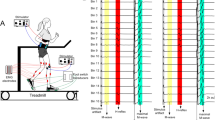
Abstract
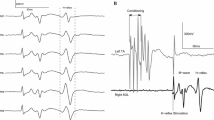
The human soleus H-reflex is commonly tested as an indicator of the reflex excitability of the calf muscles with infrequent stimuli to a subject seated and at rest. However, the reflex varies widely with the level of voluntary contraction and with the time history of stimulation. We studied two aspects of this variation. Antagonist (tibialis anterior) activation decreases the response, while increasing agonist (soleus) activation increases the H-reflex to a peak after which it declines. In subjects with large H-reflexes at rest, the reflex peaked at low levels of contraction. In contrast, in subjects with small H-reflexes at rest, the reflex peaked at higher levels of contraction for reasons that were elucidated using a realistic computer model. A parabolic curve fitted the maximum amplitude of the H-reflex in the model and over the entire range of contractile levels studied. The second aspect studied was post-activation depression or homosynaptic depression (HD), which has been described previously as a reduction of a second H-reflex elicited shortly after an initial reflex. We confirmed the presence of HD in resting, seated subjects for intervals up to 4 s. However, by voluntarily activating the soleus muscle, HD was drastically reduced when seated and abolished when standing. This suggests that HD may be absent in normal, functional movements and perhaps in clinical conditions that alter H-reflexes. Meaningful, quantitative measurements of reflex excitability can only be made under voluntary activity that mimics the condition of interest.






Similar content being viewed by others
References
Aagaard P, Simonsen EB, Andersen JL, Magnusson P, Dyhre-Poulsen P (2002) Neural adaptation to resistance training: changes in evoked V-wave and H-reflex responses. J Appl Physiol 92:2309–2318
Baldissera F, Gustafsson B (1974a) Firing behaviour of a neurone model based on the afterhyperpolarization conductance time course and algebraical summation. Adaptation and steady state firing. Acta Physiol Scand 92:27–47
Baldissera F, Gustafsson B (1974b) Firing behaviour of a neurone model based on the afterhyperpolarization conductance time course. First interval firing. Acta Physiol Scand 91:528–544
Burke D, Adams RW, Skuse NF (1989) The effects of voluntary contraction on the H reflex of human limb muscles. Brain 112:417–433
Capaday C (1997) Neurophysiological methods for studies of the motor system in freely moving human subjects. J Neurosci Methods 74:201–218
Capaday C, Stein RB (1987) A method for simulating the reflex output of a motoneuron pool. J Neurosci Methods 21:91–104
Carnevale NT, Hines ML (2006) The NEURON book. Cambridge University Press, Cambridge
Crone C, Hultborn H, Mazieres L, Morin C, Nielsen J, Pierrot-Deseilligny E (1990) Sensitivity of monosynaptic test reflexes to facilitation and inhibition as a function of the test reflex size: a study in man and the cat. Exp Brain Res 81:35–45
Devanne H, Lavoie BA, Capaday C (1997) Input–output properties and gain changes in the human corticospinal pathway. Exp Brain Res 114:329–338
Eccles JC (1964) The physiology of synapses. Springer, Berlin
Fleshman JW, Segev I, Burke RB (1988) Electrotonic architecture of type-identified alpha-motoneurons in the cat spinal cord. J Neurophysiol 60:60–85
Frigon A, Carroll TJ, Jones KE, Zehr EP, Collins DF (2007) Ankle position and voluntary contraction alter maximal M-waves in soleus and tibialis anterior. Muscle Nerve 35:756–766
Gregory JE, Mark RF, Morgan DL, Patak A, Polus B, Proske U (1990) Effects of muscle history on the stretch reflex in cat and man. J Physiol 424:93–107
Hoehler FK, Buerger AA (1981) A quantitative model of the Hoffmann reflex. Neurol Res 3:251–266
Hoffmann P (1918) Ueber die Beziehungen der Sehnenreflexe zur willkuerlichen Bewegung und zum Tonus. Zeitschrift fuer Biologie 68:351–370
Hultborn H, Nielsen JB (1998) Modulation of transmitter release from Ia afferents by their preceding activity—a ‘postactivation depression’. In: Rudomin P, Romo R, Mendell L (eds) Presynaptic inhibition and neural control. Oxford University Press, New York, pp 178–191
Hultborn H, Pierrot-Deseilligny E (1979) Changes in recurrent inhibition during voluntary soleus contractions in man studied by an H-reflex technique. J Physiol 297:229–251
Hultborn H, Illert M, Nielsen J, Paul A, Ballegaard M, Wiese H (1996) On the mechanism of the post-activation depression of the H-reflex in human subjects. Exp Brain Res 108:450–462
Inman VT, Ralston HJ, Saunders JB, Feinstein B, Wright EW Jr (1952) Relation of human electromyogram to muscular tension. Electroencephalogr Clin Neurophysiol Suppl 4:187–194
Jones KE, Bawa P (1997) Computer simulation of the responses of human motoneurons to composite 1A EPSPS: effects of background firing rate. J Neurophysiol 77:405–420
Katz R, Meunier S, Pierrot-Deseilligny E (1988) Changes in presynaptic inhibition of Ia fibres in man while standing. Brain 111:417–437
Kearney RE, Chan CW (1982) Contrasts between the reflex responses to tibialis anterior and triceps surae to sudden ankle rotation in normal human subjects. Electroencephalogr Clin Neurophysiol 54:301–310
Kido A, Tanaka N, Stein RB (2004) Spinal excitation and inhibition decrease as humans age. Can J Physiol Pharmacol 82:238–248
Lamy JC, Wargon I, Baret M, Ben Smail D, Milani P, Raoul S, Penicaud A, Katz R (2005) Post-activation depression in various group I spinal pathways in humans. Exp Brain Res 166:248–262
Linnamo V, Strojnik V, Komi PV (2001) Electromyogram power spectrum and features of the superimposed maximal M-wave during voluntary isometric actions in humans at different activation levels. Eur J Appl Physiol 86:28–33
Luscher HR, Ruenzel P, Henneman E (1983) Composite EPSPs in motoneurons of different sizes before and during PTP: implications for transmission failure and its relief in Ia projections. J Neurophysiol 49:269–289
Martin PG, Gandevia SC, Taylor JL (2006) Output of human motoneuron pools to corticospinal inputs during voluntary contractions. J Neurophysiol 95:3512–3518
Masakado Y, Kagamihara Y, Takahashi O, Akaboshi K, Muraoka Y, Ushiba J (2005) Post-activation depression of the soleus H-reflex in stroke patients. Electromyogr Clin Neurophysiol 45:115–122
Matthews PB (1999) The effect of firing on the excitability of a model motoneurone and its implications for cortical stimulation. J Physiol 518:867–882
Nagata A, Christianson JC (1995) M-wave modulation at relative levels of maximal voluntary contraction. Eur J Appl Physiol Occup Physiol 71:77–86
Nielsen J, Crone C, Hultborn H (1993a) H-reflexes are smaller in dancers from The Royal Danish Ballet than in well-trained athletes. Eur J Appl Physiol Occup Physiol 66:116–121
Nielsen J, Petersen N, Ballegaard M, Biering-Sorensen F, Kiehn O (1993b) H-reflexes are less depressed following muscle stretch in spastic spinal cord injured patients than in healthy subjects. Exp Brain Res 97:173–176
Pierrot-Deseilligny E, Burke D (2005) The circuitry of the human spinal cord: its role in motor control and movement disorders. Cambridge University Press, Cambridge
Robertson CT, Koceja DM (2003) Post-activation depression of the soleus H-reflex in the elderly. Electromyogr Clin Neurophysiol 43:103–111
Simonsen EB, Dyhre-Poulsen P (1999) Amplitude of the human soleus H reflex during walking and running. J Physiol 515:929–939
Slot PJ, Sinkjaer T (1994) Simulations of the alpha motoneuron pool electromyogram reflex at different preactivation levels in man. Biol Cybern 70:351–358
Stein RB, Capaday C (1988) The modulation of human reflexes during functional motor tasks. Trends Neurosci 11:328–332
Stein R, Thompson A (2006) Muscle reflexes in motion: how, what and why? Exerc Sport Sci Rev 34:145–153
Taborikova H, Sax DS (1969) Conditioning of H-reflexes by a preceding subthreshold H-reflex stimulus. Brain 92:203–212
Voigt M, Chelli F, Frigo C (1998) Changes in the excitability of soleus muscle short latency stretch reflexes during human hopping after 4 weeks of hopping training. Eur J Appl Physiol Occup Physiol 78:522–532
Wood SA, Gregory JE, Proske U (1996) The influence of muscle spindle discharge on the human H reflex and the monosynaptic reflex in the cat. J Physiol 497:279–290
Zehr EP, Stein RB (1999a) Interaction of the Jendrássik maneuver with segmental presynaptic inhibition. Exp Brain Res 124:474–480
Zehr EP, Stein RB (1999b) What functions do reflexes serve during human locomotion? Prog Neurobiol 58:185–205
Zucker RS, Regehr WG (2002) Short-term synaptic plasticity. Annu Rev Physiol 64:355–405
Acknowledgments
We thank Dr. Monica Gorassini for helpful comments on the manuscript. Ms SuLing Chong and Mr Robert Rolf provided technical assistance. This research was supported by grants from the Canadian Institutes of Health Research and the Christopher Reeve Foundation.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
The modeling was done using the NEURON simulation environment with 250 motor neurons in the pool (Carnevale and Hines 2006). Each MN had three sections: initial segment (threshold for spike generation), soma and an equivalent dendritic cable. The morphology of the models was illustrated previously (Jones and Bawa 1997) and is similar to those used by others (Slot and Sinkjaer 1994). To simulate a range of sizes the diameter of the stem dendrite was calculated as
where d i is diameter of the ith MN and the range of stem diameters, 1.6, was the range of diameters between the largest and smallest MNs.
The passive (leak) conductance of the pool of MNs was also varied over a range of two to generate the experimentally measured range of input resistance. The passive leak conductance (S/cm2) for each MN was calculated as
where g is specific conductance of the ith MN. This has to be combined with surface area and cable properties to generate the input resistance of the neuron. The resulting values for input resistance match the experimentally measured range (0.7–4 MΩ) for cat medial gastrocnemius MNs; e.g., (Fleshman et al. 1988). Corresponding values are not available for the human soleus muscle. After generating the pool of 250 MNs and setting the passive parameters, each section of the model was spatially divided into segments with a length constant (λ) less than 0.1 at a frequency of 100 Hz. This was done to ensure that spatial resolution meets the assumption of isopotentiality in each compartment of the model.
To simulate the composite Ia EPSP that is generated by the H-reflex stimulus a current pulse input was used rather than synaptic conductance changes; for validation see Jones and Bawa (1997). The current pulse input is located in the dendrite so that the resulting transient depolarizing potential measured in the soma has the same time course as a composite Ia EPSP generated using the observed distribution of synapses. This results in an H-reflex stimulus that evokes an EPSP that is 1.8 times larger in the lowest threshold (MN[1]) compared to the highest threshold MN (MN[250]).
Active properties were added using the Hodgkin–Huxley formalism (Jones and Bawa 1997). The initial segment and soma have fast transient Na+and delayed rectifier K+ channels and the soma also has an AHP current generated by a K+ conductance. This current is a simplified version of the calcium-dependent K+ channel that underlies the AHP and is based on previous studies (Baldissera and Gustafsson 1974a, b). Across the pool of 250 MNs the AHPs vary from a duration of 160 ms in MN[1] to 90 ms in MN[250]. The time to peak AHP is shorter in the high threshold MNs and varies monotonically across the pool of MNs.
Voluntary input to the pool of 250 MNs was simulated as a current source in the soma with equal input to all MNs. The difference in passive and active properties across the pool resulted in an exponential distribution of thresholds, i.e., relatively more low threshold units. The minimal rhythmic firing rate for the lowest threshold MN (MN[1]) was 6 imp./s and this unit increased it’s firing rate to 19 imp./s when the highest threshold MN (MN[250]) was recruited with a minimal rhythmic firing rate of 13 imp./s. The amplitude of the voluntary input needed to recruit the MNs varied exponentially over a range of 2.5 from the lowest to highest threshold MNs. The shape of the motor unit action potential (MUAP) was not simulated, since the H-reflex is quite synchronous. MNs that responded to the stimulus occurred at a fixed latency after the stimulus within a window of 3–4 ms. The MUAP size had an exponential distribution across the pool with a range of 10.
Simulations were done to match the experimental paradigm; the MN pool was either at rest or subject to voluntary input that would recruit a subset of the pool. Stimuli were delivered after the onset of voluntary contraction at a time when the firing rates of the active MNs had accommodated to a steady state. The voluntary current input needed to recruit the lowest threshold MN was 2.5 nA and the highest threshold MN was recruited with an input of 6.25 nA. Currents were used from −2 nA (to simulate antagonist contraction) up to +5 nA for agonist contractions. This is a rather narrow range of currents because of the narrow range of thresholds chosen. However, the range of thresholds was increased to 2.5–35 nA while maintaining the relative spacing between MNs. The results were similar, except that the scale in Fig. 8 was extended.
As the stimulus amplitude increased at rest the response increased until all 250 MNs were recruited via the reflex pathway, i.e., reflex curve at rest in Fig. 7a. With a simulated low level of voluntary (Vol) contraction (Vol 3) the reflex curve was shifted to the left and the final plateau amplitude was similar to rest. The reflex curve continued to shift to the left at higher levels of simulated voluntary contraction and the final plateau level decreased (Vol 4 = 89.8%, Vol 5 = 82.5% of M max). In contrast, simulations of antagonist contraction shifted the reflex curve to the right, but did not change the final plateau level. In the model all 250 MNs were still recruited, although this may not be plausible physiologically.
a Stimulus–response curves for the calculated H-reflexes (y-axis) with different levels of agonist (Vol > 0) or antagonist activity (Vol < 0). The stimulus in the model can be made arbitrarily large, so that full activation is achieved even with antagonist voluntary activity. b Adding a curve for the M-wave will occlude the firing of some motor neurons and therefore reduce the observed H-reflex. The magnitude of the H-reflex has been normalized relative to the maximum M-wave
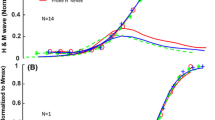
Thus, in the simulations with the MN pool model the recruitment curve shifts to the left and the final amplitude decreases as voluntary contraction increases. With antagonist contraction, the curve shifts to the right. Since the M-wave curve changes little with voluntary contraction level experimentally (Fig. 2), an M-wave can be added to the simulation results and the H-reflex calculated by subtraction (Fig. 7b). With this set of parameters H max is 86% of M max at rest. With moderate contraction (Vol 3) the H max increases to almost 100% but with further increases in voluntary contraction the peak of the H-reflex curve goes down slightly (Vol 4 and 5). The stimulus amplitude for evoking the maximum H-reflex systematically decreases as voluntary contraction levels increase. H max declines much more precipitously with antagonist contraction (Vol = −1) but is elicited with a stimulus amplitude similar to that evoking H max in the rest condition. Figure 8a shows that H max/M max at different levels of voluntary contraction is well fitted by a second order polynomial, as was done for the experimental data (Fig. 5). Assuming that the only difference between individuals is the relative position of the M-wave and H-reflex curves, one can generate a range of resting values of H max/M max from 0.2 to 0.8. The peak of the curve also shifts with the value of H max at rest, as was found experimentally (compare Fig. 5 with Fig. 8b). In conclusion, a realistic model of the motor pool is able to reproduce the changes in H-reflexes with voluntary contraction that are found experimentally.
a The calculated ratio H max/M max varies with the level of voluntary contraction in a parabolic fashion, as was observed experimentally (Fig. 5). b Shifting the relative positions of the M-wave and H-reflex curves changes the ratio H max/M max at rest and the voluntary contraction level at which the maximum level is reached (cf. Fig. 5)
Rights and permissions
About this article
Cite this article
Stein, R.B., Estabrooks, K.L., McGie, S. et al. Quantifying the effects of voluntary contraction and inter-stimulus interval on the human soleus H-reflex. Exp Brain Res 182, 309–319 (2007). https://doi.org/10.1007/s00221-007-0989-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-007-0989-x