Abstract
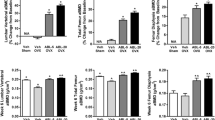
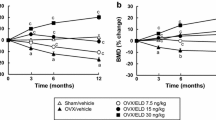
The goal of the study was to compare the effects of minodronic acid on bone mineral density (BMD) and bone turnover in a rat ovariectomized (OVX) osteoporosis model, using two intermittent treatment regimens (weekly and 4 continuous days every 4 weeks) and a daily regimen. Female F344 rats (age 14 weeks) underwent ovariectomy or a sham operation. Minodronic acid was orally administered at 0.042, 0.21, and 1.05 mg/kg in the intermittent regimens, and at 0.03 and 0.15 mg/kg in the daily regimen for 12 weeks from the day after surgery. Minodronic acid dose-dependently ameliorated the decreases in areal BMD of the lumbar vertebrae and femur, and volumetric BMD of total and trabecular bone in the distal femur. Minodronic acid also suppressed the increase in urinary deoxypyridinoline levels and reduced serum osteocalcin levels. In bone histomorphometry, all three minodronic acid regimens suppressed OVX-induced increases in bone turnover at the tissue level and ameliorated all structural indices, except that an effect on trabecular thickness only occurred with daily treatment. In conclusion, minodronic acid administered weekly or for 4 continuous days every 4 weeks suppressed increased bone resorption and BMD to a similar extent to that of a similar total dose given daily in a rat OVX model.

Similar content being viewed by others
References
Black DM, Cummings SR, Karpf DB, Cauley JA, DE Thompson, Nevitt MC, Bauer DC, Genant HK, Haskell WL, Marcus R, Ott SM, Torner JC, Quandt SA, Reiss TF, Ensrud KE, Fracture Intervention Trial Research Group (1996) Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Lancet 348:1535–1541. doi:10.1016/S0140-6736(96)07088-2
Reginster J, Minne HW, Sorensen OH, Hooper M, Roux C, Brandi ML, Lund B, Ethgen D, Pack S, Roumagnac I, Eastell R, Vertebral Efficacy with Risedronate Therapy (VERT) Study Group (2000) (2000) Randomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Osteoporos Int 11:83–91. doi:10.1007/s001980050010
Delmas PD, Recker RR, Chesnut CH 3rd, Skag A, Stakkestad JA, Emkey R, Gilbride J, Schimmer RC, Christiansen C (2004) Daily and intermittent oral ibandronate normalize bone turnover and provide significant reduction in vertebral fracture risk: results from the BONE study. Osteoporos Int 15:792–798. doi:10.1007/s00198-004-1602-9
Black DM, Kelly MP, Genant HK, Palermo L, Eastell R, Bucci-Rechtweg C, Cauley J, Leung PC, Boonen S, Santora A, de Papp A, Bauer DC, Fracture Intervention Trial Steering Committee; HORIZON Pivotal Fracture Trial Steering Committee (2010) Bisphosphonates and fractures of the subtrochanteric or diaphyseal femur. N Engl J Med 362:1761–1771. doi:10.1056/NEJMoa1001086
Matsumoto T, Hagino H, Shiraki M, Fukunaga M, Nakano T, Takaoka K, Morii H, Ohashi Y, Nakamura T (2009) Effect of daily oral minodronate on vertebral fractures in Japanese postmenopausal women with established osteoporosis: a randomized placebo-controlled double-blind study. Osteoporos Int 20:1429–1437. doi:10.1007/s00198-008-0816-7
Fleisch H (1998) Bisphosphonates: mechanisms of action. Endocr Rev 19:80–100
Ebetino FH, Hogan AM, Sun S, Tsoumpra MK, Duan X, Triffitt JT, Kwaasi AA, Dunford JE, Barnett BL, Oppermann U, Lundy MW, Boyde A, Kashemirov BA, McKenna CE, Russell RG (2011) The relationship between the chemistry and biological activity of the bisphosphonates. Bone 49:20–33. doi:10.1016/j.bone.2011.03.774
Takeuchi M, Sakamoto S, Kawamuki K, Kurihara H, Nakahara H, Isomura Y (1998) Studies on novel bone resorption inhibitors. II. Synthesis and pharmacological activities of fused aza-heteroarylbisphosphonate derivatives. Chem Pharm Bull 46:1703–1709
Dunford JE, Thompson K, Coxon FP, Luckman SP, Hahn FM, Poulter CD, Ebetino FH, Rogers MJ (2001) Structure-activity relationships for inhibition of farnesyl diphosphate synthase in vitro and inhibition of bone resorption in vivo by nitrogen-containing bisphosphonates. J Pharmacol Exp Ther 296:235–242
Ohno K, Mori K, Orita M, Takeuchi M (2011) Computational insights into binding of bisphosphates to farnesyl pyrophosphate synthase. Curr Med Chem 18:220–233. doi:10.2174/192986711794088335
Rizzoli R, Greenspan SL, Bone G III, Schnitzer TJ, Watts NB, Adami S, Foldes AJ, Roux C, Levine MA, Uebelhart B, Santora AC 2nd, Kaur A, Peverly CA, Orloff JJ, Alendronate Once-Weekly Study Group (2002) Two-year results of once-weekly administration of alendronate 70 mg for the treatment of postmenopausal osteoporosis. J Bone Miner Res 17:1988–1996. doi:10.1359/jbmr.2002.17.11.1988
Brown JP, Kendler DL, McClung MR, Emkey RD, Adachi JD, Bolognese MA, Li Z, Balske A, Lindsay R (2002) The efficacy and tolerability of risedronate once a week for the treatment of postmenopausal osteoporosis. Calcif Tissue Int 71:103–111. doi:10.1007/s00223-002-2011-8
Miller PD, McClung MR, Macovei L, Stakkestad JA, Luckey M, Bonvoisin B, Reginster JY, Recker RR, Hughes C, Lewiecki EM, Felsenberg D, Delmas PD, Kendler DL, Bolognese MA, Mairon N, Cooper C (2005) Monthly oral ibandronate therapy in postmenopausal osteoporosis: 1-year results from the MOBILE study. J Bone Miner Res 20:1315–1322. doi:10.1359/JBMR.050313
Hagino H, Nishizawa Y, Sone T, Morii H, Taketani Y, Nakamura T, Itabashi A, Mizunuma H, Ohashi Y, Shiraki M, Minamide T, Matsumoto T (2009) A double-blinded head-to-head trial of minodronate and alendronate in women with postmenopausal osteoporosis. Bone 44:1078–1084. doi:10.1016/j.bone.2009.02.016
Cotté FE, Fardellone P, Mercier F, Gaudin AF, Roux C (2010) Adherence to monthly and weekly oral bisphosphonates in women with osteoporosis. Osteoporos Int 21:145–155. doi:10.1007/s00198-009-0930-1
Roerholt C, Eiken P, Abrahamsen B (2009) Initiation of anti-osteoporotic therapy in patients with recent fractures: a nationwide analysis of prescription rates and persistence. Osteoporos Int 20:299–307. doi:10.1007/s00198-008-0651-x
Okazaki R, Hagino H, Ito M, Sone T, Nakamura T, Mizunuma H, Fukunaga M, Shiraki M, Nishizawa Y, Ohashi Y, Matsumoto T (2012) Efficacy and safety of monthly oral minodronate in patients with involutional osteoporosis. Osteoporos Int 23:1737–1745. doi:10.1007/s00198-011-1782-z
Usui T, Kawakami R, Watanabe T, Higuchi S (1994) Sensitive determination of a novel bisphosphonate, YM529, in plasma, urine and bone by high-performance liquid chromatography with fluorescence detection. J Chromatogr. 652:67–72
Kimoto A, Tanaka M, Nozaki K, Mori M, Fukushima S, Mori H, Shiroya T, Nakamura T (2013) Intermittent minodronic acid treatment with sufficient bone resorption inhibition prevents reduction in bone mass and strength in ovariectomized rats with established osteopenia comparable with daily treatment. Bone 55:189–197. doi:10.1016/j.bone.2008.07.242
Tanaka M, Mori H, Kayasuga R, Ochi Y, Kawada N, Yamada H, Kishikawa K (2008) Long-term minodronic acid (ONO-5920/YM529) treatment suppresses increased bone turnover, plus prevents reduction in bone mass and bone strength in ovariectomized rats with established osteopenia. Bone. 43(5):894–900. doi:10.1016/j.bone.2008.07.002
Monma Y, Funayama H, Mayanagi H, Endo Y (2004) Effects of weekly administrations of alendronate + clodronate on young mouse tibia: localized action at the proximal growth plate. Calcif Tissue Int 74:115–121. doi:10.1007/s00223-002-2156-5
Christiansen C, Tankó LB, Warming L, Moelgaard A, Christgau S, Qvist P, Baumann M, Wieczorek L, Hoyle N (2003) Dose dependent effects on bone resorption and formation of intermittently administered intravenous ibandronate. Osteoporos Int. 14:609–613. doi:10.1007/s00198-003-1409-0
Wronski TJ, Cintrón M, Dann LM (1988) Temporal relationship between bone loss and increased bone turnover in ovariectomized rats. Calcif Tissue Int 43:179–183
Engelke K, Kemmler W, Lauber D, Beeskow C, Pintag R, Kalender WA (2006) Exercise maintains bone density at spine and hip EFOPS: a 3-year longitudinal study in early postmenopausal women. Osteoporos Int. 17(1):133–142
Usui T, Kamimura H (2008) Pharmacokinetics of minodronic acid hydrate, a novel bisphonate, in rats and dogs. Clin Pharmacol Ther (Japansese) 18:S-129–S-142
Fleisch H (2000) Bisphosphonates in bone disease from the laboratory to the patient. Academic Press, San Diego
Acknowledgments
We thank H. Tsusaki (Shin Nippon Biomedical Laboratories, Ltd.) for their expertise and technical support in animal care and a BMD assessments, and staff of SkeleTech Inc. for performing histomorphometric analysis.
Human and Animal Rights and Informed Consent
This study was ethically approved by the Institutional Animal Care and Use Committee of Shin Nippon Biomedical Laboratories, Ltd. (Kagoshima, Japan) and performed in accordance with the criteria defined by the rules of the committee.
Author information
Authors and Affiliations
Corresponding author
Additional information
All authors are research scientists at Ono Pharmaceutical Co., Ltd. Minodronic acid was launched by Ono Pharmaceutical Co., Ltd. and Astellas Pharma Inc. in Japan. MT and HM are applicants for use patent of intermittent regimen.
Rights and permissions
About this article
Cite this article
Tanaka, M., Mori, H., Kayasuga, R. et al. Effect of Intermittent and Daily Regimens of Minodronic Acid on Bone Metabolism in an Ovariectomized Rat Model of Osteoporosis. Calcif Tissue Int 95, 166–173 (2014). https://doi.org/10.1007/s00223-014-9876-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-014-9876-1