Abstract
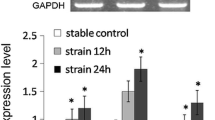
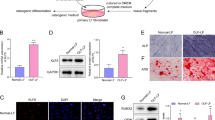
Ossification of the posterior longitudinal ligament of the spine (OPLL) is characterized by ectopic bone formation in the spinal ligaments. We previously reported that P2 purinoceptor Y1 (P2Y1) expression is elevated in the spinal ligament cells of OPLL patients, but the role of P2Y1 in the spinal ligament calcification process is unknown. To verify the hypothesis that P2Y1 expression causes ossification of the spinal ligaments, we forced expression of P2Y1 in spinal ligament cells obtained from OPLL and non-OPLL patients using a cytomegaloviral vector. The expression of mRNA and protein was investigated by quantitative real-time polymerase chain reaction and immunofluorescence staining, respectively. After transfection, bone morphogenetic protein-2 (BMP-2) and Sox9 mRNA expression was significantly increased in spinal ligament cells derived from OPLL patients (4.36- and 6.44-fold, respectively) compared with cells from non-OPLL patients (0.57- and 3.64-fold, respectively) 2 days after P2Y1 transient transfection. Furthermore, a statistically significant correlation was observed between BMP-2 and P2Y1 mRNA expression levels in cells obtained from OPLL patients but not from non-OPLL patients. Immunofluorescence analysis showed that BMP-2 and P2Y1 expression was increased in OPLL patients only, while Sox9 expression was increased in OPLL and non-OPLL patients. MRS2279, a selective P2Y1 antagonist, blocked the upregulation of Sox9 and BMP-2 after forced expression of P2Y1. Furthermore, 4 days after transient transfection of P2Y1, mineralization was observed only in spinal ligament cells from OPLL patients. These results suggest that P2Y1 expression plays an important role in ectopic bone formation in the spinal ligaments of OPLL patients.





Similar content being viewed by others
References
Trojan DA, Pouchot J, Pokrupa R, Ford RM, Adamsbaum C, Hill RO, Esdaile JM (1992) Diagnosis and treatment of ossification of the posterior longitudinal ligament of the spine. Am J Med 92:296–306
Tanaka T, Ikari K, Furushima K, Okada A, Tanaka H, Furukawa K, Yoshida K, Ikeda T, Ikegawa S, Hunt SC, Takeda J, Toh S, Harata S, Nakajima T, Inoue I (2003) Genomewide linkage and linkage disequilibrium analyses identify COL6A1, on chromosome 21, as the locus for ossification of the posterior longitudinal ligament of the spine. Am J Hum Genet 73:812–822
Kobashi G, Washio M, Okamoto K, Sasaki S, Yokoyama T, Miyake Y, Sakamoto N, Ohta K, Inaba Y, Tanaka H (2004) High body mass index after age 20 and diabetes mellitus are independent risk factors for ossification of the posterior longitudinal ligament of the spine in Japanese subjects: a case-control study in multiple hospitals. Spine 29:1006–1010
Akune T, Ogata N, Seichi A, Ohnishi I, Nakamura K, Kawaguchi H (2001) Insulin secretory response is positively associated with the extent of ossification of the posterior longitudinal ligament of the spine. J Bone Joint Surg Am 83:1537–1544
Furukawa K-I (2008) Pharmacological aspect of ectopic ossification in spinal ligament tissues. Pharmacol Ther 118:352–358
Iwasaki M, Kawaguchi Y, Kimura T, Yonenobu K (2002) Long-term results of expansive laminoplasty for ossification of the posterior longitudinal ligament of the cervical spine: more than 10 years follow up. J Neurosurg 96(2 Suppl):180–189
Kawaguchi H, Kurokawa T, Hoshino Y, Kawahara H, Ogata E, Matsumoto T (1992) Immunohistochemical demonstration of bone morphogenetic protein-2 and transforming growth factor-β in the ossification of the posterior longitudinal ligament of the cervical spine. Spine 17(3 Suppl):S33–S36
Sato R, Uchida K, Kobayashi S, Yayama T, Kokubo Y, Nakajima H, Takamura T, Bangirana A, Itoh H, Baba H (2007) Ossification of the posterior longitudinal ligament of the cervical spine: histopathological findings around the calcification and ossification front. J Neurosurg Spine 7:174–183
Tanno M, Furukawa K, Ueyama K, Harata S, Motomura S (2003) Uniaxial cyclic stretch induces osteogenic differentiation and synthesis of bone morphogenetic proteins of spinal ligament cells derived from patients with ossification of the posterior longitudinal ligaments. Bone 22:475–484
Ohishi H, Furukawa K, Iwasaki K, Ueyama K, Okada A, Motomura S, Harata S, Toh S (2003) Role of prostaglandin I2 in the gene expression induced by mechanical stress in spinal ligament cells derived from patients with ossification of the posterior longitudinal ligament. J Pharmacol Exp Ther 305:818–824
Iwasaki K, Furukawa K, Tanno M, Kusumi T, Ueyama K, Tanaka M, Kudo H, Toh S, Harata S, Motomura S (2004) Uni-axial cyclic stretch induces Cbfa1 expression in spinal ligament cells derived from patients with ossification of the posterior longitudinal ligament. Calcif Tissue Int 74:448–457
Iwasawa T, Iwasaki K, Sawada T, Okada A, Ueyama K, Motomura S, Harata S, Inoue I, Toh S, Furukawa K (2006) Pathophysiological role of endothelin in ectopic ossification of human spinal ligaments induced by mechanical stress. Calcif Tissue Int 79:422–430
Graff RD, Lazerowski ER, Banes AJ, Lee GM (2000) ATP release by mechanically loaded porcine chondrons in pellet culture. Arthritis Rheum 43:1571–1579
Romanello M, Pani B, Bicego M, D’Andrea P (2001) Mechanically induced ATP release from human osteoblastic cells. Biochem Biophys Res Commun 289:1275–1281
Riddle RC, Taylor AF, Rogers JR, Donahue HJ (2007) ATP release mediates fluid flow-induced proliferation of human bone marrow stromal cells. J Bone Miner Res 22:589–600
Ralevic V, Burnstock G (1998) Receptors for purines and pyrimidines. Pharmacol Rev 50:413–492
North RA (2002) Molecular physiology of P2X receptors. Physiol Rev 82:1013–1067
Burnstock G (2004) Introduction: P2 receptors. Curr Top Med Chem 4:793–803
von Kügelgen I (2006) Pharmacological profiles of cloned mammalian P2Y-receptor subtypes. Pharmacol Ther 110:415–432
Kanno T, Takahashi T, Tsujusawa T, Ariyoshi W, Nishihara T (2007) Mechanical stress-mediated Runx2 activation is dependent on Ras/ERK1/2 MAPK signaling in osteoblasts. J Cell Biochem 101:1266–1277
Liu D, Genetos DC, Shao Y, Geist DJ, Li J, Ke HZ, Turner CH, Duncan RL (2008) Activation of extracellular-signal regulated kinase (ERK1/2) by fluid shear is Ca2+- and ATP-dependent in MC3T3-E1 osteoblasts. Bone 42:644–652
Qi MC, Hu J, Zou SJ, Chen HQ, Zhou HX, Han LC (2008) Mechanical strain induces osteogenic differentiation: Cbfa1 and Ets-1 expression in stretched rat mesenchymal stem cells. Int J Oral Maxillofac Surg 37:453–458
Sawada T, Kishiya M, Kanemaru K, Seya K, Yokoyama T, Ueyama K, Motomura S, Toh S, Furukawa K (2008) Possible role of extracellular nucleotides in ectopic ossification of human spinal ligaments. J Pharmacol Sci 106:152–161
Shen J, DiCorleto PE (2008) ADP stimulates human endothelial cell migration via P2Y1 nucleotide receptor-mediated mitogen-activated protein kinase pathways. Circ Res 102:448–456
Kobayashi K, Fukuoka T, Yamanaka H, Dai Y, Obata K, Tokunaga A, Noguchi K (2006) Neurons and glial cells differentially express P2Y receptor mRNAs in the rat dorsal root ganglion and spinal cord. J Comp Neurol 498:443–454
Baurand A, Gachet C (2003) The P2Y1 receptor as a target for new antithrombotic drugs: a review of the P2Y1 antagonist MRS-2179. Cardiovasc Drug Rev 21:67–76
Léon C, Freund M, Latchoumanin O, Farret A, Petit P, Cazenave JP, Gachet C (2005) The P2Y1 receptor is involved in the maintenance of glucose homeostasis and insulin secretion in mice. Purinergic Signal 1:145–151
Orriss IR, Knight GE, Ranasinghe S, Burnstock G, Arnett TR (2006) Osteoblast responses to nucleotides increase during differentiation. Bone 39:300–309
Waldo GL, Harden TK (2004) Agonist binding and Gq-stimulating activities of the purified human P2Y1 receptor. Mol Pharmacol 65:426–436
Alvarenga EC, Rodrigues R, Caricati-Neto A, Silva-Filho FC, Paredes-Gamero EJ, Ferreira AT (2010) Low-intensity pulsed ultrasound-dependent osteoblast proliferation occurs by via activation of the P2Y receptor: role of the P2Y1 receptor. Bone 46:355–362
Wozney JM, Rosen V, Celeste AJ, Mitsock LM, Whitters MJ, Kriz RW, Hewick RM, Wang EA (1988) Novel regulators of bone formation: molecular clones and activities. Science 242:1528–1534
Liu T, Gao Y, Sakamoto K, Minamizato T, Furukawa K, Tsukazaki T, Shibata Y, Bessho K, Komori T, Yamaguchi A (2007) BMP-2 promotes differentiation of osteoblasts and chondroblasts in Runx2-deficient cell lines. J Cell Physiol 211:728–735
Matsubara T, Kida K, Yamaguchi A, Hata K, Ichida F, Meguro H, Aburatani H, Nishimura R, Yoneda T (2008) BMP2 regulates Osterix through Msx2 and Runx2 during osteoblast differentiation. J Biol Chem 283:29119–29125
Moon SH, Park SR, Kim H, Kwon UH, Kim KH, Kim HS, Lee HM (2004) Biologic modification of ligamentum flavum cells by marker gene transfer and recombinant human bone morphogenetic protein-2. Spine 29:960–965
Kon T, Yamazaki M, Tagawa M, Goto S, Terakado A, Moriya H, Fujimura S (1997) Bone morphogenetic protein-2 stimulates differentiation of cultured spinal ligament cells from patients with ossification of the posterior longitudinal ligament. Calcif Tissue Int 60:291–296
Akiyama H, Chaboissier MC, Martin JF, Schedl A, de Crombrugghe B (2002) The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6. Genes Dev 16:2813–2828
Zehentner BK, Dony C, Burtscher H (1999) The transcription factor Sox9 is involved in BMP-2 signaling. J Bone Miner Res 14:1734–1741
Pan Q, Yu Y, Chen Q, Li C, Wu H, Wan Y, Ma J, Sun F (2008) Sox9, a key transcription factor of bone morphogenetic protein-2-induced chondrogenesis, is activated through BMP pathway and a CCAAT box in the proximal promoter. J Cell Physiol 217:228–241
Bais MV, Wigner N, Young M, Toholka R, Graves DT, Morgan EF, Gerstenfeld LC, Einhorn TA (2009) BMP2 is essential for post natal osteogenesis but not for recruitment of osteogenic stem cells. Bone 45:254–266
Amano K, Hata K, Sugita A, Takigawa Y, Ono K, Wakabayashi M, Kogo M, Nishimura R, Yoneda T (2009) Sox9 family members negatively regulate maturation and calcification of chondrocytes through up-regulation of parathyroid hormone-related protein. Mol Biol Cell 20:4541–4551
Acknowledgments
The authors acknowledge Drs. Kazumasa Ueyama, Naoki Echigoya, Akio Sannohe, Toshihiro Tanaka, Toru Yokoyama, Takuya Numasawa, Kanichiro Wada, Yoshihito Yamasaki, Tomoyuki Irie, and Taisuke Nitobe for their contributions to sample collection. We also thank Yasuyuki Ishibashi and Kazuhiko Seya of the Hirosaki University Graduate School of Medicine for their valuable suggestions. This work was supported by grants-in-aid from the Health Labour Sciences Research of Japan.
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors have stated that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Tanaka, S., Kudo, H., Asari, T. et al. P2Y1 Transient Overexpression Induced Mineralization in Spinal Ligament Cells Derived from Patients with Ossification of the Posterior Longitudinal Ligament of the Cervical Spine. Calcif Tissue Int 88, 263–271 (2011). https://doi.org/10.1007/s00223-010-9456-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-010-9456-y