sinensis (500 mg/kg) significantly prevented the reduction of the bone volume fraction, connectivity density, trabeculae number, and thickness as well as improved the trabeculae separation and structure model index in HLS rats.
Conclusions
The present study demonstrates that administration of C. sinensis at higher doses over an 8-week period can prevent the disuse osteoporosis in rats. It implies that C. sinensis might be an alternative therapy for prevention of disuse-induced osteoporosis also in humans.





Similar content being viewed by others
References
Berecki-Gisolf J, Spallek M, Hockey R, Dobson A (2010) Height loss in elderly women is preceded by osteoporosis and is associated with digestive problems and urinary incontinence. Osteoporos Int 21:479–485
Oinuma T, Sakuma M, Endo N (2010) Secular change of the incidence of four fracture types associated with senile osteoporosis in Sado, Japan: the results of a 3-year survey. J Bone Miner Metab 28:55–59
Li CY, Price C, Delisser K, Nasser P, Laudier D, Clement M, Jepsen KJ, Schaffler MB (2005) Long-term disuse osteoporosis seems less sensitive to bisphosphonate treatment than other osteoporosis. J Bone Miner Res 20:117–124
Garber MA, McDowell DL, Hutton WC (2000) Bone loss during simulated weightlessness: a biomechanical and mineralization study in the rat model. Aviat Space Environ Med 71:586–592
van der Meulen MC, Morey-Holton ER, Carter DR (1995) Hindlimb suspension diminishes femoral cross-sectional growth in the rat. J Orthop Res 13:700–707
Caillot-Augusseau A, Vico L, Heer M, Voroviev D, Souberbielle JC, Zitterman A, Alexandre C, Lafage-Proust MH (2000) Space flight is associated with rapid decreases of undercarboxylated osteocalcin and increases of markers of bone resorption without changes in their circadian variation: observations in two cosmonauts. Clin Chem 46:1136–1143
Kiratli BJ, Smith AE, Nauenberg T, Kallfelz CF, Perkash I (2000) Bone mineral and geometric changes through the femur with immobilization due to spinal cord injury. J Rehabil Res Dev 37:225–233
Lang T, LeBlanc A, Evans H, Lu Y, Genant H, Yu A (2004) Cortical and trabecular bone mineral loss from the spine and hip in long-duration spaceflight. J Bone Miner Res 19:1006–1012
Shackelford LC, LeBlanc AD, Driscoll TB, Evans HJ, Rianon NJ, Smith SM, Spector E, Feeback DL, Lai D (2004) Resistance exercise as a countermeasure to disuse-induced bone loss. J Appl Physiol 97:119–129
Yang Li C, Majeska RJ, Laudier DM, Mann R, Schaffler MB (2005) High-dose risedronate treatment partially preserves cancellous bone mass and microarchitecture during long-term disuse. Bone 37:287–295
Zhu JS, Halpern GM, Jones K (1998) The scientific rediscovery of an ancient Chinese herbal medicine: Cordyceps sinensis: part I. J Altern Complement Med 4:289–303
Zhu JS, Halpern GM, Jones K (1998) The scientific rediscovery of a precious ancient Chinese herbal regimen: Cordyceps sinensis: part II. J Altern Complement Med 4:429–457
Deitch AD, Sawicki SG (1979) Effects of cordycepin on microtubules of cultured mammalian cells. Exp Cell Res 118:1–13
Lee HS, Kim MK, Kim YK, Jung EY, Park CS, Woo MJ, Lee SH, Kim JS, Suh HJ (2011) Stimulation of osteoblastic differentiation and mineralization in MC3T3-E1 cells by antler and fermented antler using Cordyceps militaris. J Ethnopharmacol 133:710–717
Huang LF, Liang YZ, Guo FQ, Zhou ZF, Cheng BM (2003) Simultaneous separation and determination of active components in Cordyceps sinensis and Cordyceps militarris by LC/ESI-MS. J Pharm Biomed Anal 33:1155–1162
Shin S, Lee S, Kwon J, Moon S, Lee S, Lee CK, Cho K, Ha NJ, Kim K (2009) Cordycepin suppresses expression of diabetes regulating genes by inhibition of lipopolysaccharide-induced inflammation in macrophages. Immune Netw 9:98–105
Jeong JW, Jin CY, Kim GY et al (2010) Anti-inflammatory effects of cordycepin via suppression of inflammatory mediators in BV2 microglial cells. Int Immunopharmacol 10:1580–1586
Wang Y, Wang M, Ling Y, Fan W, Yin H (2009) Structural determination and antioxidant activity of a polysaccharide from the fruiting bodies of cultured Cordyceps sinensis. Am J Chin Med 37:977–989
Huang BM, Hsiao KY, Chuang PC, Wu MH, Pan HA, Tsai SJ (2004) Upregulation of steroidogenic enzymes and ovarian 17beta-estradiol in human granulosa-lutein cells by Cordyceps sinensis mycelium. Biol Reprod 70:1358–1364
Qi W, Yan YB, Wang PJ, Lei W (2010) The co-effect of Cordyceps sinensis and strontium on osteoporosis in ovariectomized osteopenic rats. Biol Trace Elem Res 141(1–3):216–223
Qi W, Wang PJ, Guo WJ, Yan YB, Zhang Y, Lei W (2010) The mechanism of Cordyceps sinensis and strontium in prevention of osteoporosis in rats. Biol Trace Elem Res 143(1):302–309
Chang Y, Jeng KC, Huang KF, Lee YC, Hou CW, Chen KH, Cheng FY, Liao JW, Chen YS (2008) Effect of Cordyceps militaris supplementation on sperm production, sperm motility and hormones in Sprague–Dawley rats. Am J Chin Med 36:849–859
Morey-Holton ER, Globus RK (2002) Hindlimb unloading rodent model: technical aspects. J Appl Physiol 92:1367–1377
Liu Z, Li P, Zhao D, Tang H, Guo J (2010) Protective effect of extract of Cordyceps sinensis in middle cerebral artery occlusion-induced focal cerebral ischemia in rats. Behav Brain Funct 6:61
Wronski TJ, Schenck PA, Cintron M, Walsh CC (1987) Effect of body weight on osteopenia in ovariectomized rats. Calcif Tissue Int 40:155–159
FDA (2002) Guidance for industry and reviews: estimating the safe starting dose in clinical trials for therapeutics in adult healthy volunteers [S]. 12
Huang JH HX, Chen ZY et al (2004) Dose conversion among different animals and healthy volunteers in pharmacological study. Chin J Clin Pharmacol Ther 9:1069–1072
Changrani NR, Chonkar A, Adeghate E, Singh J (2006) Effects of streptozotocin-induced type 1 diabetes mellitus on total protein concentrations and cation contents in the isolated pancreas, parotid, submandibular, and lacrimal glands of rats. Ann N Y Acad Sci 1084:503–519
Ma Z, Fu Q (2010) Comparison of the therapeutic effects of yeast-incorporated gallium with those of inorganic gallium on ovariectomized osteopenic rats. Biol Trace Elem Res 134:280–287
Zhang G, Qin L, Hung WY, Shi YY, Leung PC, Yeung HY, Leung KS (2006) Flavonoids derived from herbal Epimedium Brevicornum Maxim prevent OVX-induced osteoporosis in rats independent of its enhancement in intestinal calcium absorption. Bone 38:818–825
Havill LM, Hale LG, Newman DE, Witte SM, Mahaney MC (2006) Bone ALP and OC reference standards in adult baboons (Papio hamadryas) by sex and age. J Med Primatol 35:97–105
Ross PD, Kress BC, Parson RE, Wasnich RD, Armour KA, Mizrahi IA (2000) Serum bone alkaline phosphatase and calcaneus bone density predict fractures: a prospective study. Osteoporos Int 11:76–82
Halleen JM, Ylipahkala H, Alatalo SL, Janckila AJ, Heikkinen JE, Suominen H, Cheng S, Vaananen HK (2002) Serum tartrate-resistant acid phosphatase 5b, but not 5a, correlates with other markers of bone turnover and bone mineral density. Calcif Tissue Int 71:20–25
Qin YJ, Zhang ZL, Zhang H, Hu WW, Liu YJ, Hu YQ, Li M, Gu JM, He JW (2008) Age-related changes of serum tartrate-resistant acid phosphatase 5b and the relationship with bone mineral density in Chinese women. Acta Pharmacol Sin 29:1493–1498
Bessey OA, Lowry OH, Brock MJ (1946) A method for the rapid determination of alkaline phosphates with five cubic millimeters of serum. J Biol Chem 164:321–329
Kanbur NO, Derman O, Sen TA, Kinik E (2002) Osteocalcin. A biochemical marker of bone turnover during puberty. Int J Adolesc Med Health 14:235–244
Burgeson RE (1988) New collagens, new concepts. Annu Rev Cell Biol 4:551–577
Guerrero R, Diaz Martin MA, Diaz Diego EM, Disla T, Rapado A, de la Piedra C (1996) New biochemical markers of bone resorption derived from collagen breakdown in the study of postmenopausal osteoporosis. Osteoporos Int 6:297–302
Madyastha PR, Yang S, Ries WL, Key LL Jr (2000) IFN-gamma enhances osteoclast generation in cultures of peripheral blood from osteopetrotic patients and normalizes superoxide production. J Interferon Cytokine Res 20:645–652
Mundy GR (1993) Role of cytokines in bone resorption. J Cell Biochem 53:296–300
Petricevich VL, Lebrun I (2005) Immunomodulatory effects of the Tityus serrulatus venom on murine macrophage functions in vitro. Mediators Inflamm 2005:39–49
Bagi CM, Miller SC (1994) Comparison of osteopenic changes in cancellous bone induced by ovariectomy and/or immobilization in adult rats. Anat Rec 239:243–254
Turner RT, Riggs BL, Spelsberg TC (1994) Skeletal effects of estrogen. Endocr Rev 15:275–300
Dang ZC, van Bezooijen RL, Karperien M, Papapoulos SE, Lowik CW (2002) Exposure of KS483 cells to estrogen enhances osteogenesis and inhibits adipogenesis. J Bone Miner Res 17:394–405
Laib A, Kumer JL, Majumdar S, Lane NE (2001) The temporal changes of trabecular architecture in ovariectomized rats assessed by MicroCT. Osteoporos Int 12:936–941
Tsunoo A, Takemoto NN, Tsuboi H, Kamijo MA et al. (1995) Cordyceps sinensis: its diverse effects on mammals in vitro and in vivo. In: New initiatives in mycological research. Proceedings of the Third International Symposium of the Mycological Society of Japan, pp. 1–10
Yao W, Tian XY, Chen J, Setterberg RB, Lundy MW, Chmielzwski P, Froman CA, Jee WS (2007) Rolipram, a phosphodiesterase 4 inhibitor, prevented cancellous and cortical bone loss by inhibiting endosteal bone resorption and maintaining the elevated periosteal bone formation in adult ovariectomized rats. J Musculoskelet Neuronal Interact 7:119–130
Delmas PD, Eastell R, Garnero P, Seibel MJ, Stepan J (2000) The use of biochemical markers of bone turnover in osteoporosis. Committee of Scientific Advisors of the International Osteoporosis Foundation. Osteoporos Int 11(Suppl 6):S2–S17
Gaumet N, Seibel MJ, Coxam V, Davicco MJ, Lebecque P, Barlet JP (1997) Influence of ovariectomy and estradiol treatment on calcium homeostasis during aging in rats. Arch Physiol Biochem 105:435–444
Akhter MP, Alvarez GK, Cullen DM, Recker RR (2010) Disuse-related decline in trabecular bone structure. Biomech Model Mechanobiol 10(3):423–429
Bouxsein ML (2003) Mechanisms of osteoporosis therapy: a bone strength perspective. Clin Cornerstone Suppl 2:S13–S21
Li SP, Su ZR, Dong TT, Tsim KW (2002) The fruiting body and its caterpillar host of Cordyceps sinensis show close resemblance in main constituents and anti-oxidation activity. Phytomedicine 9:319–324
Won SY, Park EH (2005) Anti-inflammatory and related pharmacological activities of cultured mycelia and fruiting bodies of Cordyceps militaris. J Ethnopharmacol 96:555–561
Hefferan TE, Evans GL, Lotinun S, Zhang M, Morey-Holton E, Turner RT (2003) Effect of gender on bone turnover in adult rats during simulated weightlessness. J Appl Physiol 95:1775–1780
Lindsay R (1993) Pathogenesis of postmenopausal osteoporosis. Baillieres Clin Rheumatol 7:499–513
Funding
Grant support from The National High Technology Research and Development Program of China (863 Program) (2007AA02Z468) was received in support of this work. No benefits in any form have been or will be received from a commercial party directly or indirectly by the authors of this manuscript.
Ethics statement
All the procedures involving use of animals were conducted according to the ethics guidelines established by the Fourth Military Medical University.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Wei Qi and Ya-bo Yan contributed equally to this work as co-first authors.
Rights and permissions
About this article
Cite this article
Qi, W., Yan, YB., Lei, W. et al. Prevention of disuse osteoporosis in rats by Cordyceps sinensis extract. Osteoporos Int 23, 2347–2357 (2012). https://doi.org/10.1007/s00198-011-1842-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-011-1842-4