Abstract
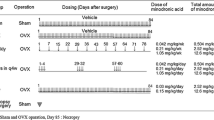
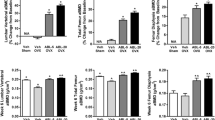
The purpose of this study was to evaluate the effects of withdrawal of minodronic acid (MIN) for 3 months after 12 months of treatment in ovariectomized (OVX) rat. OVX rats were orally treated with MIN (6, 30, and 150 µg/kg/day) for 12 months and necropsied on the day after the last dosing or following 3 months of withdrawal. Lumbar and femoral BMD were decreased in OVX controls. MIN dose-dependently increased BMD. Withdrawal eliminated the effect of MIN on BMD loss after treatment at 6 µg/kg, but not after treatment at 30 and 150 µg/kg. In MIN-treated rats, trabecular thinning occurred during withdrawal after treatment at 6 µg/kg, but the trabecular microstructure was maintained at 30 and 150 µg/kg. In a mechanical test of the femoral diaphysis, stiffness of in OVX controls was decreased but ultimate load was similar to that in sham after withdrawal. MIN increased ultimate load and stiffness, but endosteal length decreased after withdrawal. Suppression of bone turnover by MIN based on bone turnover markers and histomorphometric indices was attenuated by withdrawal after treatment at 6 and 30 µg/kg and partially at 150 µg/kg. The MIN concentration in the humerus decreased during withdrawal, and half-life at 30 µg/kg was shorter than that at 150 µg/kg. These results show that the antiresorptive action of MIN was dose-dependently attenuated by 3-month withdrawal in a rat OVX model. An absence of BMD increase was only observed at a low dose but decreases in antiresorptive activity occurred over a wide dose range.

Similar content being viewed by others
References
Black DM, Cummings SR, Karpf DB, Cauley JA, Thompson DE, Nevitt MC, Bauer DC, Genant HK, Haskell WL, Marcus R, Ott SM, Torner JC, Quandt SA, Reiss TF, Ensrud KE (1996) Fracture Intervention Trial Research Group Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Lancet 348:1535–1541
Reginster J, Minne HW, Sorensen OH, Hooper M, Roux C, Brandi ML, Lund B, Ethgen D, Pack S, Roumagnac I, Eastell R. Vertebral Efficacy with Risedronate Therapy (VERT) Study Group (2000) Randomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Osteoporos Int 11:83–91
Delmas PD, Recker RR, Chesnut CH 3rd, Skag A, Stakkestad JA, Emkey R, Gilbride J, Schimmer RC, Christiansen C (2004) Daily and intermittent oral ibandronate normalize bone turnover and provide significant reduction in vertebral fracture risk: results from the BONE study. Osteoporos Int 15:792–798
Black DM, Kelly MP, Genant HK, Palermo L, Eastell R, Bucci-Rechtweg C, Cauley J, Leung PC, Boonen S, Santora A, de Papp A, Bauer DC, Fracture Intervention Trial Steering; Committee; HORIZON Pivotal Fracture Trial Steering Committee (2010) Bisphosphonates and fractures of the subtrochanteric or diaphyseal femur. N Engl J Med 362:1761–1771
Matsumoto T, Hagino H, Shiraki M, Fukunaga M, Nakano T, Takaoka K, Morii H, Ohashi Y, Nakamura T (2009) Effect of daily oral minodronate on vertebral fractures in Japanese postmenopausal women with established osteoporosis: a randomized placebo-controlled double-blind study. Osteoporos Int 20:1429–1437
Rogers MJ, Crockett JC, Coxon FP, Mönkkönen J (2011) Biochemical and molecular mechanisms of action of bisphosphonates. Bone 49:34–41
Fleisch H (2000) Bisphosphonates in bone disease from the laboratory to the patient. Academic Press, San Diego
Ohno K, Mori K, Orita M, Takeuchi M (2011) Computational insights into binding of bisphosphates to farnesyl pyrophosphate synthase. Curr Med Chem 18:220–233
Hagino H, Shiraki M, Fukunaga M, Nakano T, Takaoka K, Ohashi Y, Nakamura T, Matsumoto T (2012) Three years of treatment with minodronate in patients with postmenopausal osteoporosis. J Bone Miner Metab 30:439–446
Hagino H, Nishizawa Y, Sone T, Morii H, Taketani Y, Nakamura T, Itabashi A, Mizunuma H, Ohashi Y, Shiraki M, Minamide T, Matsumoto T (2009) A double-blinded head-to-head trial of minodronate and alendronate in women with postmenopausal osteoporosis. Bone 44:1078–1084
Rizzoli R, Greenspan SL, Bone G 3rd, Schnitzer TJ, Watts NB, Adami S, Foldes AJ, Roux C, Levine MA, Uebelhart B, Santora AC 2nd, Kaur A, Peverly CA, Orloff JJ, Alendronate Once-Weekly Study Group (2002) Two-year results of once-weekly administration of alendronate 70 mg for the treatment of postmenopausal osteoporosis. J Bone Miner Res 17:1988–1996
Okazaki R, Hagino H, Ito M, Sone T, Nakamura T, Mizunuma H, Fukunaga M, Shiraki M, Nishizawa Y, Ohashi Y, Matsumoto T (2012) Efficacy and safety of monthly oral minodronate in patients with involutional osteoporosis. Osteoporos Int 23:1737–1745
Black DM, Delmas PD, Eastell R, Reid IR, Boonen S, Cauley JA, Cosman F, Lakatos P, Leung PC, Man Z, Mautalen C, Mesenbrink P, Hu H, Caminis J, Tong K, Rosario-Jansen T, Krasnow J, Hue TF, Sellmeyer D, Eriksen EF, Cummings SR, HORIZON Pivotal Fracture Trial (2007) Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med 356:1809–1822
Ensrud KE, Barrett-Connor EL, Schwartz A, Santora AC, Bauer DC, Suryawanshi S, Feldstein A, Haskell WL, Hochberg MC, Torner JC, Lombardi A, Black DM, Fracture Intervention Trial Long-Term Extension Research Group (2004) Randomized trial of effect of alendronate continuation versus discontinuation in women with low BMD: results from the Fracture Intervention Trial long-term extension. J Bone Miner Res 19:1259–1269
Black DM, Reid IR, Boonen S, Bucci-Rechtweg C, Cauley JA, Cosman F, Cummings SR, Hue TF, Lippuner K, Lakatos P, Leung PC, Man Z, Martinez RL, Tan M, Ruzycky ME, Su G, Eastell R (2012) The effect of 3 versus 6 years of zoledronic acid treatment of osteoporosis: a randomized extension to the HORIZON-Pivotal Fracture Trial (PFT). J Bone Miner Res 27:243–254
Watts NB, Chines A, Olszynski WP, McKeever CD, McClung MR, Zhou X, Grauer A (2008) Fracture risk remains reduced one year after discontinuation of risedronate. Osteoporos Int 19(3):365–372
Eastell R, Hannon RA, Wenderoth D, Rodriguez-Moreno J, Sawicki A (2011) Effect of stopping risedronate after long-term treatment on bone turnover. J Clin Endocrinol Metab 96:3367–3373
McClung M, Harris ST, Miller PD, Bauer DC, Davison KS, Dian L, Hanley DA, Kendler DL, Yuen CK, Lewiecki EM (2013) Bisphosphonate therapy for osteoporosis: benefits, risks, and drug holiday. Am J Med 126:13–20
Seedor JG, Quartuccio HA, Thompson DD (1991) The bisphosphonate alendronate (MK-217) inhibits bone loss due to ovariectomy in rats. J Bone Miner Res 6:339–346
Bauss F, Russell RG (2004) Ibandronate in osteoporosis: preclinical data and rationale for intermittent dosing. Osteoporos Int 15:423–433
Hornby SB, Evans GP, Hornby SL, Pataki A, Glatt M, Green JR (2003) Long-term zoledronic acid treatment increases bone structure and mechanical strength of long bones of ovariectomized adult rats. Calcif Tissue Int 72:519–527
Kimoto A, Tanaka M, Nozaki K, Mori M, Fukushima S, Mori H, Shiroya T, Nakamura T (2013) Intermittent minodronic acid treatment with sufficient bone resorption inhibition prevents reduction in bone mass and strength in ovariectomized rats with established osteopenia comparable with daily treatment. Bone 55:189–197
Tanaka M, Mori H, Kayasuga R, Ochi Y, Yamada H, Kawada N, Kawabata K (2014) Effect of intermittent and daily regimens of minodronic Acid on bone metabolism in an ovariectomized rat model of osteoporosis. Calcif Tissue Int 95:166–173
Fuchs RK, Phipps RJ, Burr DB (2008) Recovery of trabecular and cortical bone turnover after discontinuation of risedronate and alendronate therapy in ovariectomized rats. J Bone Miner Res 23:1689–1697
Wronski TJ, Dann LM, Qi H, Yen CF (1993) Skeletal effects of withdrawal of estrogen and diphosphonate treatment in ovariectomized rats. Calcif Tissue Int 53:210–216
Ebetino FH, Hogan AM, Sun S, Tsoumpra MK, Duan X, Triffitt JT, Kwaasi AA, Dunford JE, Barnett BL, Oppermann U, Lundy MW, Boyde A, Kashemirov BA, McKenna CE, Russell RG (2011) The relationship between the chemistry and biological activity of the bisphosphonates. Bone 49:20–33
Cremers S, Papapoulos S (2011) Pharmacology of bisphosphonates. Bone 49:642–649
Mori H, Tanaka M, Kayasuga R, Kishikawa K, Ito M (2008) Efficacy of preventive and therapeutic treatments with minodronic acid on ovariectomized rat model of osteoporosis. Clin Pharmacol Ther (Japanese) 18:S-33–S-48
Tanaka M, Mori H, Kayasuga R, Ochi Y, Kawada N, Yamada H, Kishikawa K (2008) Long-term minodronic acid (ONO-5920/YM529) treatment suppresses increased bone turnover, plus prevents reduction in bone mass and bone strength in ovariectomized rats with established osteopenia. Bone 43:894–900
Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR (1987) Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res 2:595–610
Usui T, Kawakami R, Watanabe T, Higuchi S (1994) Sensitive determination of a novel bisphosphonate, YM529, in plasma, urine and bone by high-performance liquid chromatography with fluorescence detection. J Chromatogr 652:67–72
Kasra M, Vanin CM, MacLusky NJ, Casper RF, Grynpas MD (1997) Effects of different estrogen and progestin regimens on the mechanical properties of rat femur. J Orthop Res 15:118–123
Fox J, Miller MA, Newman MK, Recker RR, Turner CH, Smith SY (2007) Effects of daily treatment with parathyroid hormone 1–84 for 16 months on density, architecture and biomechanical properties of cortical bone in adult ovariectomized rhesus monkeys. Bone 41:321–330
Nagira K, Hagino H, Kameyama Y, Teshima R (2013) Effects of minodronate on cortical bone response to mechanical loading in rats. Bone 53:277–283
Ito M, Nishida A, Nakamura T, Uetani M, Hayashi K (2002) Differences of three-dimensional trabecular microstructure in osteopenic rat models caused by ovariectomy and neurectomy. Bone 30:594–598
Iwamoto J, Seki A, Sato Y (2014) Effect of combined teriparatide and monthly minodronic acid therapy on cancellous bone mass in ovariectomized rats: a bone histomorphometry study. Bone 64:88–94
Usui T, Kamimura H (2008) Pharmacokinetics of minodronic acid hydrate, a novel bisphosphonate, in rats and dogs. Clin Pharmacol Ther (Japansese) 18:S-129–S-142
Khan SA, Kanis JA, Vasikaran S, Kline WF, Matuszewski BK, McCloskey EV, Beneton MN, Gertz BJ, Sciberras DG, Holland SD, Orgee J, Coombes GM, Rogers SR, Porras AG (1997) Elimination and biochemical responses to intravenous alendronate in postmenopausal osteoporosis. J Bone Miner Res 12:1700–1707
Germann PG, Ockert D, Heinrichs M (1998) Pathology of the oropharyngeal cavity in six strains of rats: predisposition of Fischer 344 rats for inflammatory and degenerative changes. Toxicol Pathol 26:283–289
Maita K, Hirano M, Harada T, Mitsumori K, Yoshida A, Takahashi K, Nakashima N, Kitazawa T, Enomoto A, Inui K, Shirasu Y (1986) An Outbreak of Esophagectasis in F344 Rats Jpn. J Vet Sci 48:i539–i546
Naito Y, Kuroda M, Uchiyama K, Mizushima K, Akagiri S, Takagi T, Handa O, Kokura S, Yoshida N, Ichikawa H, Yoshikawa T (2006) Inflammatory response of esophageal epithelium in combined-type esophagitis in rats: a transcriptome analysis. Int J Mol Med 18:821–828
Varma M, Chai JK, Meguid MM, Laviano A, Gleason JR, Yang ZJ, Blaha V (1999) Effect of estradiol and progesterone on daily rhythm in food intake and feeding patterns in Fischer rats. Physiol Behav 68:99–107
Yamauchi H, Kushida K, Yamazaki K, Inoue T (1995) Assessment of spine bone mineral density in ovariectomized rats using DXA. J Bone Miner Res 10:1033–1039
Acknowledgments
We thank Soh-ichi Takashima and Norio Muto (Ina Research Inc.) for their expertise and technical support in animal care and assessment. We are also grateful to Chihiro Hasegawa for his critical review of the manuscript.
Conflict of Interest
Makoto Tanaka, Hiroshi Mori, and Kazuhito Kawabata are research scientists at Ono Pharmaceutical Co., Ltd. Minodronic acid was launched by Ono Pharmaceutical Co., Ltd. and Astellas Pharma Inc.
Human and Animal Rights and Informed Consent
This study was approved by Ina Research Inc. (Nagano, Japan) and performed in accordance with the by-rules of the company, including all ethical aspects related to animal treatment.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tanaka, M., Mori, H. & Kawabata, K. Attenuation of Antiresorptive Action in Withdrawal of Minodronic Acid for Three Months After Treatment for Twelve Months in Ovariectomized Rats. Calcif Tissue Int 97, 402–411 (2015). https://doi.org/10.1007/s00223-015-0017-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-015-0017-2