Abstract
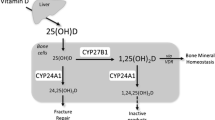
The source and function of C-3α epimer of 25(OH)D (C-3 epimer) is unknown. The objectives were to (1) establish if increasing doses of vitamin D (VD) results in a proportionate dose–response in C-3 epimer; and (2) determine the biological response of bone to C-3 epimer treatment. Sprague Dawley rats (12 weeks, n = 36 female, n = 36 male) were randomized to control AIN93-M diet (1 IU VD3/g diet) or an experimental diet for 8 weeks containing VD3 at 2 or 4 IU/g diet, C-3 epimer at 0.5 or 1 IU/g diet or 25(OH)D (0.5 IU/g diet). BW and food consumption were measured weekly. Blood was sampled at week 0, 4, and 8 for assessment of VD metabolites and bone metabolism biomarkers. DXA (week 0, 4, and 8) and in vivo micro CT (μCT) (week 0 and 8) were performed in vivo plus ex vivo μCT imaging and bone biomechanics. Dietary intake and anthropometry did not differ among diet groups. The dose–response of VD generated significantly elevated C-3 epimer only in females with concentrations of 4 IU VD diet group [mean 84.6 (62.5) nmol/L] exceeding control [mean 21.4 (18.5) nmol/L, p = 0.005]. Both sexes in the 25(OH)D group did not show significant increases in C-3 epimer, whereas 0.5 and 1 IU epimer groups exceeded 100 nmol/L of C-3 epimer by 8 weeks. These data suggest C-3 epimer is endogenously generated with higher intakes of VD. Endogenous and exogenous C-3 epimer accumulates in serum without impact upon bone health outcomes in a healthy young adult model over 8 weeks.




Similar content being viewed by others
References
Bailey D, Veljkovic K, Yazdanpanah M, Adeli K (2013) Analytical measurement and clinical relevance of vitamin D(3) C3-epimer. Clin Biochem 46(3):190–196
Genazzani AR, Palumbo MA, de Micheroux AA, Artini PG, Criscuolo M, Ficarra G, Guo A-L, Benelli A, Bertolini A, Petraglia F (1995) Evidence for a role for the neurosteroid allopregnanolone in the modulation of reproductive function in female rats. Eur J Endocrinol 133(3):375–380
Reddy GS, Muralidharan KR, Okamura WH, Tserng KY, McLane JA (2001) Metabolism of 1alpha,25-dihydroxyvitamin D(3) and its C-3 epimer 1alpha,25-dihydroxy-3-epi-vitamin D(3) in neonatal human keratinocytes. Steroids 66(3–5):441–450
Kamao M, Hatakeyama S, Sakaki T, Sawada N, Inouye K, Kubodera N, Reddy GS, Okano T (2005) Measurement and characterization of C-3 epimerization activity toward vitamin D3. Arch Biochem Biophys 436(1):196–205
Fleet JC, Bradley J, Reddy GS, Ray R, Wood RJ (1996) 1 alpha,25-(OH)2-vitamin D3 analogs with minimal in vivo calcemic activity can stimulate significant transepithelial calcium transport and mRNA expression in vitro. Arch Biochem Biophys 329(2):228–234
Kamao M, Tatematsu S, Hatakeyama S, Sakaki T, Sawada N, Inouye K, Ozono K, Kubodera N, Reddy GS, Okano T (2004) C-3 epimerization of vitamin D3 metabolites and further metabolism of C-3 epimers: 25-hydroxyvitamin D3 is metabolized to 3-epi-25-hydroxyvitamin D3 and subsequently metabolized through C-1alpha or C-24 hydroxylation. J Biol Chem 279(16):15897–15907
Rehan VK, Torday JS, Peleg S, Gennaro L, Vouros P, Padbury J, Rao DS, Reddy GS (2002) 1Alpha,25-dihydroxy-3-epi-vitamin D3, a natural metabolite of 1alpha,25-dihydroxy vitamin D3: production and biological activity studies in pulmonary alveolar type II cells. Mol Genet Metab 76(1):46–56
Rhieu SY, Annalora AJ, Wang G, Flarakos CC, Gathungu RM, Vouros P, Sigueiro R, Mourino A, Schuster I, Palmore GT, Reddy GS (2013) Metabolic stability of 3-Epi-1alpha,25-dihydroxyvitamin D3 over 1 alpha 25-dihydroxyvitamin D3: metabolism and molecular docking studies using rat CYP24A1. J Cell Biochem 114(10):2293–2305
Gallo S, Comeau K, Vanstone C, Agellon S, Sharma A, Jones G, L’Abbe M, Khamessan A, Rodd C, Weiler H (2013) Effect of different dosages of oral vitamin D supplementation on vitamin D status in healthy, breastfed infants: a randomized trial. JAMA 309(17):1785–1792
Anderson PH, Sawyer RK, Moore AJ, May BK, O’Loughlin PD, Morris HA (2008) Vitamin D depletion induces RANKL-mediated osteoclastogenesis and bone loss in a rodent model. J Bone Miner Res 23(11):1789–1797
Lee AM, Anderson PH, Sawyer RK, Moore AJ, Forwood MR, Steck R, Morris HA, O’Loughlin PD (2010) Discordant effects of vitamin D deficiency in trabecular and cortical bone architecture and strength in growing rodents. J Steroid Biochem Mol Biol 121(1–2):284–287
Fleet JC, Gliniak C, Zhang Z, Xue Y, Smith KB, McCreedy R, Adedokun SA (2008) Serum metabolite profiles and target tissue gene expression define the effect of cholecalciferol intake on calcium metabolism in rats and mice. J Nutr 138(6):1114–1120
Pye KM, Wakefield AP, Aukema HM, House JD, Ogborn MR, Weiler HA (2009) A high mixed protein diet reduces body fat without altering the mechanical properties of bone in female rats. J Nutr 139(11):2099–2105
Reeves PG, Nielsen FH, Fahey GC Jr (1993) AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr 123(11):1939–1951
Brouwers JE, Van Rietbergen B, Huiskes R (2007) No effects of in vivo micro-CT radiation on structural parameters and bone marrow cells in proximal tibia of wistar rats detected after eight weekly scans. J Orthop Res 25(10):1325–1332
Meganck JA, Kozloff KM, Thornton MM, Broski SM, Goldstein SA (2009) Beam hardening artifacts in micro-computed tomography scanning can be reduced by X-ray beam filtration and the resulting images can be used to accurately measure BMD. Bone 45(6):1104–1116
Crenshaw TD, Peo ER, Lewis AJ, Moser BD (1981) Bone strength as a trait for assessing mineralization in swine: a critical review of techniques involved. J Anim Sci 53(3):827–835
Brown AJ, Ritter CS, Weiskopf AS, Vouros P, Sasso GJ, Uskokovic MR, Wang G, Reddy GS (2005) Isolation and identification of 1alpha-hydroxy-3-epi-vitamin D3, a potent suppressor of parathyroid hormone secretion. J Cell Biochem 96(3):569–578
Lensmeyer G, Poquette M, Wiebe D, Binkley N (2012) The C-3 epimer of 25-hydroxyvitamin D(3) is present in adult serum. J Clin Endocrinol Metabol 97(1):163–168
Schleicher RL, Encisco SE, Chaudhary-Webb M, Paliakov E, McCoy LF, Pfeiffer CM (2011) Isotope dilution ultra performance liquid chromatography-tandem mass spectrometry method for simultaneous measurement of 25-hydroxyvitamin D2, 25-hydroxyvitamin D3 and 3-epi-25-hydroxyvitamin D3 in human serum. Clin Chim Acta 412(17–18):1594–1599
Yazdanpanah M, Bailey D, Walsh W, Wan B, Adeli K (2013) Analytical measurement of serum 25-OH-vitamin D(3), 25-OH-vitamin D(2) and their C3-epimers by LC-MS/MS in infant and pediatric specimens. Clin Biochem 46(13–14):1264–1271
Engelman CD, Bo R, Zuelsdorff M, Steltenpohl H, Kirby T, Nieto FJ (2014) Epidemiologic study of the C-3 epimer of 25-hydroxyvitamin D(3) in a population-based sample. Clin Nutr 33(3):421–425
Lensmeyer G, Poquette M, Wiebe D, Binkley N (2011) The C-3 epimer of 25-hydroxyvitamin D3 is present in adult serum. J Clin Endocrinol Metabol 97(1):163–168
Witschi AK, Liesegang A, Gebert S, Weber GM, Wenk C (2011) Effect of source and quantity of dietary vitamin D in maternal and creep diets on bone metabolism and growth in piglets. J Anim Sci 89(6):1844–1852
Rhieu SY, Annalora AJ, Wang G, Flarakos CC, Gathungu RM, Vouros P, Sigueiro R, Mourino A, Schuster I, Palmore GT, Reddy GS (2013) Metabolic stability of 3-epi-1alpha,25-dihydroxyvitamin D3 over 1 alpha 25-dihydroxyvitamin D3: metabolism and molecular docking studies using rat CYP24A1. J Cell Biochem 114(10):2293–2305
Masuda S, Kamao M, Schroeder NJ, Makin HL, Jones G, Kremer R, Rhim J, Okano T (2000) Characterization of 3-epi-1alpha,25-dihydroxyvitamin D3 involved in 1alpha,25-dihydroxyvitamin D3 metabolic pathway in cultured cell lines. Biol Pharm Bull 23(2):133–139
Gallo S, Comeau K, Agellon S, Vanstone C, Sharma A, Jones G, L’Abbe M, Khamessan A, Weiler H, Rodd C (2014) Methodological issues in assessing plasma 25-hydroxyvitamin D concentration in newborn infants. Bone 61:186–190
Olfert ED, Cross BM, McWilliam AA (1993) Guide to the care and use of experimental animals, vol 2. Canadian Council on Animal Care, Ottawa
Acknowledgments
This work was supported by a Collaborative Research and Development Grant from Dairy Farmers of Canada and the Natural Sciences and Engineering Research Council of Canada. Infrastructure support from the Canadian Foundation for Innovation and Canada Research Chairs programs awarded to HW is acknowledged. Vitamin D metabolites were measured by Warnex, Laval (Bioanalytical Services, Laval, QC). C-3 epimer and 25(OH)D was purchased from Isosciences (Isosciences, LCC. Pennsylvania, USA) for diet production by Harlan (Harlan Laboratories, Madison, WI). This study was reviewed and approved by the Macdonald Campus Facility Animal Care Committee and in accordance with the Canadian Council on Animal Care [28].
Conflict of Interest
Dr. Hope A. Weiler has received research Grants from a Collaborative Research and Development Grant from Dairy Farmers of Canada and the Natural Sciences and Engineering Research Council of Canada. Christina Bianchini, Paula Lavery, and Sherry Agellon declare they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human participants performed by any of the authors. All procedures performed in studies involving animals were reviewed and approved by the Macdonald Campus Facility Animal Care Committee and in accordance with the Canadian Council on Animal Care the ethical standards.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bianchini, C., Lavery, P., Agellon, S. et al. The Generation of C-3α Epimer of 25-Hydroxyvitamin D and Its Biological Effects on Bone Mineral Density in Adult Rodents. Calcif Tissue Int 96, 453–464 (2015). https://doi.org/10.1007/s00223-015-9973-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-015-9973-9