Abstract
The path that the hand takes to intercept an elongated moving target depends on the target’s orientation. How quickly do people respond to changes in the moving target’s orientation? In the present study, participants were asked to intercept moving targets that sometimes abruptly changed orientation shortly after they started moving. It took the participants slightly more than 150 ms to adjust their hands’ paths to a change in target orientation. This is about 50 ms longer than it took them to respond to a 5-mm jump in the moving target’s position. It is only slightly shorter than it took them to initiate the movement. We propose that responses to changes in visually perceived orientation are not exceptionally fast, because there is no relationship between target orientation and direction of hand movement that is sufficiently general in everyday life for one to risk making an inappropriate response in order to respond faster.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In everyday life we interact with a wide variety of objects. Occasionally the objects are moving and usually we ourselves are moving. Sometimes there are also other moving objects. This is most evident in team sports such as basketball, where a running player may want to intercept a thrown ball while avoiding several opponents. Since sensory resolution is limited, predicting future positions from all these movements is prone to error, even for the theoretically predictable trajectory of a thrown ball. Moreover, many movements are clearly not predictable. For instance, another player’s hand may deflect the ball in an attempt to catch it. Moreover, the opponents themselves may move differently than the player expected, or even push him so that he himself moves differently than he expected. In such cases it is clearly advantageous to be able to respond quickly to new visual information.
The results of a recent study suggested that it might be so advantageous to react quickly that people take the risk of responding incorrectly in order to respond faster. In that study obstacles sometimes jumped to new positions while the subjects moved their hand towards a target (Aivar et al. 2008). In one condition, colliding with the obstacle could only be avoided by moving in the opposite direction than the one in which the obstacle had jumped. Subjects initially responded by following the obstacle with their hand. About 50 ms later a response in the appropriate direction replaced this initial incorrect one.
Inappropriate initial responses in the direction in which a target moved have been found when the task is to move in the opposite direction than the target (Day and Lyon 2000), and when the task is to stop the movement if the target changes colour at the moment that it is displaced (Pisella et al. 1998). Inappropriate responses have also been found when there is irrelevant motion in the background (Brenner and Smeets 1997; Whitney et al. 2003). These responses may even be controlled sub-cortically (Day and Brown 2001), although the posterior parietal cortex also seems to be involved (Pisella et al. 2000). All this suggests that the initial response is a direct response to certain visual information (the motion or displacement), rather than being based on a new evaluation of the circumstances.
In recent years it has become evident that successful interception relies on a large number of visual and other sources of information (reviewed in Zago et al. 2009). Are exceptionally fast responses only possible for changes in position? In order to answer this question one must examine other kinds of information for which such fast responses are likely to have evolved. Fast responses are most likely to be found for attributes that are important for action. The most obvious attribute to consider (besides position and change in position) is orientation. A target’s orientation influences the trajectory that one takes to reach it, both for static targets (Brenner and Smeets 1995) and for moving ones (Brenner and Smeets 2007). Moreover, having to judge the target’s orientation does not delay the response to a change in target position—in the way that having to judge colour or texture does—when the orientation is used to recognize the target rather than to select a path (Veerman et al. 2008). The present study examines how people respond to a change in the orientation of a moving target that they are trying to intercept. If they respond directly to the visually perceived orientation we expect such responses to be exceptionally fast and reproducible, as they are for changes in position.
Methods
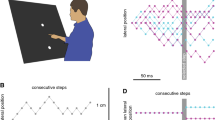
We used a simple interception task in which the target (an oriented ellipse) always appeared at the same place and moved in the same manner. The only difference between trials was the target’s orientation (Fig. 1). The task was always to hit the target as quickly as possible. On some trials the target orientation changed instantaneously near the moment that the hand started moving. We examined how much time it took to adjust the hand’s path. More specifically, we compared the direction in which the hand was moving at each moment in time on trials in which the target orientation did and did not change. Of course the change must take place early during the movement because otherwise there may be no possibility to adjust the path adequately because the hand will have already moved too far. Moreover, later in the movement the direction of motion is likely to be more variable.
Subjects moved a stylus from a starting point across the surface of a graphics tablet to intercept an elongated target. Sometimes the target’s orientation changed. The six conditions are described schematically on the right. In half of the trials the luminance gradient within the target was as shown and in the other half it was exactly the opposite. Counter-clockwise target rotation is considered positive
To be able to determine whether a change in movement direction is an automatic response to the target’s rotation or a re-evaluation of the circumstances, we used asymmetrically coloured targets (see Fig. 1) so that we could distinguish between −90° (clockwise) and +90° (counter-clockwise) changes in orientation. For −90° and +90° changes in orientation the targets appear to rotate in opposite directions, but the rotations result in the same target orientation. Of course an instantaneous −90° rotation is still equivalent to an instantaneous +270° rotation, but the percept is that of the smaller change in orientation. A control experiment was conducted in which the target jumped 5 mm rather than its orientation changing. The purpose of the control experiment was to determine the latency of fast responses to target displacement under similar conditions and for a comparable magnitude of effect and analysis of the data as for changes in target orientation.
Subjects and sessions
Altogether, there were four sessions. In the three sessions of the main experiment the targets sometimes rotated. The sessions differed in the moment at which this happened. In the single session of the control experiment the target sometimes jumped, either in the direction of motion or in the opposite direction. Eight subjects took part in each session. Several subjects, including one of the authors, took part in more than one session. Only the authors were aware of the purpose of the experiments, but the presence of different target orientations and of the changes in target orientation in the main experiment was evident to all. In the control experiment the perturbations were not readily visible. On questioning after having performed the experiment none of the seven (non-author) subjects reported having noticed a jump in target position, but two believed that the speed differed across trials, which may be the result of the jumps. All subjects noticed that the asymmetry in the colour within the target differed across trials.
Equipment
Images (1024 by 768 pixels; 85 Hz) were projected from above onto a back-projection screen, 20 cm above a half-silvered mirror. There was a large (WACOM A2) drawing tablet 20 cm below the mirror, positioned so that it coincided precisely with the apparent position of the screen as seen through the mirror. Thus the images appeared to the subject to be on the tablet. Subjects moved a stylus across the surface of the tablet. The stylus looked and felt like a normal pen, and was held like a normal pen, but it did not leave any trace when it was moved. Instead, the tablet determined the position of its tip at 200 Hz. Lamps between the half-silvered mirror and the drawing tablet ensured that subjects could clearly see the stylus and their hand as well as the projected images. A simple calibration whereby the experimenter aligned the tip of the stylus with small disks presented on the screen allowed us to later relate any position in the image to a position on the surface of the drawing tablet, and vice versa.
Stimulus and procedure
Subjects had to intercept the moving targets by moving the stylus across the drawing tablet. The instruction was to hit the targets as quickly as possible without lifting the stylus off the surface. The stylus had to make contact with the target, but it did not have to stop when it did so. Due to the elongated shape of the targets, and the time pressure that encouraged subjects to intercept the targets by moving the stylus across them without slowing down substantially, it was clearly advantageous to aim for the less curved sides when approaching the targets. However we did not explicitly instruct subjects to do so.
Subjects started a trial by moving the stylus to a 0.5-cm diameter green disk. At a random time between 0.5 and 2 s after they did so, if the stylus was still at the starting point, the target appeared. If the stylus had started moving in anticipation of the target appearing nothing happened, and the subject had to return the stylus to the starting point to start the trial. The targets moved at 20 cm/s from left to right across a red background. They first appeared 12 cm to the left of, and 20 cm further away than, the starting point. They were ellipses that were 3 cm long and 0.6 cm wide, and were black at one end and white at the other, with a gradual change in luminance along the long axis. The target stopped and a short tone could be heard if the target was hit.
There were 50 trials for each of the six conditions within each session of the main experiment (see Fig. 1). The conditions only differed in the orientation of the target. In five of the six conditions the target was initially oriented at −30° (whereby zero degrees means that the target’s long axis is aligned with the direction of motion, and a counter-clockwise rotation is considered to be positive). In one of these five conditions the orientation did not change, and in the others it changed by −45°, 45°, −90° and 90°. In the sixth condition the target was oriented at 60° from the start. This condition was included to encourage the subjects to attend to the orientation as soon as the target appeared. In half the trials of each condition one of the ends of the ellipse was black, and in the other half the other end of the ellipse was black. The condition on each trial was chosen at random from all trials that remained to be done.
The only difference between the three sessions of the main experiment is whether the target’s orientation changed 100, 200 or 300 ms after the target appeared (in the four conditions in which it changed). The control experiment consisted of a single session with seven conditions. The target was always oriented at −30°. After 100, 200 or 300 ms the target could be displaced by 5 mm either in the direction of motion or in the opposite direction. Each of the six combinations of the time and direction of the displacement was presented 30 times. The seventh condition, in which there was no displacement, was presented 90 times (it was presented more often in order to have the same fraction of perturbed trials as in the main experiment). Again half the targets of each condition were black at one end and half at the other, although in this experiment the gradient served no purpose other than to keep the stimuli identical to those of the main experiment. The task and procedure were identical to those of the main experiment, except that we asked subjects whether they noticed anything about the target after the session (in the main experiment there was no need ask whether they noticed the change in orientation because it was obvious that they did).
Analysis
Our main interest was in the responses to the perturbations: the ±45° and ±90° target rotations in the main experiment and the 5-mm target shifts in the control experiment. Our prediction was that if the target rotated −45° subjects would veer to the left, opposite the target’s overall direction of motion, in order to approach the target further from the side. By doing so they would approach the target at an angle that is closer to 90° than it would be if they continued on the path that they would have taken if the orientation had not changed. They could also achieve the latter by moving more slowly and hitting the target later, and thus further along its path. If the target rotated +45° we predict the opposite response: veering to the right and moving faster to hit the target earlier, and thus further to the left. If the direction in which the hand moves is directly linked to the direction of rotation, we expect to see responses in the same directions for ±90° changes in target orientation as for ±45° changes. If only the final target orientation matters −90° and +90° rotations will give identical responses. For the control experiment we expect the hand to veer in the direction of the target displacement.
Thus, we expect to see an effect on the direction in which the stylus moves. We determined the stylus’ direction of motion (as well as its speed) in the simplest possible manner from consecutively recorded positions. Rather than smoothing the data by filtering in time, we did so by averaging across trials. The advantage of doing so is that the temporal resolution is maintained. We used the median rather than the mean to reduce the sensitivity to outliers. Since we are particularly interested in fast direct responses to the visual images, that can be expected to be consistent across subjects, we conduct our further analysis on the basis of the subjects’ average values rather than on the basis of individual trials. To nevertheless get some idea of the variability between trials, we also analysed the lateral variability (the inter-quartile range) in the position of the stylus when it was half way to the target (in the sagittal direction). Since the starting point was always the same, the lateral position can be considered to represent the cumulative direction of motion during the first half of the movement. Since we cannot be completely sure that subjects do not change the timing of their movements rather than the trajectory, despite the instruction to hit as quickly as possible, we also examined the median reaction and movement times.
The appearance of the first image of the target within a trial (or of the first image with a new target orientation a fixed time later) did not necessarily coincide with a measurement of the stylus’ position. Moreover, images were presented at a different rate (85 Hz) than the rate at which the stylus’ position was determined (200 Hz). In order to precisely align the timing of all movement traces to the moment that the target appeared, we used linear interpolation to estimate the stylus’ position within the 5-ms intervals between the individual measurements. For each subject in each condition we then determined the median direction in which the stylus was moving at each moment from when the target appeared (at 1000 Hz based on the above-mentioned interpolation). We used the median value because it automatically gives little weight to occasional trials in which the subject moved very differently, for instance because he or she started moving much too late and therefore in quite a different direction. Consequently, there was no need to remove any trials from the analysis. To isolate the response to the perturbation, we subtracted the median direction on unperturbed trials from that on perturbed ones. We present the average of these differences with the standard error across subjects.
Results
On average, subjects hit 79% of the targets in the main experiment. A mixed factor ANOVA for session (i.e. the time at which the perturbation took place) and condition revealed a significant influence of condition (P < 0.0001), but no interaction with, or main effect of, session. Table 1 shows the average percentages of hit targets for each condition of each session. The number of hit targets appears to mainly depend on the final target orientation, probably because of the benefits of approaching surfaces orthogonally while moving slightly along with the target, but considering the cost of moving the stylus on a very curved path (Brenner and Smeets 2007).
Reaction time was defined as the median time from when the target appeared until the stylus moved at 2 cm/s. The overall average of these median reaction times (across subjects, conditions and sessions) was 211 ms. As was to be expected, a mixed factor ANOVA revealed no significant effects of session or condition (Table 2). The inset in Fig. 2 shows that at least for some subjects the stylus was moving on at least half the trials after only about 170 ms. It is also evident from the inset in Fig. 2 that perturbations that occurred 100 ms after the target appeared generally took place before the movement started, ones that occurred 200 ms after the target appeared took place when the stylus was just starting to move, and ones that occurred 300 ms after the target appeared took place when the stylus was already moving.
Average paths towards targets that rotated −45° (red dots) or +45° (black dots), 200 ms after they appeared. The average path on trials in which the target kept its −30° orientation are shown in blue. The horizontal dashed lines indicate the average sagittal position of the stylus at the indicated time. The vertical dashed lines indicate the lateral position of the target (with the part before the rotation indicated in yellow). The inset shows the median tangential velocity of the stylus. For each subject, the median velocity was determined for each moment after the target appeared, irrespective of the condition or session. The plot shows the mean and standard error across subjects (colour figure online)
Movement time was defined as the time from when the stylus moved at 2 cm/s until it reached the target’s path (even if it missed the target). The overall average of the median movement times for each subject, condition and session was 403 ms. A mixed factor ANOVA for session and condition revealed a significant influence of condition (P < 0.001), but no interaction with, or main effect of, session. Table 3 shows the average movement times for each condition and session. The movements probably took longer in conditions in which the orientation changed to a more difficult one (so that fewer targets were hit) because adjustments to the path made it longer. Figure 2 shows an example of how subjects adjusted the trajectory to the change in target orientation. The adjustments increased the angle at which the stylus approached the targets (as we expected).
From Fig. 2 we can guess that it takes less than 200 ms to respond to a 45° target rotation because the paths clearly start to diverge before the stylus reaches its average position 200 ms later (400 ms after the target appears). However in Fig. 2 the paths are averaged for equivalent traversed percentages of the trajectory rather than for equivalent moments. To get a better estimate of the latency of the response, we examined the direction in which the stylus moved as a function of time.
In order to make it easier to see the response we do not show the direction itself, but the difference between the median direction (at each moment) on trials with and without a perturbation (Fig. 3). It would appear from the figure that it takes about 150 ms to respond to a 45° change in target orientation (for rotations at 100 or 200 ms). This is the moment at which the average values start to systematically diverge, both from the value of zero (i.e. relative to unperturbed trials) and from each other. A latency of 150 ms means that the response takes place 250, 350 and 450 ms after the target appears for rotations at 100, 200 and 300 ms. In order to ensure that the differences that we see in Fig. 3 are consistent across subjects, we used Wilcoxon signed rank tests to compare the individual median responses to clockwise and counter-clockwise target rotations (at each moment). We used one-tailed tests because we can predict the direction of the response. We selected this test because it does not entail assuming that all subjects will respond equally vigorously to the perturbation. We only considered differences that remained significant for at least 50 ms. The vertical dashed lines indicate the moments at which the curves start to differ significantly. This was the case after about 190 ms, which confirms that the response must have consistently started shortly before that time.
Difference in movement direction between trials in which the target rotated −45° (red) or +45° (black) and trials in which the target did not rotate. A deviation to the right is considered positive. Zero is indicated by the horizontal dotted line. Values are shown from the moment of the perturbation. Only values based on at least half of the subjects and on at least half of the trials per subject (velocity threshold of 2 cm/s) are shown. Shaded areas show the standard error across subjects. The vertical dashed lines (in the top two panels) indicate the moment at which the difference between the responses to the two directions of target rotation reaches statistical significance (and remains significant for at least 50 ms; one-tailed Wilcoxon signed rank test; P < 0.05) (colour figure online)
It is not clear that there is a response after about 150 ms for the rotation at 300 ms. A quick look at Fig. 2 suggests why. We expect an initial deflection to the left (downwards in Fig. 3) for a −45° rotation (red traces) because the stylus has to initially move to the left to hit the target from the left (and therefore at a larger angle). However, by 450 ms after the target appeared the stylus has started curving back towards the target, so the expected influence of the target’s orientation reverses (see red dotted path in Fig. 2). We see such a reversal in the bottom panel of Fig. 3, although none of the differences are statistically significant.
The systematic difference between responses to −45° and +45° target rotations, with a latency of about 150 ms, was not found for −90° and +90° rotations (Fig. 4). There appears to be no difference between responses to −90° and +90° rotations (Wilcoxon signed rank tests revealed a significant difference for rotation at 300 ms, but its latency was too short for the difference to be taken seriously: less than 100 ms). When the change in orientation took place at 100 ms there does seem to be an effect of the new orientation after about 200 ms. At that time the hand starts moving more to the right (upwards in the figure). This is the case for both directions of rotation, so it is not revealed by our statistical test. A similar but much less clear inflection after about 200 ms (at about 400 and 500 ms) can be seen for the rotations at 200 and 300 ms. Thus, there appears to be a (slow) response to the new direction, but no response to the rotation itself.
Response to a −90° (red) or +90° (black) target rotation. Further details as in Fig. 3 (colour figure online)
Another change that we found is that the variability across trials increases after ±90° rotations. For targets that maintain a −30° angle, the mean (across subjects, for the session with rotation at 100 ms) inter-quartile range of the lateral position of the stylus when half way to the target was 1.01 cm. After −90° and +90° rotations the mean inter-quartile ranges were 1.81 and 1.80 cm, respectively (both larger than the ranges on unperturbed trials: P < 0.05; one-tailed Wilcoxon signed rank test). After −45° and +45° rotations the inter-quartile ranges also increased, but much more modestly (to 1.28 and 1.08 cm, respectively; only the first being statistically significant). The larger variability is probably mainly related to the new orientation, rather than to responding to the rotation, because the mean inter-quartile range is 2.06 cm for targets that are oriented at 60° from the start.
The larger variability is not surprising because the optimal path depends much more on details such as the timing of the hit for the more awkwardly oriented target. For instance, for the 60° target even the side that one aims for may differ between trials: if one were to hit it early it would be advantageous to initially curve to the right and hit it from the ‘front’, whereas if one were to hit it late it would be advantageous to initially curve to the left and hit it from ‘behind’. Rotations at 200 and 300 ms had very little effect on the variability when half way to the target, but considering a response latency of about 200 ms this is not surprising because the stylus will have already reached the distance in question before there has been enough time to respond.
In order to judge whether a response is exceptionally fast one must consider how the response latency was judged, because the latency that is estimated depends on the way one analyses the data. It can depend on the measure used (position, velocity, acceleration, direction) as well as how one extracts the latency from this measure. For instance, our estimate based on statistical testing across subjects determines the moment at which most subjects are certainly responding. The estimate based on visual inspection of the curves could potentially identify the true onset of the response, but it is biased towards the response of the fastest subject. The latency may also depend on details of the task such as the amplitude and direction of the perturbation (Sarlegna et al. 2003) and on contrast levels (Veerman et al. 2008). Our control experiment provides a direct comparison with the response to a target jump, using the same target, the same analysis and a comparable anticipated response. Previous studies that found exceptionally fast responses to a sudden change in target position used static targets, but in order to keep the conditions as similar as possible to those of the main experiment we displaced the target while it was moving.
The direction in which the stylus moved changed about 100 ms after the moving target jumped (Fig. 5). Wilcoxon signed rank tests revealed that there were significant differences between the responses to 5-mm shifts in the two directions after 132, 127 and 111 ms when the shifts occurred at 100, 200 and 300 ms, respectively. The apparent decrease in latency when the jump occurred later cannot be taken to indicate that responses are faster for changes that occur during the movement because when the jump occurred 100 ms after the target appeared the difference was visible as soon as the stylus started moving. Moreover, such modest differences in latency under specific conditions should not be taken too seriously anyway, because they could easily arise from details that are specific to the experiment such as the retinal eccentricity of the target when it changes orientation or how well it is pursued at that time. A repeated-measures ANOVA showed that there were no significant differences between the seven conditions (no shift, or a 5-mm shift in either direction after 100, 200 or 300 ms) in the percentage of targets hit (on average 96%), in the median reaction time (224 ms) or in the median movement time (351 ms).
Difference in movement direction between trials in which the moving target jumped 5 mm to the left (red) or to the right (black) and trials in which the moving target did not jump. Further details as in Fig. 3 (colour figure online)
Discussion
The responses to the rotation are neither exceptionally fast nor direct responses to a single aspect of the visual stimulation. Subjects hardly took less time to respond to a 45° change in target orientation (Fig. 3) than to initiate the movement when the target first appeared (inset in Fig. 2). This could partly be because the reaction times in the present study are quite short, perhaps as a result of the target always appearing at the same place and moving at the same speed. However, the response to 45° rotations took about 50 ms longer than that to additional 5-mm changes in position under similar conditions (Fig. 5). As already mentioned, it also takes about 50 ms longer to respond adequately to new circumstances after an obstacle is displaced than to respond directly to the displacement (Aivar et al. 2008). Thus, altogether we see no indication of any special mechanism for responding especially quickly to a change in orientation during the movement. Moreover, the responses were not driven directly by the rotation, but by the new target orientation after the change, because we find similar responses for the −90° and +90° target rotations (Fig. 4). It did not appear to matter for the latency to respond to either perturbation whether the hand was already moving or not.
The latency of approximately 100 ms for responding to a shift in target position is consistent with estimates from previous studies in which a static target was displaced (Brenner and Smeets 1997; Pisella et al. 1998; Veerman et al. 2008) and with improved performance when the hand reappears after having been occluded until about 135 ms before reaching the target (Carlton 1981). That the displacement was very small (only 5 mm), the target was moving, and subjects did not notice the displacement (as in Goodale et al. 1986; Prablanc and Martin 1992) does not seem to be important. The modest variability across subjects in the initial response to the shifts (see width of shaded areas in Fig. 5) is consistent with this being an automatic response to the changed visual information.
Desmurget et al. (1996) found that subjects could respond to a change in the orientation of a bar that they were trying to grasp in less than 130 ms. However, that response may have been driven by changes in the positions of the grasping points. When an object rotates around a point between the grasping points, the latter move in opposite directions at the two sides of the object (in their case the changes in position would each be about 2 cm). If grasping emerges from bringing the digits to grasping points (Smeets and Brenner 1999), then moving the grasping points in opposite directions will result in a change in hand orientation as a response to the changing positions rather than as a response to the change in orientation. However, we obviously cannot exclude the possibility that the orientation of the hand is adjusted as a direct response to the change in target orientation when grasping.
Intercepting a moving target is far from simple. To successfully make contact one must be at the right place at the right time. To increase ones accuracy, one could choose a path that minimizes the motion relative to the target near the moment of contact and one could approach the target’s surface more or less orthogonally, but one will not want to follow a too curved path and one may want to make contact in a particular manner, for instance in order to hit the target in a certain direction (Brenner and Smeets 2007). One may also want to consider the likelihood of certain perturbations (Teixeira et al. 2006). Thus, many issues need to be considered when selecting a trajectory. The results of the present study suggest that it takes at least 150 ms to take all these issues into account, including the time taken to obtain the necessary sensory information and to get the motor commands to our muscles. This does not include the time needed to make the movement or any time it takes to decide what action should be taken. Our findings confirm that considering the new circumstances, including the target’s orientation, takes about 50 ms longer than responding directly to a change in position.
References
Aivar MP, Brenner E, Smeets JBJ (2008) Avoiding moving obstacles. Exp Brain Res 190:251–264
Brenner E, Smeets JBJ (1995) Moving one’s finger to a visually specified position: target orientation influences the finger’s path. Exp Brain Res 105:318–320
Brenner E, Smeets JBJ (1997) Fast responses of the human hand to changes in target position. J Mot Behav 29:297–310
Brenner E, Smeets JBJ (2007) Flexibility in intercepting moving objects. J Vis 7:14
Carlton LG (1981) Processing visual feedback information for movement control. J Exp Psychol Hum Percept Perform 7:1019–1030
Day BL, Brown P (2001) Evidence for subcortical involvement in the visual control of human reaching. Brain 124:1832–1840
Day BL, Lyon IN (2000) Voluntary modification of automatic arm movements evoked by motion of a visual target. Exp Brain Res 130:159–168
Desmurget M, Prablanc C, Arzi M, Rossetti Y, Paulignan Y, Urquizar C (1996) Integrated control of hand transport and orientation during prehension movements. Exp Brain Res 110:265–278
Goodale MA, Pelisson D, Prablanc C (1986) Large adjustments in visually guided reaching do not depend on vision of the hand or perception of target displacement. Nature 320:748–750
Pisella L, Arzi M, Rossetti Y (1998) The timing of color and location processing in the motor context. Exp Brain Res 121:270–276
Pisella L, Gréa H, Tilikete C, Vighetto A, Desmurget M, Rode G, Boisson D, Rossetti Y (2000) An ‘automatic pilot’ for the hand in human posterior parietal cortex: toward reinterpreting optic ataxia. Nat Neurosci 3:729–736
Prablanc C, Martin O (1992) Automatic control during hand reaching at undetected two-dimensional target displacements. J Neurophysiol 67:455–469
Sarlegna F, Blouin J, Bresciani JP, Bourdin C, Vercher JL, Gauthier GM (2003) Target and hand position information in the online control of goal-directed arm movements. Exp Brain Res 151:524–535
Smeets JBJ, Brenner E (1999) A new view on grasping. Mot Control 3:237–271
Teixeira LA, Chua R, Nagelkerke P, Franks IM (2006) Reprogramming of interceptive actions: time course of temporal corrections for unexpected target velocity change. J Mot Behav 38:467–477
Veerman MM, Brenner E, Smeets JBJ (2008) The latency for correcting a movement depends on the visual attribute that defines the target. Exp Brain Res 187:219–228
Whitney D, Westwood DA, Goodale MA (2003) The influence of visual motion on fast reaching movements to a stationary object. Nature 423:869–873
Zago M, McIntyre J, Senot P, Lacquaniti F (2009) Visuo-motor coordination and internal models for object interception. Exp Brain Res 192:571–604
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Brenner, E., Smeets, J.B.J. Modifying one’s hand’s trajectory when a moving target’s orientation changes. Exp Brain Res 196, 375–383 (2009). https://doi.org/10.1007/s00221-009-1857-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-009-1857-7