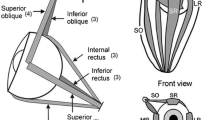
Abstract
The most conspicuous feature of the rabbit retina is the visual streak that extends along the horizontal azimuth from the nasal margin to the temporal limit of the retina. We believe the streak processes movement vision and that the temporal region (area centralis) is responsible for pattern perception. Both anatomical and behavioural experiments were used to test this hypothesis. Behavioural measures of pattern vision in normal and chiasma-sectioned rabbits revealed both to have the same visual acuity. Using OKN as a measure of movement vision, normal rabbits showed both a directional and velocity-tuned response. The chiasma-sectioned rabbits, with only uncrossed fibre projections remaining, showed a total loss of movement detection. The injection of HRP into the vitreal chamber of one eye in normal rabbits revealed extensive uptake throughout the contralateral thalamus. In the ipsilateral thalamus, there was uptake solely from the ipsilateral retinal projection to a restricted wafer of the lateral geniculate nucleus (LGN). The chiasma cut rabbits showed a very different distribution of HRP in the thalamus. The uptake was restricted to a thin wafer of the LGN, with no contralateral uptake. Thus, the thalamic projections from the retinal area centralis were strictly segregated from the thalamic target areas for the visual streak without any overlap. These findings provide strong evidence for separate retinal origins with anatomically separate pathways for pattern and movement vision in the rabbit.






Similar content being viewed by others
Abbreviations
- ANOVA:
-
Analysis of variance
- AOS:
-
Accessory optic system
- DPFl:
-
Dorsal paraflocculus
- HVI:
-
Cerebellar vermal lobus simplex
- HVII:
-
Vermal lobule
- LDN:
-
Lateral dorsal nucleus of the accessory optic system
- LGN:
-
Lateral geniculate nucleus of the thalamus
- LTN:
-
Lateral terminal nucleus of the accessory optic system
- MST:
-
Region in parietal extrastriate cortex anterior to MT
- MT:
-
Region in parietal extrastriate cortex
- MTN:
-
Medial terminal nucleus of the accessory optic system
- M cells:
-
Retinal ganglion cells which in monkey and humans project to the magnocellular layers of the LGN
- P cells:
-
Retinal ganglion cells which in monkey and human project to the parvocellular layers of the LGN
- NOT:
-
Nucleus of the optic tract
- NP:
-
Pontine nucleus
- SPEM:
-
Smooth pursuit eye movements
- V1:
-
Striate cortex or Brodmann area of 17 of the cerebral cortex
- V2:
-
Brodmann area 18 of the cortex
- VOR:
-
Vestibulo-ocular reflex
- X cells:
-
Retinal ganglion cells with small receptive fields—tuned to patterns
- Y cells:
-
Retinal ganglion cells with large receptive fields—tuned to motion
References
Alley K, Baker R, Simpson JI (1975) Afferents to the vestibulo-cerebellum and the origin of the visual climbing fibers in the rabbit. Brain Res 98:582–589
Amthor ES, Takahashi ES, Oyster CW (1989) Morphologies of rabbit retinal ganglion cells with complex receptive fields. J comp Neurol 280:97–121
Baker J, Gibson A, Glickstein M, Stein J (1976) Visual cells in the pontine nuclei of the cat. J Physiol 255:415–433
Barash S, Melikyan A, Sivakov A, Zhang M, Glickstein M, Thier P (1999) Saccadic dysmetria and adaptation after lesions of the cerebellar cortex. J Neurosci 19:10931–10939
Barlow RM, Hill RM (1963) Selective sensitivity to direction of movement in ganglion of the rabbit retina. Science 139:412–414
Barlow HB, Hill RM, Levick WR (1964) Retinal ganglion cell responding selectively to direction and speed of image motion in the rabbit. J Physiol Lond 173:377–407
Buhl EH, Peichl I (1986) morphology of rabbit retinal ganglion cells projecting to the medial terminal nucleus of the optic system. J Comp Neurol 253:163–174
Bunt AH, Minckler DS, Johanson GW (1977) Demonstration of bilateral projection of the central retina of the monkey with horseradish peroxidase neuronography. J Comp Neurol 171:619–630
Bushnell MC, Goldberg ME, Robinson DL (1981) Behavioral enhancement of visual responses in monkey cerebral cortex. I. Modulation in the posterior parietal cortex related to selective attention. J Neurophysiol 46:755–772
Caldwell JH, Daw NW (1978a) New properties of rabbit retinal ganglion cells. J Physiol Lond 276:257–276
Caldwell JH, Daw NW (1978b) Effects of picrotoxin and strychnine on rabbit retinal ganglion cells: changes in centre surround receptive fields. J. Physiol Lond 276:299–310
Caldwell JH, Daw NW, Wyatt HJ (1978) Effects of picrotoxin and strychnine on rabbit ganglion cells: lateral interaction for cells with more complex receptive fields. J Physiol Lond 276:288–298
Chievitz JH (1891) Ueber das Vorkommen der area centralis retinae in den Hoeneren Wirbetierklassen. R Anat Entwichlingsgesch Suppl 139:311–334
Chow KL, Douville A, Mascetti GG, Grobstein P (1977) Receptive field characteristics of neurons in a visual area of the rabbit temporal cortex. J Comp Neurol 171:135–146
Christie D, Steele-Russell I (1989) The effects of anteromedial cortex lesions on pattern discrimination learning. Behav Brain Res 35:135–146
Collewijn H (1975) Direction-selective units in the rabbit’s nucleus of the otic tract. Brain Res 100:489–508
Collewijn H (1977) Head and eye movements in freely moving rabbits. J Physiol (Lond) 266:47–498
Collewijn H (1981) The occulomotor system of the rabbit and its plasticity. Springer, Berlin, pp 1–237
DeVries SH, Baylor DA (1997) Mosaic arrangement of ganglion cell receptive fields in the rabbit retina. J Neurophysiol 78:2048–2060
Famiglietti EV (2005) Small-tufted ganglion cells and two visual systems for the detection of object motion in rabbit retina. Vis Neurosci 22:509–534
Famiglietti EV (2009) Bistratified ganglion cells of rabbit retina: neural architecture for contrast independent visual responses. Vis Neurosci 26:195–213
Fuchs AF, Robinson FR, Straube A (1993) Role of the caudal fastigial nucleus in saccade generation. I. Neuronal discharge pattern. J Neurophysiol 70:1723–1740
Fuchs AF, Robinson FR, Straube A (1994) Participation of the caudal fastigial nucleus in smooth-pursuit eye movements. I. Neuronal activity. J Neurophysiol 72:2714–2728
Ghelarducci B, Ito M, Yagi N (1975) Impulse discharges from the flocculus Purkinje cells of alert rabbit during visual stimulation combined with horizontal head rotation. Brain Res 87:66–72
Giolli RA, Guthrie MD (1969) The primary optic projections in the rabbit. An experimental degeneration study. J Comp Neurol 136:99–126
Giolli RA, Guthrie MD (1971) Organization of the subcortical projections of visual areas I and II in the rabbit. An experimental degeneration study. J Comp Neurol 142:351–376
Giolli RA, Towns LC, Takahashi TT, Karamanlis AN, Williams DD (1978) An autoradiographic study of the projection of visual cortical area I to the thalamus, pretectum and superior colliculus of the rabbit. J Comp Neurol 180:743–752
Glickstein M, Cohen JL, Dixon B, Gibson A, Hollins M, Labossiere E, Robinson F (1980) Corticopontine visual projections in macaque monkeys. J Comp Neurol 190:209–229
Glickstein M, May JG, Mercier BE (1985) Corticopontine projection in the macaque: the distribution of labelled cortical cells after large injections of horseradish peroxidase in the pontine nuclei. J Comp Neurol 235:343–359
Glickstein M, May J, Mercier B (1990) Visual corticopontine and tectopontine projections in the macaque. Arch Ital Biol 128:273–293
Glickstein M, Gerrits N, Kralj-Hans I, Mercier B, Stein J, Voogd J (1994) Visual pontocerebellar projections in the macaque. J Comp Neurol 349:51–72
Graybiel AM (1975) Anatomical organisation of the retinotectal afferents in the cat: an autoradiographic study. Brain Res 96:1–23
Graybiel AM (1976) Evidence for banding of the cat’s ipsilateral retinotectal connection. Brain Res 114:318–327
Hall RD, Lindholm EP (1974) Organisation of the motor and somato- sensory neocortex in the albino rat. Brain Res 66:23–38
Harting JK (1977) Descending pathways from the superior colliculus: an autoradiographic analysis in the rhesus monkey (Macaca mulatta). J Comp Neurol 173:583–612
Hayhow WR (1959) An experimental study of the accessory optic fiber system in the cat. J Comp Neurol 113:281–314
Hughes A (1971) Topographical relationships between anatomy and physiology of the rabbit visual system. Doc Ophthalmol 30:33–159
Hughes A (1977) Topography of vision in mammals of contrasting life style: comparative optics and retinal organisation. In: Crescitelli F (ed) Handbook of sensory physiology, vol VII/5, the visual system in vertebrates, Springer-Verlag, pp 613–756
Hughes A, Vaney DI (1982) The organisation of the binocular cortex in the primary visual area of the rabbit. J Comp Neurol 204(1982):151–164
Hughes A, Wässle H (1976) The cat optic nerve. Fibre total count and diameter spectrum. J Comp Neurol 169(1976):171–184
Hughes A, Wilson ME (1969) Callosal terminations along the boundary between visual areas I and II in the rabbit. Brain Res 12:19–25
Julesz B (1964) Binocular depth perception without familiarity cues. Science 145:356–362
Kanjhan R, Sivyer B (2010) Two types of ON direction-selective ganglion cells in rabbit retina. Neurosci Lett 483:105–109
Kirk DL, Levick WR, Cleland BG, Wässle H (1976) Crossed and uncrossed representation of the visual field by brisk sustained and brisk transient cat retinal ganglion cells. Vis Res 16:225–231
Kralj-Hans I, Baiser JS, Glickstein M (2007) Independent roles for the dorsal paraflocculus and vermal lobule VII of the cerebellum invisuomotor coordination. Exp Brain Res 177:209–222
Krauzlis RJ, Miles FA (1998) Role of the oculomotor vermis in generating pursuit and saccades: effects of microstimulation. J Neurophysiol 80:2046–2062
Krettik JE, Price JL (1977) The cortical projections of the mediodorsal nucleus and adjacent thalamic nuclei in the rat. J Comp Neurol 171:157–192
Leonard CM (1969) The prefrontal cortex of the rat. I. Cortical projection of the mediodorsal nucleus. II. Efferent connections. Brain Res 12:321–343
Levick W (1967) Receptive fields and trigger features of ganglion cells in the visual streak of the rabbit’s retina. J Physiol (Lond) 188:285–307
Lisberger SG, Fuchs AF (1974) Response of flocculus Purkinje cells to adequate vestibular stimulation in the alert monkey: fixation vs compensatory eye movements. Brain Res 69:347–353
Lynch JC (1980) The functional organisation posterior parietal association cortex. Behav Brain Sci 3:485–534
Lynch JC, McLaren JW (1982) The contribution of parieto-occipital association cortex to the control of slow eye movements. In: Lennerstrand G, Zee DS, Keller E (eds) Functional basis of ocular mobility disorders. Pergamon Press, Oxford, pp 501–510
Maekawa K, Simpson JI (1972) Climbing fiber activation of Purkinje cells in the flocculus by impulses transferred through the visual pathway. Brain Res 39:245–251
Maekawa K, Simpson JI (1973) Climbing fiber responses evoked in vestibulo cerellum of rabbit from visual system. J Neurophysiol 36:649–666
Maekawa K, Takeda T (1975) Mossy fiber responses evaoked in the cerebellar flocculus of rabbits by stimulation of the optic pathway. Brain Res 98:590–595
Maekawa R, Takeda T (1976) Electrophysiological identification of the climbing and mossy fiber from the rabbit’s retina to the contralateral cerebellar floccus. Brain Res 109:169–174
Mathers LH, Douville A, Chow KL (1977) Anatomical studies of a temporal visual area in the rabbit. J Comp Neurol 17:147–156
Matsuzaki R, Kyuhou S (1997) Pontine neurons which relay pro- jections from the superior colliculus to the posterior vermis of the cerebellum in the cat: distribution and visual properties. Neurosci Lett 236:99–102
Mesulam MM (1978) Tetramethylbenzidine for horseradish peroxidase neurohistochemistry: a noncarcinogenic blue reaction product with superior sensitivity for visualising neural afferents and efferents. J Neurohistochem Cytochem 26:106–117
Mesulam MM, Mufson EJ (1980) The rapid anterograde transport of horseradish peroxidase. Neuroscience 6:231–246
Miles FA, Fuller JH (1975) Adaptive plasticity in the vestibulo-ocular responses of the rhesus monkey. Brain Res 80:512–516
Mizuno N, Mochizuli K, Akimoto C, Matsushima R (1973) Pretectal projections to the inferior in the rabbit. Exp Neurol 39:498–506
Montarolo PG, Precht W, Strata P (1981) Functional organisation of the mechanisms subserving optokinetic nystagmus in the cat. Neurosci 6:231–246
Montero VM, Murphy EH (1976) Cortico-cortical connections from the striate cortex in the rabbit. Anat Rec 184:483
Mower G, Gibson A, Glickstein M (1979) Tectopontine pathway in the cat: laminar distribution of cells of origin and visual properties of target cells in dorsolateral pontine nucleus. J Neurophysiol 42:1–15
Mustari MJ, Fuchs AF, Wallman J (1988) Response properties of dorsolateral pontine units during smooth pursuit in the rhe- sus macaque. J Neurophysiol 60:664–686
Noda H, Fujikado T (1987) Topography of the oculomotor area of the cerebellar vermis in macaques as determined by microstimulation. J Neurophysiol 58:359–378
Optican LM, Robinson DA (1980) Cerebellar-dependent adaptive control of primate saccadic system. J Neurophysiol 44:1058–1076
Oyster CW (1968) The analysis of image motion by the rabbit retina. J Physiol Lond 199:613–635
Oyster CW, Simpson JI, Takahashi ES, Soodak RE (1980) Retinal ganglion cells projecting to the rabbit accessory optical system. J Comp Neurol 190:49–61
Pasik T, Pasik P (1964) Optokinetic nystagmus: an unlearned response altered by section of the chiasma and corpus callosum in monkeys. Nature 203:609–611
Pasik P, Pasik T, Krieger HP (1959) The effects of cerebral lesions upon opto- kinetic nystagmus in monkeys. J Neurophysiol 22:297–304
Provis JM (1979) The distribution and size of ganglion cells in the retina of the pigmented rabbit: a quantitative analysis. J Comp Neurol 185:121–138
Pu MI, Amthor FR (1990) Dendritic morphologies of retinal ganglion cells projecting to the lateral geniculate nucleusin the rabbit. J comp Neurol 302:675–693
Reep RL, Corvin JV, Hashimoto A, Watson RT (1984) Afferent connections of medial precentral cortex in the rat. Neurosci Lett 44:247–252
Ritchie L (1976) Effects of cerebellar lesions on saccadic eye movements. J Neurophysiol 39:1246–1256
Robinson DA, Fuchs AF (1969) Eye movements evoked by stimulation of the frontal eye fields. J Neurophysiol 32:637–648
Rockhill RL, Daly FI, MacNeil MA, Brown SP, Masland RH (2002) The diversity of ganglion cells in the mammalian retina. J Neuriosci 22:3831–3843
Simpson JI, Alley KE (1974) Visual climbing fiber input to rabbit vestiblocerebellum: a source of direction-specific information. Brain Res 82:302–308
Simpson JI, Hess R (1977) Complex and simple visual messages in the flocculus. In: Baker R, Berthoz A (eds) Control of gaze by brain stem neurons (developments in neuroscience, vol 1). Elsevier, Amsterdam, pp 351–360
Sivyer B, Vaney DL (2010) Dendritic morphology and tracer-coupling pattern of physiologically identified transient uniformity detector ganglion cells in rabbit retina. Vis Neurosci 27:159–170
Soodack RE, Simpson JI (1988) The accessory optic system of rabbit. I. Basic visual response properties. J Neurophysiol 60(6):2037–2054
Steele-Russell I (1982) Some considerations of functional recovery following brain damage. Human Neurobiol 1:68–72
Steele-Russell I (2011) The use of commissurotomy in studies of interhemispheric communication. In: Lane EL, Dunnett SB (eds) Contemporary animal models of movement disorders. Neuromethods. Springer, Berlin
Steele-Russell I, van Hof MW, Pereira SC (1983) Angular acuity in normal and commissure-sectioned rabbits. Behav Brain Res 8(3):167–176
Steele-Russell I, Van Hof MW, Pereira SC, James G (1984) The role of the uncrossed optic fibres in rabbit vision. Behav Brain Res 12:232–233
Steele-Russell I, Van Hof MW, Van der Steen J, Collewijn H (1987) Visual and oculomotor function in optic chiasma-sectioned rabbits. Exp Brain Res 66:61–73
Steele-Russell I, Russell MI, Castiglioni JA, Swetlow B, Werka T (2008) The role of sensory pathways in Pavlovian conditioning in the rabbit. Exp Brain Res 185:199–213
Stone JA (1965) A quantitative analysis of the distribution of ganglion cells in cat’s retina. J comp Neurol 124:337–352
Stone J (1978) The number and distribution of ganglion cells in the cat’s retina. J Comp Neurol 180:753–772
Stone J (1981) The whole mount handbook: a guide to the preparation and analysis of retinal whole mounts. Maitland Publications, Sydney
Stone J, Fukuda Y (1974) Properties of cat retinal ganglion cells: a comparison W-cells with X- and Y-cells. J Neurophysiol 37:722–748
Stone J, Leicester J, Sherman SM (1973) The naso-temporal division of the monkey retina. J Comp Neurol 150:333–348
Suzuki DA, Noda H, Kase M (1981) Visual and pursuit eye movement-related activity in the posterior vermis of monkey cerebellum. J Neurophysiol 46:1120–1139
Suzuki DA, May JG, Keller EL, Yee RD (1990) Visual motion response properties of neurons in dorsolateral pontine nucleus of alert monkey. J Neurophysiol 63:37–59
Takeda T, Maekawa K (1976) The origin of the pretecto-tract. A study the horse raddish peroxidase method. Brain Res 117:319–325
Van Hof MW (1967) Visual acuity in the rabbit. Vision Res 7:749–751
Van Hof MW, Steele-Russell I (1976) Binocular vision in the rabbit. Physiol Behav 19:121–128
Van Wyk M, Roland Taylor W, Vaney DL (2006) Local edge detectors: a substrate for fine spatial vision at low temporal frequencies in rabbit retina. J Neurosci 26:13250–13263
Vaney DI, Hughes A (1976) The rabbit optic nerve: fiber diameter spectrum, fiber count, and comparison with retinal cell count. J comp Neurol 170:241–252
Vaney DI, Wässle Peichl, Illing RB (1981) Almost all ganglion cells in the rabbit retina project to the superior colliculus. Brain Res 212:447–453
Wagman IH, Krieger HP, Papatheodorou CA, Bender M (1961) Eye movements elicited by surface and depth stimulation of the frontal lobe of Macaca mulata. J Comp Neurol 117:179–188
Walley RE (1967) Receptive fields in the accessory optic system of the rabbit. Exp Neurol 17:27–43
Wässle H (2004) Parallel processing in the mammalian retina. Nat Rev Neurosci 5:1–11
Wässle H, Levick WR, Cleland BR (1975) The distribution of the alpha type of ganglion cells in the cat retina. J Comp Neurol 159:419–438
Winfield JA, Hendrickson A, Kim J (1978) Anatomical evidence that the medial terminal nucleus of the accessory optic tract in mammals provides a visual mossy fiber input to the flocculus. Brain Res 151:175–182
Zuidam I, Collewijn H (1979) Vergence eye movements of the rabbit in visuomotor behavior. Vision Res 19:185–194
Acknowledgments
This research was supported by Scott &White research grants ID# 1810 to ISR and ID# 7985 to JAC.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Steele-Russell, I., Russell, M.I., Castiglioni, J.A. et al. Differential retinal origins of separate anatomical channels for pattern and motion vision in rabbit. Exp Brain Res 222, 99–111 (2012). https://doi.org/10.1007/s00221-012-3198-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-012-3198-1