Abstract
During amelogenesis, extracellular matrix proteins interact with growing hydroxyapatite crystals to create one of the most architecturally complex biological tissues. The process of enamel formation is a unique biomineralizing system characterized first by an increase in crystallite length during the secretory phase of amelogenesis, followed by a vast increase in crystallite width and thickness in the later maturation phase when organic complexes are enzymatically removed. Crystal growth is modulated by changes in the pH of the enamel microenvironment that is critical for proper enamel biomineralization. Whereas the genetic bases for most abnormal enamel phenotypes (amelogenesis imperfecta) are generally associated with mutations to enamel matrix specific genes, mutations to genes involved in pH regulation may result in severely affected enamel structure, highlighting the importance of pH regulation for normal enamel development. This review summarizes the intra- and extracellular mechanisms employed by the enamel-forming cells, ameloblasts, to maintain pH homeostasis and, also, discusses the enamel phenotypes associated with disruptions to genes involved in pH regulation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Tooth enamel is formed by tightly packed hydroxyapatite-like (Hap-like) crystals creating the hardest and one of the most architecturally complex biological tissues [1, 2]. However, during its development, enamel is mostly composed of soft gel-like matrix proteins secreted by specific enamel cells known as ameloblasts. The environment surrounding the crystals and the chemical processes involved in their growth are exquisitely regulated by the ameloblasts [3]. Crystal growth and secretion of organic matrix proteins are a continuous and near-synchronous process. When the appositional growth of enamel has been completed, the organic components in the tissue are enzymatically removed [4]. The spaces that originally contained water and matrix proteins are then filled by the crystals, which vastly increase in width and thickness at this point, leaving almost no spaces between the enamel crystallites when enamel is fully mature [1].
The process of enamel formation requires the tight control of extracellular pH and bicarbonate concentration [1, 5]. Crystal growth and proteinase activity in the extracellular space are thought to be pH-dependent phenomena requiring that cellular transport processes controlling the extracellular fluid pH be coordinated in a highly controlled fashion [1, 6]. However, as will be discussed later, it is not yet clear whether gene expression (i.e. bicarbonate secretion by ameloblasts) generates the necessary local conditions for crystal growth or if gene expression acts in response to changing conditions during crystal formation. This review summarizes previous work on regulation of pH during amelogenesis, focusing on the known genes expressed by ameloblasts whose function is thought to be involved in pH homeostasis.
Ameloblast Function and Amelogenesis
Ameloblasts cells perform a variety of biological roles, changing their cellular organization and function in a complex fashion throughout their lifespan. Ameloblasts synthesize and secrete a host of enamel matrix proteins within spatially and temporally constrained domains. These ameloblast products are actively involved in regulating the structural development of enamel (see below). The role of ameloblasts as pH regulators is perhaps less well studied, but recent advances are now allowing for the construction of a mechanistic model for maintenance of pH homeostasis by ameloblasts during enamel formation (e.g., Refs. [7, 8]) that we expand on in the next sections.
Amelogenesis is generally subdivided into three main functional phases, commonly referred to as the presecretory, secretory, and maturation stages [9]. These stages can be largely characterized by the morphology of the ameloblast cells, as well as by the appearance of the extracellular matrix they are associated with. During the presecretory stage, ameloblasts differentiate, change polarity, develop a junctional complex at their new apex, and assemble an extensive protein synthetic apparatus. Secretory ameloblasts then become tall columnar cells with a polarized organization. The nucleus and mitochondria are found at the basal pole of the cells, whereas the endoplasmic reticulum and Golgi complex are positioned near the apical (secretory) pole. The apical pole of secretory ameloblasts is characterized by the presence of an extended cell process called the Tomes’ process that plays an important role in organizing the enamel crystals into a complex network of rod (prism) and interrod enamel. During the secretory stage, ameloblasts create the full thickness of the enamel by matrix apposition, but this matrix remains only partly mineralized until the ensuing maturation stage [4, 10].
Prior to the maturation phase of the enamel matrix, ameloblasts undergo cellular changes that involve a decrease in height, a reduction in protein synthesizing organelles, and the loss of their apical cell extension (Tomes’ process) [11, 12], although high levels of lipids detected in maturation may be indicative of Tomes’ process remnants [13]. Interestingly, recent studies have elucidated that during maturation, a number of extracellular proteins are secreted by ameloblasts, and whereas no evident appositional growth of the enamel tissue takes place at that point, ameloblasts remain active secretory cells. Some of these secreted products are proteolytic enzymes whose function is to remove organic matrix [4, 14, 15]. Some others, including amelotin [16, 17] and Apin/ODAM [18], accumulate at the interface between the maturing enamel and the ameloblasts. They may mediate adhesion of the cells to the enamel surface, thereby creating a restricted environment for proper matrix removal and crystal growth to occur. It is yet unclear whether crystal growth stimulates removal of proteins or if protein removal is necessary for crystal growth [10, 19]. Furthermore, during the maturation stage, ameloblasts change morphology in a unique series of modulations from a ruffle-ended shape characterized by infolded plasma membranes at the apical end, to a smooth-ended shape [20–23]. Ruffle-ended ameloblasts typically are tightly bound by junctional complexes at their apices and show considerable endocytotic activity. On the other hand, smooth-ended ameloblasts show little endocytotic activity and have no apical tight junctions, making them leaky to small proteins and molecules. It should be pointed out that how or what triggers ameloblasts to switch between ruffle- and smooth-ended functional stages remains unknown, but the ruffle-ended phase predominates during maturation [1, 24]. It is also noteworthy that ameloblasts change morphology in coordinated groups noticeably appearing as bands of similar morphology around the circumference of the crown [20, 25, 26]. These waves of ameloblast modulations seem to play a role in pH regulation and bicarbonate transport as discussed later.
Enamel Microstructure and Crystal Growth
The main structural feature of mature enamel is the enamel prism or enamel rod, each containing thousands of crystallites arranged in bundles [27]. Enamel rods are separated from one another by interrod enamel, which is also formed by crystallites. The differences between rod and interrod enamel reflect different orientations of crystallites [27]. At the rod sites, very long crystallites are found parallel to each other. These crystallites develop at the apicalmost portion of the Tomes’ process, which is inclined with respect to the long axis of the cell. In contrast, the crystals constituting the interrod enamel are formed at the proximal portion of the Tomes’ process. Each rod is thought to reflect the path of a single ameloblast as it moves from the dentinoenamel junction (DEJ) to the outer surface of the enamel [27]. Enamel growth rates vary depending on the species and the anatomical region of the tooth crown in which these are measured [28]. For instance, mouse enamel has the fast growth average of 13.5 μm a day [1], whereas human enamel grows at an increasing rate from inner to outer enamel, ranging from ~2.8 μm at the start of secretion near the DEJ to ~5.2 μm at the outer areas in the occlusal enamel [28].
The first stage of crystal formation is nucleation, which can be defined as a “chain of collision events leading to the formation of a stable cluster of ions capable of survival and growth as a crystal” [29]. Enamel crystallites first form at the DEJ as ribbon-like structures only a few nanometers across, uniform in size, and parallel to each other along the c-axis [30, 31]. The length of crystals in fully formed enamel, however, likely nears that of the entire thickness of the enamel layer [32].
The atomic structure of enamel crystals is a variation of the classic hydroxyapatite (Hap) lattice in that it incorporates other types of ions (i.e., carbonates), and thus it is better described as a nonstochiometric carbonated calcium hydroxyapatite [33–35]. It has been argued that for every unit cell, or the minimum repeating structure of hydroxyapatite crystals that are formed, ~8 H+ protons are released in the extracellular environment, lowering the pH [1]. Simmer and Fincham [35] suggested that these free protons could reverse the deposition of ions on the crystal surface while increasing the deposition of crystal growth inhibitors such as calcium phosphate. These free protons may diffuse away or alternatively, enamel cells may use acid–base transport systems to regulate the extracellular pH [36]. The main extracellular buffering mechanism used by ameloblasts appears to be the bicarbonate buffer system [1]. Compared with other tissues, such as bone and dentine, enamel has a higher acid loading capacity [37, 38].
pH Changes During Amelogenesis
Earlier works on the analysis of extra- and intracellular pH on the developing mouse dentition used autoradiography, injecting [14C]DMO (dimethyloxazolidinedione) compound in 10- to 19-day-old C57/Bl/6 mice [39]. This compound is distributed throughout the body tissues, depending on local concentrations of water and pH. [14C]DMO concentrations increase in areas of high pH [39]. Rat maxillary incisors were divided into four regions of study, from the mature to the developing end of the crown. Higher pH levels were detected at the mature end (pH 8.0–8.5), whereas the less calcified zones of the crown showed a lower pH (pH 7.3−7.4). Unlike the variation in extracellular pH, the intracellular pH of ameloblasts remained between 7.2 and 7.1. Lyman and Waddell [39] suggested that such changes in enamel extracellular pH were related to calcification levels, as the binding of calcium ions to protein matrix creates a high local pH. This high pH permits an accumulation of PO4 3− and OH− ions to levels that permit the initiation of crystal nucleation.
The glyoxal bis (2-hydroxyanil) method (GBHA)—a calcium-chelator dye—was first used by Takano et al. [40] to determine pH differences in tooth enamel. This study showed bands of GBHA red staining, which marks alkaline conditions, on maturation-stage surface enamel devoid of the enamel organ in whole bovine, dog, monkey, and rat incisors. In a separate experiment, after perfusion, rat and monkey incisors were extracted and the saggital half of each tooth was cleaned off the enamel organ while the other half was left intact [40]. The samples were immersed in GBHA, and red bands appeared on the half of the crown devoid of enamel organ. These bands were used to mark their location on the half containing enamel organ in order to examine ameloblast morphology from histological sections. Ameloblasts under the GBHA bands showed a smooth-ended morphology under the light microscope, indicating that alkaline conditions dominate ameloblasts during the smooth-ended phase. It was also shown that autoradiographic analysis of 45Ca-labeled rat incisors displayed a distribution pattern matching the GBHA staining, suggesting that Ca entry into the enamel area increased during smooth-ended transitions, as previously observed [21, 22, 41]. It was then concluded that it is likely that the maturation process of ameloblasts represents a common mechanism of enamel formation in mammals, and therefore, pH conditions similarly change during this phase.
Following these findings, Sasaki et al. [5] used three different pH indicator solutions to cross-reference details of pH changes in unerupted whole bovine incisors after removal of the enamel organ. Results of that study showed alternating bands of acidic to neutral extracellular pH along the crown. The enamel corresponding to acidic or neutral zones was then carefully removed and suspended in distilled deionized water. The resulting pH was measured from the supernatants using a glass-electrode pH meter. In addition, a number of halved incisors were stained with GBHA and a pH indicator mixture to perform an overall cross-checking of each pH indicator for each zone of the incisors. Results from using a variety of pH staining solutions indicated an alternating and matching pattern of extracellular pH conditions ranging from acidic (pH 5.5–6.0) to neutral (pH ~ 7.2) zones. Sasaki et al. [5] labeled the acidic zone located in the occlusal half of the crown as “forming or maturing enamel” and hypothesized that the acidic conditions related to the release of protons by forming crystals. Those authors proposed that physiological neutralizing conditions are required in vivo during ameloblast shift from smooth- to ruffle-ended. Interestingly, this study also suggested that an unnamed proteinase found in the acidic enamel zone was pH dependent, with optimal activity at ~pH 6.0 [5].
More recently, Tagaki and collaborators investigated changes in pH on bovine incisors and its relationship with crystal growth and carbonate levels [6]. Developing bovine incisors were stained with pH indicator solutions identifying four different and alternating stages of acidic and neutral pH along the crown, with the first neutral stage located near the root. The erupted portion of the crown, the occlusal enamel, was used as a standard. Samples of each of the alternating zones in the developing enamel were mechanically extracted, and proteins were purified from the supernatants. Results of that study showed that neutral zones of enamel were characterized by the presence of full molecular weight forms of amelogenin and enamelin, as shown in SDS-PAGE gel electrophoresis. In acidic zones, a low molecular weight form of these proteins was detected. The authors interpreted this difference as possible enzymatic processing of enamel matrix, given that a low pH may activate an enamel proteinase previously identified [5]. Furthermore, it was shown that the composition of crystals changed between the alternating acidic and neutral stages. Inductively coupled plasma (ICP) emission spectrometry showed that ratios of (Ca + Mg)/P changed in the alternating acidic-neutral phases along the crown, increasing under acidic conditions compared to the preceding neutral phase [6]. Erupted enamel had a higher (Ca + Mg)/P ratio than either the acidic or the neutral unerupted enamel.
Smith et al. [42] investigated the role that the pH of the aqueous fluid present in enamel during the secretory and maturation phases plays in controlling the activity and solubility of enamel proteases. Their study used enamel organ cells as well as underlying enamel derived from rat incisors that had been previously freeze-dried. Extraction of these materials from the sectioned incisor followed a protocol previously described by Smith and Nanci [43] in which whole incisors are divided into various stages of amelogenesis based on a distance from the tooth apex and using a molar reference line. This protocol enables material from specific developmental stages to be dissected. Strips of material (containing both cells and enamel) were cut from each of the five sequential stages defined by this protocol. Results from this mixture of intra- and extracellular material indicated that the pH was relatively uniform during the secretory stage, with values clustering around ~7.23. Strips of material from maturation stage showed greater variability in extracellular pH values, ranging from neutral to weakly acidic conditions (pH 6.2–7.2). An interesting aspect of that work was that it showed a rise in pH values toward the end of the maturation stage [44]. Values obtained by this method were in line with the work by Sasaki et al. [5] described above and were also consistent with the notion that pH oscillates from neutral to acidic during maturation.
At this point, it would be useful to summarize some aspects of the works detailed in this section. Based on the descriptions of sample preparation in which the enamel organ had been removed, it is highly likely, then, that the pH staining solutions in the studies discussed above are indicative of extracellular pH [5, 40]. It is also unlikely that intracellular pH drops to acidic levels such as those recorded during maturation (e.g., pH of 6.2), thus one should expect that the acidic conditions measured from tissue and enamel homogenates using electrodes [5, 40] largely reflect extracellular pH. The works discussed above also demonstrate the irregular pH constancy throughout amelogenesis. During the secretory phase, pH values cluster around neutral conditions (~7.2). In contrast, pH values during maturation stage show a considerable range of values from acidic to near neutral, rising to higher values in the more advanced matured enamel. These changes are correlated with the functional stage of ameloblasts. Alkaline conditions predominate during the smooth-ended stage, whereas acidic conditions likely dominate during the ruffle-ended stage. pH conditions also affect the stochiometry of the crystals, as mineral ratios differ between crystals grown in acidic versus alkaline environments.
pH Regulation in Amelogenesis
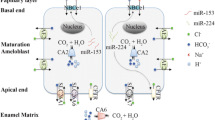
Ameloblast cells play an active role in pH regulation by using various acid–base transport mechanisms that may be found in a variety of tissues and cells. Others may be unique to the ameloblasts, as, for instance, the potential buffering role that amelogenins play during the initial phase of enamel development when crystal growth is moderate [35]. As pointed out [35], the nucleation of hydroxyapatite-like crystals produces large quantities of hydrogen protons throughout amelogenesis, but this activity peaks during the maturation stage. It is known that hydrogen protons acidify the microenvironment of the ameloblasts, requiring tight regulation of pH to prevent disruptions to crystal growth. However, the sequence of events altering extracellular pH during amelogenesis remains to be resolved. While most studies suggest that proton release by growing crystals acidifies the cell microenvironment and that ameloblasts then act in response to low extracellular pH by secreting bicarbonate (e.g., Refs. 1, 5, 35) (Fig. 1, scenario A), at least one study suggests that this working model may be more complex [39]. Specifically, the high extracellular pH recorded in calcifying enamel, well above neutral conditions (pH 8.5), by [14C]DMO autoradiography was interpreted as resulting from the binding of calcium ions with protein matrix [39]. It could be hypothesized that ameloblasts specifically regulate the magnitude of bicarbonate secretion as a primary event (independent of changes in extracellular pH) in order to induce crystal nucleation by creating a microenvironment that decreases the solubility of calcium phosphate (Fig. 1, scenario B). In this second scenario, the concomitant release of protons due to hydroxyapatite formation would in turn lower the extracellular pH toward neutrality. Further studies are needed to address which of these models accounting for the changes in extracellular pH is correct. It may also be possible that a combination of these two models is correct.
Given the known changes in crystal growth between secretion and maturation and the pH shifts associated particularly with the maturation phase, it should also be considered whether the same mechanism of pH homeostasis is used throughout amelogenesis, or if there are variations in pH regulation at each stage. In the following section, evidence is provided for the involvement in pH regulation of carbonic anhydrases (CAs) and the cystic fibrosis (CF) conductance transmembrane regulator, and finally, we discuss the role of bicarbonate cotransporters. None of these pathways is unique to ameloblasts.
Carbonic Anhydrases
CAs are a large family of enzymes containing numerous isozymes varying in cellular localization, kinetics, and tissue distribution, acting as catalysts for the reversible hydration of carbon dioxide to bicarbonate \( [{\text{CO}}_{ 2} {\text{ + H}}_{ 2} {\text{O}} \leftrightarrow {\text{HCO}}_{3}^{ - } {\text{ + H}}^{ + } ]. \) CAs also participate in biological processes including pH homeostasis, CO2 and \( {\text{HCO}}_{3}^{ - } \) transport, and bone resorption [45, 46]. The only two isoforms reported in dental tissues to date are CAs II and VI [47, 48]. CA II (CA2) is the most widely expressed isozyme found in all major mammalian organs and localized to the cytoplasm. CA6 appears to be the only secreted isozyme [46, 49, 50].
Earlier studies on CA expression in dental tissues did not account for isozyme class but generally reported CA activity as a single type. This issue was partly resolved during the mid-90s with the identification of various isozymes. Currently, 16 isozymes are recognized [45]. It should be pointed out that despite the role(s) that CAs may play during amelogenesis described below, no enamel defects associated with deficiencies in the expression of CAs have been reported.
The earliest report on CA activity in dental tissues was made by Kondo and Kuriaki [51] in homogenates from adult rat incisors. A later histochemical analysis of unerupted hamster molars confirmed CA expression and found staining in more mature ameloblasts near the tips of the cusps and in postsecretory ameloblasts but not in young ameloblasts [52]. Sugimoto et al. [53] reported a similar pattern in the mature ameloblasts of rat incisors.
Early studies considered that the role of CAs was to supply ions into the mineralization sites to initiate crystal nucleation [54]. This interpretation appeared to be supported by the expression of a high-activity type of CA during the early stages of enamel development whose expression decreased in maturation-stage ameloblasts [54]. However, focusing on CA2, Lin et al. [55] studied CA2 expression in maturation stage ameloblasts by immunogold labeling. Ruffle-ended ameloblasts were more strongly stained for CA2 than smooth-ended ameloblasts, showing a greater number of gold particles in the distal end of cytoplasm. The ruffled border of ruffle-ended ameloblasts was the most immunoreactive site. This observation, together with a higher expression of H+ATPase on the ruffled-border, prompted the interpretation, or hypothesis, that ruffle-ended ameloblasts have a proton pump to transport H+ ions into the enamel. While a similar H+ transport mechanism is noted in osteoclasts, the function in osteoclasts, in part, mediates the resorption of mineralized tissues [56], a function that is not apparent in ameloblasts. This hypothesis, however, drew parallels from the work of Sasaki et al. [5], which suggested that acidic pH in ruffle-ended ameloblasts may be due to proton release, and with a previous study reporting the involvement of proteases in matrix degradation during maturation which are released in the ruffle-ended stage [20]. It should be pointed out, however, that despite more recent studies confirming CA2 expression on the maturation stage of amelogenesis but not during the secretory stage [47], others have found CA2 expression in secretory-stage ameloblasts [48], which suggests that this issue remains unresolved.
CA6, also referred to as gustin in the literature, was originally described as a glycoprotein found in human saliva [46]. Two isoforms of CA6 have been reported, Types A and B, but only the former is found as a secreted protein [57]. Screening of the rat incisor cDNA library by a signal peptide trap identified a DNA fragment which matched the rat Type A of CA6 from the predicted translation sequence [48]. That study used rat incisor enamel organ samples from different developmental stages further characterized by reverse transcription PCR and Northern blotting. CA6 was found to be expressed during the maturation stage. However, which enamel organ cells express CA6, or the postsecretory localization of this enzyme, is not known. Smith et al. [48] posited that CA6 function during maturation may be associated with local buffering, providing bicarbonate ions or recycling excess of carbonic acid.
The Cystic Fibrosis Conductance Transmembrane Regulator (CFTR)
Enamel contains relatively high levels of chloride [58]. The exact role of chloride in the development of enamel remains under investigation but Odajima and Onishi [59] suggest that its function may be related to crystallite growth by acting as a transmitter of charge (cation carrier). Reports on disruption to normal chloride movement in epithelial cells, which results in CF, have also reported dental abnormalities [60, 61], and thus it has been considered that CF-null mice are a good model to investigate the role of chloride in enamel formation [62, 63].
CF is the most common and potentially lethal autosomal recessive hereditary disease affecting individuals of European origin [64]. It should be regarded as a multiorgan system disorder caused by a single biochemical abnormality [65]. This disorder is characterized by a disruption to the chloride transport channel in epithelial cells resulting in clinical manifestations such as respiratory failure, pancreatic insufficiency, and chronic sinusitis [64]. The exocrine (mucous) glands of the lungs, liver, pancreas, and intestines are the most affected. Chloride movement across epithelial cells is an important physiological regulator of salt and water [65]. The cell mechanism of such regulation involves plasma membranes on the apical surface of epithelial cells. Opening of this membrane allows chloride flow into the cell through an electrochemical gradient in response to β-adrenergic stimuli, increasing cAMP to activate protein kinase A, which mediates the opening of the channel [66]. In CF, this opening of the chloride channel is deregulated and does not respond to cAMP increases [65].
The CF gene is located in chromosome 7 and includes 27 exons. The coded protein, which contains 1480 amino acids [67], is known as the “cystic fibrosis conductance transmembrane regulator” (CFTR), which functions as a cyclic AMP-regulated chloride channel and as a regulator of other ion channels and transporters. A single mutation at position 508 accounts for the majority of the known CF disorders [68]. In the oral cavity, CF abnormalities have been related to elevated pH and an increase in calcium content [69]. Original reports relating CF with dental defects, however, presented some problems, as several of these descriptions included patients treated with therapeutic drugs including tetracycline, rendering difficult delineation of the defect’s exact etiology [62, 63]. Earlier work by Cua [70] reported mineral differences in teeth from children with CF, including individuals who had not taken tetracycline. A variety of studies examining the enamel deficiencies and alterations associated with abnormal CFTR function in a mouse model have been reported by Wright et al. [7, 62, 63, 71]. The mouse models used in those studies were originally reported by Koller et al. [72] and Snouwaert et al. [73]. The first report on enamel abnormalities from CF animals derived from this gene-targeting mutation indicated that during early secretion ameloblast cells appeared normal, displaying polarized features. However, during late secretion/early maturation these cells changed morphology to become cuboidal cells, with premature transition to a squamous epithelial stage [74]. Importantly, the enamel architecture and thickness of these CF mice appeared normal under the scanning electron microscope and crystallites had a morphology similar to that of normal mice, albeit appearing more porous in TEM [62, 63]. The gross external appearance of the CF mouse incisors was described as chalky-white, differing from the characteristic yellowish (iron-rich) pigment observed in the enamel of normal mice [62, 63]. This difference in coloration could distinguish CF mice from normal mice already at 3 weeks of age [62, 63]. It should be pointed out that this gross pigmentation difference in enamel could not be detected in molars of CF animals [62, 63]. When the amino acid composition of incisor enamel from the secretory stage was compared between CF and normal mice, it was shown to be very similar, but this was not the case in the maturing enamel, which showed a decrease in proline and histidine. Interestingly, secretory and mature zones of CF incisor enamel showed cross-reactivity with antiamelogenin antibody, but only to the secretory stage in normal mice [62, 63]. The enamel of CF animals was deemed “soft” during mechanical preparation of tooth samples. This led to the analysis of mineral composition, first by means of atomic absorption spectrophotometry, which showed that the CF enamel was considerably hypomineralized compared with that of normal mice and that it contained high levels of magnesium [62, 63]. Subsequent elemental analysis of CF enamel by energy-dispersive x-ray spectroscopy indicated decreased levels of chloride in the secretory stage, as well as a general decrease in iron and potassium, in addition to a reduction in calcium-to-phosphorus ratios [71]. The latter study also showed that the Cftr gene was expressed in normal enamel cells by RT–PCR. Based on these results, Wright et al. [62, 63] surmised that the enamel defects of CF-deficient mice could result from a loss of ameloblasts’ capacity to process extracellular matrix during the maturation stage. This abnormal cell capacity means that amelogenin, which stained positively in the maturation stage of enamel in CF mice but not in normal mice, may remain in the extracellular space affecting crystallite growth, as suggested by the irregular surface topography of CF crystallites examined using TEM.
Building from this work, a more recent study on CF-deficient mice reveals an important mechanistic role of CFTR in the regulation of pH during enamel development [7]. The relationship between pH regulation and CFTR stemmed from the role that CFTR plays in the regulation of \( {\text{Cl}}^{ - } {\text{and HCO}}_{3}^{ - } \) in intestinal cells [75] and the purported role of \( {\text{HCO}}_{3}^{ - } \) as pH buffer in enamel [1]. Sui et al. [7] demonstrated that the dissected incisors of CFTR animals stained yellow when immersed in pH indicator solution, pointing to a low or acidic pH in the transition and maturation zones of enamel.
Solute Carriers
The SLC4 family includes membrane proteins that play an important role in regulating intra- and extracellular pH and are a key base transport system in eukaryotic cells [76]. Two of these genes, SLC4A2 and SLC4A4, encode the anion exchanger (AE2) and the electrogenic bicarbonate cotransporter (NBCe1), respectively [76]. The interest in AE2 and NBCe1 was generated by studies in which mutations to SLC4A2 and SLC4A4 reported enamel abnormalities in humans and mice [77–79].
The SLC4 family is divided into three groups according to their ability to transport Na+ and Cl− simultaneously, with the SLC4A4 mediating the electrogenic transport of Na+ and and base [76]. There are three variants of the membrane protein NBCe1 (NBCe1-A, NBCe1-B and NBCe1-C; all produced from the gene locus Slc4a4), which result from two different promoter regions (NBCe1-A and NBCe1-B) or from alternative splicing (NBCe1-C) [80, 81]. There is a broad distribution of tissues that express NBCe1-B, while NBCe1-A is only expressed in the kidney and eye [80, 81]. The NBCe1-C variant has only been identified in rat brain tissues [82], and because of this, it appears unlikely to have a more widespread expression profile. In the case of AE2 (gene locus Slc4a2), five isoforms are recognized and differ in their promoter regions [83–85]. With the exception of NBCe1-C, each of the isoforms of AE2 and NBCe1 can be identified by its unique N′ terminus, while the majority of each protein, through its C′ terminus, remains constant. This use of different promoter regions allows for the distinction of each isoform by either RT–PCR (using a unique forward primer), immunolabeling (using antibodies that recognize only the N′ terminus), and in situ (using labeled RNA or DNA hybridization probes to the unique 5′ regions).
Mice lacking Slc4a2 encoding the AE2 anion exchanger are edentulous [78]. In addition, patients with SLC4A4 mutations encoding the electrogenic sodium bicarbonate cotransporter NBCe1 have enamel abnormalities [77, 79] and similar findings have recently been documented in mice lacking SLC4A4 [86]. Lyaruu et al. [87] showed that mice lacking AE2a/b have an abnormality in incisor enamel maturation, whereas molars are less severely affected. The loss of AE2 function results in a more severe tooth phenotype than loss of NBCe1 [77, 79, 86]. The incisors of mice lacking NBCe1 have a chalky-white appearance and are prone to enamel fracture [86]. In patients with loss of NBCe1 function, enamel defects have been described that appear as raised white and white-chalk-like spots [79]. The NBCe1 mutations in these patients involved all three NBCe1 variants (NBCe1A-C).
Paine et al. [8] using the LS8 ameloblast cell model system, recently showed that the variants of AE2 and NBCe1 that are expressed on the apical and basolateral membrane of secretory ameloblasts are AE2a and NBCe1-B, respectively. Moreover, the levels of AE2a and NBCe1-B mRNAs were pH dependent, with both transcripts showing the highest level of expression below pH 7.0. The results suggested that NBCe1-B variant mediates basolalateral bicarbonate transport in ameloblasts, whereas apical bicarbonate secretion is mediated by AE2a. This model is in keeping with reported calcium concentrations at the secretory and basal cell poles [88]. Importantly, given that the tooth phenotype in patients and mice with loss of NBCe1-B function is not characteristic of subjects with comparable metabolic acidosis, we hypothesized that the enamel defects in patients with SLC4A4 mutations were due to abnormal ameloblast function (loss of NBCe1-B transport per se) rather than due to systemic acidosis. To further investigate the role of NBCe1 in enamel formation we have used mice with a targeted disruption to the Slc4a4 gene locus (referred to as NBCe1−/−), which do not express any of the NBCe1 isoforms [86]. Our results show that the enamel of NBCe1−/− animals is extremely hypoplastic and thin, and that its prismatic structure is severely disorganized [89, 90]. The current model of ameloblast acid–base transport suggests that ameloblasts secrete bicarbonate across their apical membrane to buffer the proton load generated by apatite formation (see pH Regulation in Amelogenesis, above). In this scenario, both the bicarbonate secretory rate and the proton production rate likely change as a function of time depending on the ameloblast stage given that net pH various during the various maturation stages [5, 6]. We surmise that SLC4 bicarbonate transporters thus play an important role in pH regulation/bicarbonate transport in ameloblasts and other cell types [8, 76].
Model for pH Regulation
Based on the details discussed in the previous section, we propose a working model for understanding the regulation of pH by ameloblasts (Fig. 2). We recognize that whereas CA2 expression is greater during the ruffle-ended phase, at present, we have no data to support a greater affinity in the expression of CFTR, NBCe1, or AE2 with the ameloblast functional stages. Having said that, it should be highlighted that we have recently shown higher expression of NBCe1 in the more mature stages of enamel formation [89, 90], and the same applies to CA6 [48]. In the case of CFTR and AE2, this remains to be elucidated. In our working model for pH regulation thus we have made use of a polarized ameloblast during the early maturation stage at an undetermined functional stage. The proposed model for ameloblast bicarbonate transport (Fig. 2) is based on published expression profile data for NBCe1-B [8], AE2a [8], CFTR [7], CA2 [55], and CA6 [48].
Genetic Disruptions to pH Regulators Create Abnormal Enamel Phenotypes
Disruptions to normal enamel development have been commonly associated with genetic defects resulting from the abnormal expression of enamel-specific genes (e.g., Ref. [91]). Such dental anomalies are generally classified as amelogenesis imperfecta (AI) and are not often associated with syndromes or metabolic disorders [91, 92]. As discussed in previous sections, ameloblast cells synthesize and secrete a number of proteins largely restricted to the enamel environment. Amelogenin is the main secreted product of ameloblasts, forming about 90% of the total volume of the extracellular matrix proteins. Mutations to amelogenin and other ameloblast products (ameloblastin, enamelin, proteases, etc.) in humans and, in some instances, also in mice, result in AI, although there is ample variation in the severity of the resulting phenotypes for each gene, a variation that also depends on the region within each gene where the mutations occur [91]. In a recent review of the molecular etiologies of AI, Wright [91] discussed the involvement of additional genes in AI based on linkage studies. Indeed a recent addition to the AI spectrum has been the identification of mutations to the metal transporter CNNM4 [93]. Here, we focus on the dental phenotypes caused by disruptions to the expression of pH regulators during enamel formation, which, given their etiology, likely form a different group from those classically described as AI. It should be pointed out that because the expression of genes thus far identified as important players in ameloblast pH homeostasis is not restricted to the enamel environment, it is not unusual to find associations between dental anomalies and metabolic disorders or syndromes.
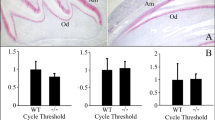
Patients with familial proximal renal tubular acidosis (pRTA) due to SLC4A4 mutations have a lower than normal level of circulating bicarbonate and frequently have short stature, bone growth delays, and ocular defects including band keratopathy, glaucoma, and cataracts [94, 95]. However, there have been documented case reports in which dental enamel abnormalities have been identified in patients with inherited pRTA [94, 96–98]. These case reports identify enamel anomalies associated with abnormal pH regulation/transport in multiple organs including teeth. Mice null or mutant for the Slc4a2 gene locus (coding for the anion exchanger AE2) have abnormal enamel [78, 87]. Furthermore, human patients with mutations in the SLC4A4 gene locus [77, 94], and mice null for Slc4a4 [86], have enamel abnormalities. These observations suggest that AE2 and NBCe1 each play an essential role in enamel formation. Two recent reports provide the first evidence that AE2 [8, 87] and NBCe1-B [8] are expressed in dental enamel cells (ameloblasts) in a polarized fashion, thereby providing a molecular mechanism for ameloblast transcellular bicarbonate secretion in the process of enamel formation. Figure 3 shows a lateral view of the molar row in wild-type and NBCe1−/− animals, clearly illustrating abnormal enamel in the latter.
A diagnosis of pRTA is most likely made by the patient’s pediatrician, a pediatric ophthalmologist, a cornea specialist, or a nephrologist. The specific tissues involved depend on which of the NBCe1 variants are affected. While unknown at this stage, it is possible that all patients with pRTA due to SLC4A4 mutations affecting the NBCe1-B variant have dental pathology. Postdiagnosis, because of the systemic nature of the disease, the primary care physician should consult with the appropriate medical specialists and, also, a pediatric or general dentist. This is a prudent approach since immediate preventative medical and dental treatment may be indicated. In the case of dental diagnosis/therapy, early preventive treatment could avoid excessive wear and disease to dental tissues. The primary dentition starts to erupt at ~6 months of age, and this is the ideal time to start ongoing dental observation and preventive treatment. Appropriate dental therapy would include fissure sealants, more frequent topical fluoride applications, and composite veneers to protect pitted or chalky enamel.
Just as mutations to the SLC4A4 gene locus may result in abnormal enamel phenotypes, mutations affecting the CFTR (cystic fibrosis) gene locus may also result in dental and enamel anomalies, however, in a human population there are only a few documented cases that have identified such a connection [60, 99]. Data from the CF mouse (targeted knockout of the Cftr gene locus) [72, 73] do suggest that a disruption to this gene activity does impact on the enamel phenotype [7, 62, 63]. Clearly, further studies on human populations and animal models are required to establish the exact role of \( {\text{HCO}}_{3}^{ - } ,{\text{Cl}}^{-} , \) and H+ movements in ameloblast cells during amelogenesis.
No reports to date have identified disruptions to enamel formation in association with defects of CAs. The only possible exception is a single report on maloclussion in patients with CA2 deficiencies [100], although the causes for such abnormality could easily be related to bone defects linked to CA2 rather than tooth malformation.
Summary
Amelogenesis is a complex and continuous process during which enamel matrix proteins interact with growing crystals. pH values are maintained in near-neutral conditions (~7.2) during secretion, whereas the extracellular pH shows considerable variation during maturation, shifting from acidic to near-neutral values, then rising to higher pH levels in more mature enamel. Alkaline conditions predominate during the smooth-ended stage of ameloblast modulation during maturation, and acidic conditions likely dominate the ruffle-ended phase. These shifts between ruffle and smooth phases appear to be a unique property of ameloblasts, playing a role in regulating the final stages of enamel crystal growth by allowing the movement of carbonates and other ions into the enamel zone [21, 22, 41]. pH affects the stoichiometry of the crystals, as the ratios of mineral content differ between acidic and alkaline regimes. The known pathways employed by ameloblasts to date in pH regulation involve CAs (to generate local bicarbonate), chloride (CFTR regulated but whose function is less clear), and bicarbonate cotransporters (permit the passage of bicarbonates from external sources, across the basal end, to the apical pole of ameloblasts). Based on the abnormal phenotypes resulting from the lack of expression of the genes or proteins associated with the pathways described above, we surmise that the development of healthy enamel requires correct maintenance of pH homeostasis at all stages of enamel formation. In the case of CAs, given that no abnormal enamel phenotypes have been associated with disruptions in gene expression to date, and given the high number of CA isoforms [45], it may be the case that isoforms other than CA2 and CA6 are expressed by ameloblasts, compensating for the loss of CA2 and CA6 activity.
In conclusion, there is growing evidence that gene regulation and protein expression maintain an enamel pH conducive to enamel biomineralization and that bicarbonate transporters, chloride channels, and CAs all play an important role in pH regulation and maintenance.
References
Smith CE (1998) Cellular and chemical events during enamel maturation. Crit Rev Oral Biol Med 9:128–161
Bartlett JD, Ganss B, Goldberg M, Moradian-Oldak J, Paine ML, Snead ML, Wen X, White SN, Zhou YL (2006) Protein-protein interactions of the developing enamel matrix. Curr Top Dev Biol 74:57–115
Paine ML, White SN, Luo W, Fong H, Sarikaya M, Snead ML (2001) Regulated gene expression dictates enamel structure and tooth function. Matrix Biol 20:273–292
Bartlett JD, Simmer JP (1999) Proteinases in developing dental enamel. Crit Rev Oral Biol Med 10:425–441
Sasaki S, Takagi T, Suzuki M (1991) Cyclical changes in pH in bovine developing enamel as sequential bands. Arch Oral Biol 36:227–231
Takagi T, Ogasawara T, Tagami J, Akao M, Kuboki Y, Nagai N, LeGeros RZ (1998) pH and carbonate levels in developing enamel. Connect Tissue Res 38:181–187
Sui W, Boyd C, Wright JT (2003) Altered pH regulation during enamel development in the cystic fibrosis mouse incisor. J Dent Res 82:388–392
Paine ML, Snead ML, Wang HJ, Abuladze N, Pushkin A, Liu W, Kao LY, Wall SM, Kim YH, Kurtz I (2008) Role of NBCe1 and AE2 in secretory ameloblasts. J Dent Res 87:391–395
Nanci A (2008) Ten Cate’s oral histology: development, structure, and function, 7th edn. Mosby Elsevier, St. Louis, MO
Nanci A, Smith CE (1992) Development and calcification of enamel. In: Bonucci E (ed) Calcification in biological systems. CRC Press, Boca Raton, FL, pp 313–343
Smith CE (1979) Ameloblasts: secretory and resorptive functions. J Dent Res 58(Spec Issue B):695–707
Warshawsky H, Josephsen K, Thylstrup A, Fejerskov O (1981) The development of enamel structure in rat incisors as compared to the teeth of monkey and man. Anat Rec 200:371–399
Goldberg M, Septier D (2002) Phospholipids in amelogenesis and dentinogenesis. Crit Rev Oral Biol Med 13:276–290
Hu JC, Sun X, Zhang C, Liu S, Bartlett JD, Simmer JP (2002) Enamelysin and kallikrein-4 mRNA expression in developing mouse molars. Eur J Oral Sci 110:307–315
Ryu O, Hu JC, Yamakoshi Y, Villemain JL, Cao X, Zhang C, Bartlett JD, Simmer JP (2002) Porcine kallikrein-4 activation, glycosylation, activity, and expression in prokaryotic and eukaryotic hosts. Eur J Oral Sci 110:358–365
Iwasaki K, Bajenova E, Somogyi-Ganss E, Miller M, Nguyen V, Nourkeyhani H, Gao Y, Wendel M, Ganss B (2005) Amelotin—a novel secreted, ameloblast-specific protein. J Dent Res 84:1127–1132
Moffatt P, Smith CE, St-Arnaud R, Simmons D, Wright JT, Nanci A (2006) Cloning of rat amelotin and localization of the protein to the basal lamina of maturation stage ameloblasts and junctional epithelium. Biochem J 399:37–46
Moffatt P, Smith CE, St-Arnaud R, Nanci A (2008) Characterization of Apin, a secreted protein highly expressed in tooth-associated epithelia. J Cell Biochem 103:941–956
Deutsch D (1989) Structure and function of enamel gene products. Anat Rec 224:189–210
Josephsen K, Fejerskov O (1977) Ameloblast modulation in the maturation zone of the rat incisor enamel organ. A light and electron microscopic study. J Anat 124:45–70
Reith EJ, Boyde A (1981) The arrangement of ameloblasts on the surface of maturing enamel of the rat incisor tooth. J Anat 133:381–388
Reith EJ, Boyde A (1981) Autoradiographic evidence of cyclical entry of calcium into maturing enamel of the rat incisor tooth. Arch Oral Biol 26:983–987
Sasaki T, Nakagawa K, Higashi S (1983) Fine structure of secretory ameloblasts in kitten tooth germs, with special regard to intercellular junctions as revealed by freeze–fracture. Arch Oral Biol 28:177–183
Smith CE, McKee MD, Nanci A (1987) Cyclic induction and rapid movement of sequential waves of new smooth-ended ameloblast modulation bands in rat incisors as visualized by polychrome fluorescent labeling and GBHA-staining of maturing enamel. Adv Dent Res 1:162–175
Boyde A, Reith EJ (1981) Display of maturation cycles in rat incisor enamel with tetracycline labelling. Histochemistry 72:551–561
Boyde A, Reith EJ (1977) Scanning electron microscopy of rat maturation ameloblasts. Cell Tissue Res 178:221–228
Boyde A (1989) Enamel. In: Oksche A, Vollrath L (eds) Handbook of microscopic anatomy. Springer-Verlag, Berlin, pp 309–473
Lacruz RS, Bromage TG (2006) Appositional enamel growth in molars of South African fossil hominids. J Anat 209:13–20
Eanes ED (1992) Dynamics of calcium phosphate precipitation. In: Bonucci E (ed) Calcification in biological systems. CRC Press, Boca Raton, FL, pp 1–18
Nylen MU, Eanes ED, Omnell KA (1963) Crystal growth in rat enamel. J Cell Biol 18:109–123
Travis DF, Glimcher MJ (1964) The structure and organization of, and the relationship between the organic matrix and the inorganic crystals of embryonic bovine enamel. J Cell Biol 23:447–497
Warshawsky H, Nanci A (1982) Stereo electron microscopy of enamel crystallites. J Dent Res Spec Issue 1504–1514
Goldberg M, Septier D, Lecolle S, Chardin H, Quintana MA, Acevedo AC, Gafni G, Dillouya D, Vermelin L, Thonemann B et al (1995) Dental mineralization. Int J Dev Biol 39:93–110
Young RA (1974) Implications of atomic substitutions and other structural details in apatites. J Dent Res 53:193–203
Simmer JP, Fincham AG (1995) Molecular mechanisms of dental enamel formation. Crit Rev Oral Biol Med 6:84–108
Smith CE, Chong DL, Bartlett JD, Margolis HC (2005) Mineral acquisition rates in developing enamel on maxillary and mandibular incisors of rats and mice: implications to extracellular acid loading as apatite crystals mature. J Bone Miner Res 20:240–249
Aoba T, Tanabe T, Moreno EC (1987) Proteins in the enamel fluid of immature porcine teeth. J Dent Res 66:1721–1726
Aoba T (1996) Recent observations on enamel crystal formation during mammalian amelogenesis. Anat Rec 245:208–218
Lyman GE, Waddell WJ (1977) pH gradients in the developing teeth of young mice from autoradiography of [14C]DMO. Am J Physiol 232:364–367
Takano Y, Crenshaw MA, Bawden JW, Hammarstrom L, Lindskog S (1982) The visualization of the patterns of ameloblast modulation by the glyoxal bis(2-hydroxyanil) staining method. J Dent Res Spec Issue 1580–1587
McKee MD, Warshawsky H (1986) Modification of the enamel maturation pattern by vinblastine as revealed by glyoxal bis(2-hydroxyanil) staining and 45 calcium radioautography. Histochemistry 86:141–145
Smith CE, Issid M, Margolis HC, Moreno EC (1996) Developmental changes in the pH of enamel fluid and its effects on matrix-resident proteinases. Adv Dent Res 10:159–169
Smith CE, Nanci A (1989) A method for sampling the stages of amelogenesis on mandibular rat incisors using the molars as a reference for dissection. Anat Rec 225:257–266
Smith CE, Nanci A (1996) Protein dynamics of amelogenesis. Anat Rec 245:186–207
Supuran CT (2008) Carbonic anhydrases—an overview. Curr Pharm Des 14:603–614
Sly WS, Hu PY (1995) Human carbonic anhydrases and carbonic anhydrase deficiencies. Annu Rev Biochem 64:375–401
Toyosawa S, Ogawa Y, Inagaki T, Ijuhin N (1996) Immunohistochemical localization of carbonic anhydrase isozyme II in rat incisor epithelial cells at various stages of amelogenesis. Cell Tissue Res 285:217–225
Smith CE, Nanci A, Moffatt P (2006) Evidence by signal peptide trap technology for the expression of carbonic anhydrase 6 in rat incisor enamel organs. Eur J Oral Sci 114(Suppl 1):147–153
Pastorekova S, Parkkila S, Pastorek J, Supuran CT (2004) Carbonic anhydrases: current state of the art, therapeutic applications and future prospects. J Enzyme Inhib Med Chem 19:199–229
Pan P, Leppilampi M, Pastorekova S, Pastorek J, Waheed A, Sly WS, Parkkila S (2006) Carbonic anhydrase gene expression in CA II-deficient (Car2−/−) and CA IX-deficient (Car9−/−) mice. J Physiol 571:319–327
Kondo K, Kuriaki K (1961) Carbonic anhydrases in dental tissue and effect of parathyroid hormone and fluoride on its activity. J Dent Res 40:971–974
Dogterom AA, Bronckers AL (1983) Carbonic anhydrase in developing hamster molars. J Dent Res 62:789–791
Sugimoto T, Ogawa Y, Kuwahara H, Shimazaki M, Yagi T, Sakai A (1988) Histochemical demonstration of carbonic anhydrase activity in the odontogenic cells of the rat incisor. J Dent Res 67:1271–1274
Kakei M, Nakahara H (1996) Aspects of carbonic anhydrase and carbonate content during mineralization of the rat enamel. Biochim Biophys Acta 1289:226–230
Lin HM, Nakamura H, Noda T, Ozawa H (1994) Localization of H(+)-ATPase and carbonic anhydrase II in ameloblasts at maturation. Calcif Tissue Int 55:38–45
Supanchart C, Kornak U (2008) Ion channels and transporters in osteoclasts. Arch Biochem Biophys 473:161–165
Sok J, Wang XZ, Batchvarova N, Kuroda M, Harding H, Ron D (1999) CHOP-dependent stress-inducible expression of a novel form of carbonic anhydrase VI. Mol Cell Biol 19:495–504
Retief DH, Cleaton-Jones PE, Turkstra J, De Wet WJ (1971) The quantitative analysis of sixteen elements in normal human enamel and dentine by neutron activation analysis and high-resolution gamma-spectrometry. Arch Oral Biol 16:1257–1267
Odajima T, Onishi M (1989) The state of chlorine in human enamel and dentine. In: Fearnhead R (ed) Tooth enamel V. Florence Publishers, Barbera, pp 360–366
Primosch RE (1980) Tetracycline discoloration, enamel defects, and dental caries in patients with cystic fibrosis. Oral Surg Oral Med Oral Pathol 50:301–308
Primosch RE (1980) Dental and skeletal maturation in patients with cystic fibrosis. J Oral Med 35:7–13
Wright JT, Hall KI, Grubb BR (1996) Enamel mineral composition of normal and cystic fibrosis transgenic mice. Adv Dent Res 10:270–274
Wright JT, Kiefer CL, Hall KI, Grubb BR (1996) Abnormal enamel development in a cystic fibrosis transgenic mouse model. J Dent Res 75:966–973
Collins FS (1992) Cystic fibrosis: molecular biology and therapeutic implications. Science 256:774–779
Sferra TJ, Collins FS (1993) The molecular biology of cystic fibrosis. Annu Rev Med 44:133–144
Drumm ML, Collins FS (1993) Molecular biology of cystic fibrosis. Mol Genet Med 3:33–68
Riordan JR, Rommens JM, Kerem B et al (1989) Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science 245:1066–1073
Frizzell RA (1995) Functions of the cystic fibrosis transmembrane conductance regulator protein. Am J Respir Crit Care Med 151:S54–S58
Mandel ID, Kutscher A, Denning CR, Thompson RH Jr, Zegarelli EV (1967) Salivary studies in cystic fibrosis. Am J Dis Child 113:431–438
Cua FT (1991) Calcium and phosphorous in teeth from children with and without cystic fibrosis. Biol Trace Elem Res 30:277–289
Arquitt CK, Boyd C, Wright JT (2002) Cystic fibrosis transmembrane regulator gene (CFTR) is associated with abnormal enamel formation. J Dent Res 81:492–496
Koller BH, Kim HS, Latour AM, Brigman K, Boucher RC Jr, Scambler P, Wainwright B, Smithies O (1991) Toward an animal model of cystic fibrosis: targeted interruption of exon 10 of the cystic fibrosis transmembrane regulator gene in embryonic stem cells. Proc Natl Acad Sci USA 88:10730–10734
Snouwaert JN, Brigman KK, Latour AM, Malouf NN, Boucher RC, Smithies O, Koller BH (1992) An animal model for cystic fibrosis made by gene targeting. Science 257:1083–1088
Kiefer CL, Hall KI, Grubb BR, Wright JT (1995) Abnormal enamel development in a cystic fibrosis transgenic mouse model. J Dent Res 74:178 (abstract)
Illek B, Fischer H, Machen TE (1998) Genetic disorders of membrane transport. II. Regulation of CFTR by small molecules including HCO3. Am J Physiol 275:G1221–G1226
Pushkin A, Kurtz I (2006) SLC4 base (HCO3 −, CO3 2−) transporters: classification, function, structure, genetic diseases, and knockout models. Am J Physiol Renal Physiol 290:F580–F599
Dinour D, Chang MH, Satoh J, Smith BL, Angle N, Knecht A, Serban I, Holtzman EJ, Romero MF (2004) A novel missense mutation in the sodium bicarbonate cotransporter (NBCe1/SLC4A4) causes proximal tubular acidosis and glaucoma through ion transport defects. J Biol Chem 279:52238–52246
Gawenis LR, Ledoussal C, Judd LM, Prasad V, Alper SL, Stuart-Tilley A, Woo AL, Grisham C, Sanford LP, Doetschman T, Miller ML, Shull GE (2004) Mice with a targeted disruption of the AE2 Cl-/HCO3-exchanger are achlorhydric. J Biol Chem 279:30531–30539
Inatomi J, Horita S, Braverman N, Sekine T, Yamada H, Suzuki Y, Kawahara K, Moriyama N, Kudo A, Kawakami H, Shimadzu M, Endou H, Fujita T, Seki G, Igarashi T (2004) Mutational and functional analysis of SLC4A4 in a patient with proximal renal tubular acidosis. Pflugers Arch 448:438–444
McAlear SD, Liu X, Williams JB, McNicholas-Bevensee CM, Bevensee MO (2006) Electrogenic Na/HCO3 cotransporter (NBCe1) variants expressed in Xenopus oocytes: functional comparison and roles of the amino and carboxy termini. J Gen Physiol 127:639–658
Abuladze N, Lee I, Newman D, Hwang J, Boorer K, Pushkin A, Kurtz I (1998) Molecular cloning, chromosomal localization, tissue distribution, and functional expression of the human pancreatic sodium bicarbonate cotransporter. J Biol Chem 273:17689–17695
Bevensee MO, Schmitt BM, Choi I, Romero MF, Boron WF (2000) An electrogenic Na(+)-HCO(−)(3) cotransporter (NBC) with a novel COOH-terminus, cloned from rat brain. Am J Physiol Cell Physiol 278:C1200–C1211
Kurschat CE, Shmukler BE, Jiang L, Wilhelm S, Kim EH, Chernova MN, Kinne RK, Stewart AK, Alper SL (2006) Alkaline-shifted pHo sensitivity of AE2c1-mediated anion exchange reveals novel regulatory determinants in the AE2N-terminal cytoplasmic domain. J Biol Chem 281:1885–1896
Lecanda J, Urtasun R, Medina JF (2000) Molecular cloning and genomic organization of the mouse AE2 anion exchanger gene. Biochem Biophys Res Commun 276:117–124
Medina JF, Lecanda J, Acin A, Ciesielczyk P, Prieto J (2000) Tissue-specific N-terminal isoforms from overlapping alternate promoters of the human AE2 anion exchanger gene. Biochem Biophys Res Commun 267:228–235
Gawenis LR, Bradford EM, Prasad V, Lorenz JN, Simpson JE, Clarke LL, Woo AL, Grisham C, Sanford LP, Doetschman T, Miller ML, Shull GE (2007) Colonic anion secretory defects and metabolic acidosis in mice lacking the NBC1 Na+/HCO3-cotransporter. J Biol Chem 282:9042–9052
Lyaruu DM, Bronckers AL, Mulder L, Mardones P, Medina JF, Kellokumpu S, Oude Elferink RP, Everts V (2008) The anion exchanger Ae2 is required for enamel maturation in mouse teeth. Matrix Biol 27:119–127
Bawden JW (1989) Calcium transport during mineralization. Anat Rec 224:226–233
Lacruz RS, Paine ML, Kurtz I (2009) The ultrastructural and mechanical analysis of the dentition of mice lacking the NBCe1 Na+/HCO3-cotransporter. FASEB J 23:800–806 (abstract)
Paine ML, Snead ML, Lacruz RS, Wang HJ (2009) A relationship between proximal renal tubular acidosis and dental disease. J Dent Res 88 (Spec Issue A):2099
Wright JT (2006) The molecular etiologies and associated phenotypes of amelogenesis imperfecta. Am J Med Genet A 140:2547–2555
Crawford PJ, Aldred M, Bloch-Zupan A (2007) Amelogenesis imperfecta. Orphanet J Rare Dis 2:17
Parry DA, Mighell AJ, El-Sayed W, Shore RC, Jalili IK, Dollfus H, Bloch-Zupan A, Carlos R, Carr IM, Downey LM, Blain KM, Mansfield DC, Shahrabi M, Heidari M, Aref P, Abbasi M, Michaelides M, Moore AT, Kirkham J, Inglehearn CF (2009) Mutations in CNNM4 cause Jalili syndrome, consisting of autosomal-recessive cone-rod dystrophy and amelogenesis imperfecta. Am J Hum Genet 84:266–273
Demirci FY, Chang MH, Mah TS, Romero MF, Gorin MB (2006) Proximal renal tubular acidosis and ocular pathology: a novel missense mutation in the gene (SLC4A4) for sodium bicarbonate cotransporter protein (NBCe1). Mol Vis 12:324–330
Katzir Z, Dinour D, Reznik-Wolf H, Nissenkorn A, Holtzman E (2008) Familial pure proximal renal tubular acidosis—a clinical and genetic study. Nephrol Dial Transplant 23:1211–1215
Koppang HS, Stene T, Solheim T, Larheim TA, Winsnes A, Monn E, Stokke O (1984) Dental features in congenital persistent renal tubular acidosis of proximal type. Scand J Dent Res 92:489–495
Igarashi T, Inatomi J, Sekine T, Cha SH, Kanai Y, Kunimi M, Tsukamoto K, Satoh H, Shimadzu M, Tozawa F, Mori T, Shiobara M, Seki G, Endou H (1999) Mutations in SLC4A4 cause permanent isolated proximal renal tubular acidosis with ocular abnormalities. Nat Genet 23:264–266
Elizabeth J, Lakshmi Priya E, Umadevi KM, Ranganathan K (2007) Amelogenesis imperfecta with renal disease—a report of two cases. J Oral Pathol Med 36:625–628
Azevedo TD, Feijo GC, Bezerra AC (2006) Presence of developmental defects of enamel in cystic fibrosis patients. J Dent Child (Chic) 73:159–163
Whyte MP (1993) Carbonic anhydrase II deficiency. Clin Orthop Relat Res 294:52–63
Acknowledgments
The authors thank Dr. Gary Shull, University of Cincinnati, College of Medicine, for the Slc4a4 mutant animals and Ms. Alicia Thompson for help in generating the images at the University of Southern California’s Center for Electron Microscope and Microanalysis. This work was supported by National Institutes of Health Grants DE013404, DE019629, DK058563, and DK077162 and by the Canadian Institutes of Health Research.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors state no conflicts of interest regarding any of the work reported here.
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Lacruz, R.S., Nanci, A., Kurtz, I. et al. Regulation of pH During Amelogenesis. Calcif Tissue Int 86, 91–103 (2010). https://doi.org/10.1007/s00223-009-9326-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-009-9326-7