Abstract
Patient preferences, convenience, and bone turnover markers were evaluated for the monthly ibandronate over the weekly risedronate regimen in Korean postmenopausal osteoporotic women. This was a 6-month, prospective, randomized, open-label, multicenter study with a two-period and two-sequence crossover treatment design. After a 30-day screening period, eligible participants with postmenopausal osteoporosis were randomized to receive either monthly oral ibandronate 150 mg for 3 months followed by weekly oral risedronate 35 mg for 12 weeks (sequence A) or the same regimen in reverse order (sequence B). Patient preference and convenience were evaluated by questionnaire. The changes in serum C-telopeptide after 3 months of treatment were analyzed. A total of 365 patients were enrolled in this study (sequence A 182, sequence B 183). Of patients expressing a preference (83.4%), 74.8% preferred the monthly ibandronate regimen over the weekly regimen (25.2%). More women stated that the monthly ibandronate regimen was more convenient (84.2%) than the weekly regimen (15.8%). There was no significant difference in the change in bone turnover marker between the two treatments. The two regimens were similarly tolerable. There were fewer adverse events in the monthly ibandronate group compared to the weekly risedronate group in terms of gastrointestinal side effects (nausea and abdominal distension). This study revealed a strong preference and convenience for monthly ibandronate over weekly risedronate in Korean postmenopausal osteoporotic women. There was no significant difference in change of bone turnover marker and safety profile between the two regimens.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
With the progressive aging of the world’s population, osteoporosis has emerged as an important global health problem. Based on population growth and the current incidence of hip fractures in Asia, it is estimated that by 2050 50% of the world’s hip fractures will occur in Asian women [1].
Bisphosphonates are regarded as the treatment of choice for postmenopausal osteoporosis and have proven clinical benefits, including a significantly reduced risk of vertebral and nonvertebral fractures in many clinical trials [2–6]. However, adherence to treatment among patients with postmenopausal osteoporosis is currently suboptimal, like in other chronic diseases [7, 8]. Poor adherence leads to reduced clinical benefit, a raised incidence of secondary complications, and therefore increased health-care costs [7]. Data from a study evaluating adherence to bisphosphonate therapy show that the probability of continuing daily oral treatment is approximately 50% at 1 year [9]. This problem is largely contributed to the complex and inconvenient dosing instructions due to their low bioavailability and the potential for upper gastrointestinal (GI) side effects [8].
Patient treatment preference is an important factor in determining patient satisfaction with medical care and could provide an efficient way of maximizing the effectiveness of medical care [10]. Patient preferences for daily or weekly bisphosphonate therapy have been evaluated in prospective, open-label studies [11, 12]. Actually, numerous studies have proven that weekly dosing improves therapeutic adherence, though it remains suboptimal [13–15].
Ibandronate is a potent, new aminobisphosphonate with proven antifracture efficacy [2] and can be administered as a monthly regimen. A comparative study (Monthly Oral Iandronate in Ladies study [MOBILE]) demonstrated that monthly ibandronate was as effective and well tolerated as the currently approved daily ibandronate regimen in postmenopausal osteoporosis [6]. Furthermore, two randomized, multicenter clinical trials (the Bonviva Alendronate Trial in Osteoporosis [BALTO I and II]) found that significantly more women with postmenopausal osteoporosis preferred the monthly oral ibandronate regimen than the weekly alendronate regimen [16, 17]. However, it is difficult to apply this result in Asian countries because both studies had populations that were only about 1% Asian. Moreover, there has been no randomized multicenter clinical trial comparing patient preference between ibandronate and risedronate, which is also widely prescribed for the treatment of osteoporosis. In addition, there were few studies comparing bone turnover markers between ibandronate and risedronate.
This study was aimed at determining whether the preference results of Caucasians are similar to those of Koreans. Other objectives were comparison of preference, convenience, and bone turnover marker between monthly ibandronate and weekly risedronate.
Methods
Study Design
This was a 6-month, prospective, randomized, open-label, multicenter, two-period, and two-sequence crossover study to investigate patient preference on dosing between once-monthly ibandronate and once-weekly risedronate. The study design was similar to that used in previous studies (BALTO I and II) [16, 17] except that this study compared ibandronate with risedronate, not alendronate, and measured serum C-telopeptide of type I collagen (CTX) of participants both at baseline and 12 weeks after treatment to examine the change in bone turnover.
The study was conducted between March 2007 and May 2008 in 15 centers in South Korea and enrolled ambulatory women with postmenopausal osteoporosis who were bisphosphonate-naive. Participants were required to be able to understand and complete the Preference Questionnaire and to comply with the study protocol to be enrolled in this study. Women with upper GI disease such as reflux esophagitis uncontrolled with drugs or delayed esophageal emptying or active gastric/duodenal ulcer and who were unable to maintain an upright position for at least 60 minutes were excluded. Subjects who had hypersensitivity to ibandronate or risedronate; any other metabolic bone diseases but postmenopausal osteoporosis; any chronic diseases which could affect bone metabolism; or any abnormalities in laboratory parameters such as serum calcium, liver, or kidney function test and had been taking glucocorticoid were also excluded. All participants provided informed consent. The appropriate independent ethics committee or institutional review board approved the study protocol and all materials provided to participants.
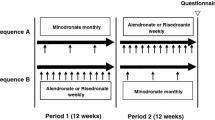
After a 30-day screening period, eligible participants were randomized to take either monthly oral ibandronate 150 mg (Bonviva; F. Hoffmann-La Roche Ltd, Basel, Switzerland) for 3 months followed by weekly oral risedronate 35 mg (Actonel; Sanofi-Aventis Korea, Seoul, Korea) for 12 weeks (sequence A) or the same regimen in reverse order (sequence B) (Fig. 1). Central randomization was used to ensure similar distribution between the two sequences. There was no washout period between the two treatment regimens. The crossover in treatment regimens occurred after 3 months’ treatment with ibandronate (sequence A) or 12 weeks’ treatment with risedronate (sequence B). Additional safety information was collected 15 days after the end of treatment. Participants were educated to take both study medications in the morning after an overnight fast (6 hours or more), to keep an upright position (sitting or standing), and not to eat or drink fluids other than water for either 60 minutes or 30 minutes after taking ibandronate or risedronate, respectively. All participants received appropriate dosing and administration instructions and were reminded by telephone contact before each medication dosing schedule (ibandronate every month, risedronate every week). All women were supplemented with daily elemental calcium 500 mg and vitamin D 125 IU (Oscal 500 D; Handok Pharmaceuticals, Seoul, Korea). Compliance was estimated by recording drugs dispensed versus drugs returned on the case record form. Adverse events and laboratory parameters were assessed by the study investigators. The use of clinically relevant concomitant medications was also recorded.
The primary end point of this study was patients’ preference. The secondary end points were convenience and bone turnover marker level.
Preference Questionnaire
All participants were asked to answer the Preference Questionnaire at the end of the study (month 6) or when they withdrew from the study. The Preference Questionnaire was adapted from the BALTO I and II [16, 17]. If patients withdrew from the study before the crossover point, i.e., after having taken only the first treatment in the sequence, they were not requested to complete the questionnaire. If they withdrew after taking at least one dose of the second treatment in the sequence, they were asked to complete the questionnaire at the time of withdrawal. Patients were asked to complete the questionnaire by themselves before any other scheduled procedure at the visit and were not assisted in completing the questionnaire.
Bone Turnover Marker
Serum levels of CTX were measured at baseline and after 3 months of treatment. Blood samples for CTX assessments were taken at the end of the dosing interval (1 month after 3-month dosing of ibandronate, one week after 12-week dosing of risedronate), after an overnight fast of at least 6 hours, between 8:00 a.m. and 10:00 a.m., and processed at the central laboratory (Green Cross Reference Lab, Seoul, Korea). CTX levels were measured by electrochemiluminescence immunoassay (Modular Analytics, E170 Modular; Roche, Mannheim, Germany).
Statistical Analysis
The significance of preference and convenience was assessed, accounting for the potential effect of treatment order, using Gart’s test [18] (excluding subjects with no preference) and Prescott’s test [19] (including all preference and no preference data). Baseline and 3-month serum CTX levels between the two groups were analyzed with Student’s t-test. The mean percentage changes in serum CTX (3 months – baseline values) between the two sequences were tested with ANCOVA and 95% confidence interval. All the tests were two-sided, with a significance level of P < 0.05.
Results
Patient Disposition and Baseline Characteristics
A total of 365 women were enrolled in this study; 182 were randomized to sequence A and 183 to sequence B. The safety population (defined as patients who had received at least one dose of trial medication and had follow-up data end point) comprised 352 women included in the modified intention-to-treat population (Fig. 2).
Participants in sequence A and sequence B were well matched for age as well as weight, height, time since menopause, and time since diagnosis of postmenopausal osteoporosis (Table 1). There were no significant differences between sequences A and B in prior fragility fracture, family history of fragility fracture in a first-degree relative, and current smoking. The most common medications received by patients prior to study enrollment for the treatment of osteoporosis were estrogen and progestin (sequence A 34.5%, sequence B 34.7%).
Preference
Most women (262/314, 83.4%) preferred one of the two regimens to the other. Of the patients who reported a preference, 74.8% (196/262) preferred the monthly regimen of ibandronate and 25.2% (66/262) preferred the weekly regimen of risedronate. The preference rate for monthly ibandronate was statistically significant (P < 0.0001). Preference for monthly ibandronate was not affected by the order in which the women took the study medications (Gart order-effect P = 0.6210). When data from all patients were included, i.e., including women who did not express a preference, 62.4% (196/314) preferred monthly ibandronate dosing and 21.0% (66/314) preferred weekly risedronate dosing (Fig. 3). Again, the preference rate for monthly ibandronate was statistically significant (P < 0.0001).
The reasons identified by patients for their preference are detailed in Fig. 4 (patients could choose more than one reason). Of the 74.8% of participants who expressed a preference for the monthly ibandronate regimen, 77.6% (152/196) chose the greater ease of long-term adherence and 52.6% (103/196) a better lifestyle fit as a reason for their preference. The proportions of participants who mentioned less stomach discomfort and more easily tolerated side effects were 24.0% (47/196) and 22.0% (43/196), respectively.
Convenience
Of the women expressing an opinion on convenience (229/314, 72.9%), 84.2% found that the monthly ibandronate regimen was more convenient and 15.8% (43/272) found that the weekly risedronate regimen was more convenient. The convenience rate for monthly ibandronate dosing was statistically significant (P < 0.0001). This opinion was not affected by treatment sequence (Gart order-effect, P = 0.8814). Similar results were shown when the analysis included patients who did not express an opinion about treatment convenience (Fig. 5): The monthly ibandronate regimen was chosen by 72.9% of the overall study population and the weekly risedronate regimen was chosen by 13.7%. Again, the convenience rate for monthly ibandronate was statistically significant (P < 0.0001).
Compliance
The compliance rate of the monthly ibandronate group was significantly higher than that of the weekly risedronate group (P < 0.01). The difference in compliance was relatively prominent at month 6 compared to month 3 (Table 2).
Bone Turnover Marker
There was no statistically significant difference between the monthly and weekly bisphosphonate groups in baseline and 3-month serum CTX levels. In addition, there was no statistical significant difference between the two treatment groups in mean percent change of serum CTX values (Table 3).
Safety
The incidence of adverse events was comparable between the two bisphosphonate regimens, with 41.0% and 40.1% of patients experiencing at least one event during ibandronate and risedronate treatment, respectively. Treatment-related adverse events were reported by 29.5% of patients during ibandronate treatment and 29.9% of patients during risedronate treatment. Treatment-related GI adverse events were reported by 19.4% of patients while taking ibandronate and 23.1% of patients while taking risedronate. More patients receiving once-weekly risedronate experienced abdominal distension and nausea (6.9% vs. 3.3% and 6.9% vs. 3.0%, respectively; P < 0.05). Other frequent adverse events were generally comparable between patients receiving ibandronate and risedronate (Table 4). The incidence of adverse events resulting in withdrawal from the study was 4.2% (n = 14) in patients receiving ibandronate and 4.5% (n = 15) in patients receiving risedronate. Clinically relevant changes in laboratory parameters were not observed in any patient during the study.
Discussion
Although bisphosphonates are regarded as the treatment of choice for postmenopausal osteoporosis, their poor adherence and persistence have limited their efficacy. It was known that patients preferred less frequent, simpler, and more convenient dosing regimens [20, 21]. A weekly dosing regimen has better therapeutic adherence than a daily dosing regimen in postmenopausal osteoporosis; however, it remains suboptimal [13–15]. Ibandronate is a potent, nitrogen-containing bisphosphonate which has been approved in Europe, the United States, and Korea to be given as a monthly regimen. The BALTO I and II studies reported that more women with postmenopausal osteoporosis preferred the monthly ibandronate regimen and expressed that the monthly ibandronate was more convenient than the weekly alendronate regimen [16, 17]. However, these findings cannot be applied in Asian countries because the participants in these studies included only about 1% Asian ethnicity. Furthermore, there have been few studies about preferences between monthly ibandronate and weekly risedronate, which have comparable efficacy and tolerability in treatment of osteoporosis [22–24]. This study has not only reconfirmed previous findings from the BALTO studies in Korea, an Asian country, but also proved that monthly ibandronate had better preference and convenience than weekly risedronate, similar to Western results, which was highly expected despite the different ethnicity.
One of the main reasons for noncompliance is the occurrence of adverse events [25]. In this study, the overall incidence of adverse events was similar between the two bisphosphonate regimens. The number of patients who were withdrawn from the study because of adverse events was also similar in both treatments. These results were also consistent with the previous BALTO studies.
Clinical trials evaluating the GI tolerability of bisphosphonates have found no significant differences in the incidence of spontaneously reported GI adverse events between placebo and bisphosphonates [2, 4, 26]. However, GI adverse symptoms have been reported with bisphosphonate use in the clinical setting and may be an important factor for discontinuing treatment [27–30]. BALTO I and II reported that the patients receiving both the monthly ibandronate and weekly alendronate regimens experienced similar GI adverse events. In this study, more patients receiving once-weekly risedronate experienced abdominal distension and nausea, though other GI adverse events occurred similarly in both treatments.
In many clinical studies, monthly ibandronate showed a significant increase in bone mineral density (BMD) and a reduction in fracture risk [2, 6, 31]. Once-monthly ibandronate was shown to be clinically comparable to weekly alendronate at increasing BMD after 12 months in both the lumbar spine and total hip [32]. Furthermore, a retrospective cohort study found that patients treated with oral monthly ibandronate or weekly bisphosphonates (alendronate and risedronate) had similar low risks of hip fracture, nonvertebral fracture, and any clinical fracture. Ibandronate patients had a significantly lower relative risk of vertebral fracture than weekly bisphosphonate patients [33]. However, there were few studies comparing the change in bone turnover marker after treatment with monthly ibandronate and weekly bisphosphonates. In the present study, although it was of relatively short duration, both treatment regimens showed a similar percentage change of bone turnover marker.
Recently, a study reported that the monthly ibandronate regimen showed a better persistence rate with therapy than the weekly regimen, even though telephone contact was provided only to the monthly ibandronate group [34]. This study was not designed to assess the persistence of treatment. Further studies on monthly ibandronate persistence will be required in Asian countries.
In conclusion, this study confirmed the patient preference for monthly ibandronate over weekly bisphosphonates in Korean. Moreover, the present study indicates that the results previously reported vs. alendronate are also applicable to risedronate. Monthly ibandronate showed similar reduction of bone turnover marker compared to a weekly regimen. Patients with a monthly ibandronate regimen experienced fewer upper GI adverse events (nausea, abdominal distension) in this study.
References
Cooper C, Compion G, Melton LJ (1992) Hip fractures in the elderly: a worldwide projection. Osteoporos Int 2:285–289
Chesnut IC, Skag A, Christiansen C, Recker R, Stakkestad JA, Hoiseth A, Felsenberg D, Huss H, Gilbride J, Schimmer RC, Delmas PD (2004) Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. J Bone Miner Res 19:1241–1249
Epstein S (2005) The roles of bone mineral density, bone turnover, and other properties in reducing fracture risk during antiresorptive therapy. Mayo Clin Proc 80:379–388
Harris ST, Watts NB, Genant HK, McKeever CD, Hangartner T, Keller M, Chesnut CH III, Brown J, Eriksen EF, Hoseyni MS, Axelrod DW, Miller PD (1999) Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy with Risedronate Therapy (VERT) Study Group. JAMA 282:1344–1352
Liberman UA, Weiss SR, Broll J, Minne HW, Quan H, Bell NH, Rodriguez-Portales J, Downs RW Jr, Dequeker J, Favus M (1995) Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. The Alendronate Phase III Osteoporosis Treatment Study Group. N Engl J Med 333:1437–1443
Miller PD, McClung MR, Macovei L, Stakkestad JA, Luckey M, Bonvoisin B, Reginster JY, Recker RR, Hughes C, Lewiecki EM, Felsenberg D, Delmas PD, Kendler DL, Bolognese MA, Mairon N, Cooper C (2005) Monthly oral ibandronate therapy in postmenopausal osteoporosis: 1-year results from the MOBILE study. J Bone Miner Res 20:1315–1322
World Health Organization (2003) Prevention and management of osteoporosis. World Health Organ Tech Rep Ser 921:1–164
Reginster JY, Rabenda V, Neuprez A (2006) Adherence, patient preference and dosing frequency: understanding the relationship. Bone 38:S2–S6
Lombas C, Hakim C, Znachetta JR (2001) Compliance with alendronate treatment in an osteoporosis clinic. J Bone Miner Res 15 Suppl 1:S529
Jahng KH, Martin LR, Golin CE, DiMatteo MR (2005) Preferences for medical collaboration: patient–physician congruence and patient outcomes. Patient Educ Couns 57:308–314
Kendler D, Kung AW, Fuleihan Gel H, Gonzalez Gonzalez JG, Gaines KA, Verbruggen N, Melton ME (2004) Patients with osteoporosis prefer once weekly to once daily dosing with alendronate. Maturitas 48:243–251
Simon JA, Lewiecki EM, Smith ME, Petruschke RA, Wang L, Palmisano JJ (2002) Patient preference for once-weekly alendronate 70 mg versus once-daily alendronate 10 mg: a multicenter, randomized, open-label, crossover study. Clin Ther 24:1871–1886
Bartl R, Goette S, Hadji P, Hammerschmidt T (2005) Persistence and compliance with daily and weekly administered bisphophonates for osteoporosis treatment in Germany. Osteoporos Int 16 Suppl 3:S45
Cramer JA, Amonkar MM, Hebborn A, Suppapanya N (2004) Does dosing regimen impact persistence with bisphosphonate theapy among postmenopausal osteoporotic women. J Bone Miner Res 19 Suppl 1:S448
Ettinger MP, Gallagher R, Amonkar M, Mahoney PM, Gilbride J (2004) Medication persistence is improved with less frequent dosing of bisphosphonates, but remains inadequate. Arthritis Rheum 50:S513
Emkey R, Koltun W, Beusterien K, Seidman L, Kivitz A, Devas V, Masanauskaite D (2005) Patient preference for once-monthly ibandronate versus once-weekly alendronate in a randomized, open-label, cross-over trial: the Boniva Alendronate Trial in Osteoporosis (BALTO). Curr Med Res Opin 21:1895–1903
Hadji P, Minne H, Pfeifer M, Bourgeois P, Fardellone P, Licata A, Devas V, Masanauskaite D, Barrett-Connor E (2008) Treatment preference for monthly oral ibandronate and weekly oral alendronate in women with postmenopausal osteoporosis: a randomized, crossover study (BALTO II). Joint Bone Spine 75:303–310
Gart JJ (1969) An exact test for comparing matched proportions in cross-over designs. Biometrika 56:75–80
Prescott RJ (1981) The comparison of success rates in cross-over trials in the presence of an order effect. Appl Stat 30:9–15
Claxton AJ, Cramer J, Pierce C (2001) A systematic review of the associations between dose regimens and medication compliance. Clin Ther 23:1296–1310
Richter A, Anton SE, Koch P, Dennett SL (2003) The impact of reducing dose frequency on health outcomes. Clin Ther 25:2306–2335
Curtis JR, Westfall AO, Cheng H, Saag KG, Delzell E (2009) Risedronate and Alendronate Intervention over Three Years (REALITY): minimal differences in fracture risk reduction. Osteoporos Int 20:973–978
Takada J, Iba K, Imoto K, Yamashita T (2007) Changes in bone resorption markers among Japanese patients with postmenopausal osteoporosis treated with alendronate and risedronate. J Bone Miner Metab 25:142–146
Umland EM, Boyce EG (2001) Risedronate: a new oral bisphosphonate. Clin Ther 23:1409–1421
Kamatari M, Koto S, Ozawa N, Urao C, Suzuki Y, Akasaka E, Yanagimoto K, Sakota K (2007) Factors affecting long-term compliance of osteoporotic patients with bisphosphonate treatment and QOL assessment in actual practice: alendronate and risedronate. J Bone Miner Metab 25:302–309
Eisman JA, Rizzoli R, Roman-Ivorra J, Lipschitz S, Verbruggen N, Gaines KA, Melton ME (2004) Upper gastrointestinal and overall tolerability of alendronate once weekly in patients with osteoporosis: results of a randomized, double-blind, placebo-controlled study. Curr Med Res Opin 20:699–705
Hamilton B, McCoy K, Taggart H (2003) Tolerability and compliance with risedronate in clinical practice. Osteoporos Int 14:259–262
Lanza FL, Hunt RH, Thomson AB, Provenza JM, Blank MA (2000) Endoscopic comparison of esophageal and gastroduodenal effects of risedronate and alendronate in postmenopausal women. Gastroenterology 119:631–638
Rossini M, Bianchi G, Di Munno O, Giannini S, Minisola S, Sinigaglia L, Adami S (2006) Determinants of adherence to osteoporosis treatment in clinical practice. Osteoporos Int 17:914–921
Tosteson AN, Grove MR, Hammond CS, Moncur MM, Ray GT, Hebert GM, Pressman AR, Ettinger B (2003) Early discontinuation of treatment for osteoporosis. Am J Med 115:209–216
Delmas PD, Recker RR, Chesnut CH III, Skag A, Stakkestad JA, Emkey R, Gilbride J, Schimmer RC, Christiansen C (2004) Daily and intermittent oral ibandronate normalize bone turnover and provide significant reduction in vertebral fracture risk: results from the BONE study. Osteoporos Int 15:792–798
Miller PD, Epstein S, Sedarati F, Reginster JY (2008) Once-monthly oral ibandronate compared with weekly oral alendronate in postmenopausal osteoporosis: results from the head-to-head MOTION study. Curr Med Res Opin 24:207–213
Harris ST, Reginster JY, Harley C, Blumentals WA, Poston SA, Barr CE, Silverman SL (2009) Risk of fracture in women treated with monthly oral ibandronate or weekly bisphosphonates: The Evaluation of Ibandronate Efficacy (VIBE) database fracture study. Bone 44:758–765
Cooper A, Drake J, Brankin E (2006) Treatment persistence with once-monthly ibandronate and patient support vs. once-weekly alendronate: results from the PERSIST study. Int J Clin Pract 60:896–905
Acknowledgement
We thank Yong Jun Choi for manuscript preparation.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was conducted as clinical trial of GSK Korea as protocol number 109393.
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Chung, YS., Lim, SK., Chung, HY. et al. Comparison of Monthly Ibandronate Versus Weekly Risedronate in Preference, Convenience, and Bone Turnover Markers in Korean Postmenopausal Osteoporotic Women. Calcif Tissue Int 85, 389–397 (2009). https://doi.org/10.1007/s00223-009-9294-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-009-9294-y