Abstract
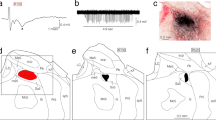
We previously reported that 6–16 weeks after forelimb amputation in adult rats, neurons in layer IV of rat first somatosensory cortex (SI) in the forepaw barrel subfield (FBS) associated with the representation of the forepaw became responsive to new input from the shoulder (Pearson et al. 1999). These new shoulder-responsive sites in deafferented FBS had longer evoked response latencies than did sites in the shoulder representation located in the posterior part of the trunk subfield, hereafter referred to as the original shoulder representation. Furthermore, projection neurons in the original shoulder representation in both intact and deafferented adults did not extend their axons into the FBS, and ablation of the original shoulder representation cortex and/or the second somatosensory cortex (SII) failed to eliminate new shoulder input in the deafferented FBS (Pearson et al. 2001). These results led us to conclude that large-scale reorganization in FBS quite likely involved a subcortical substrate. In addition, the time course for large-scale cortical reorganization following forelimb amputation was unknown, and this information could shed light on potential mechanisms for large-scale cortical reorganization. In the present study, we extended our previous findings of large-scale cortical reorganization in the FBS by investigating the time course for reorganization following forelimb amputation. The major findings are: a) deafferented forelimb cortex remained unresponsive to shoulder stimulation during the 1st week following forelimb amputation; b) new responses to shoulder stimulation were first observed in deafferented forelimb cortex 2–3 weeks after forelimb amputation; however, the new shoulder input was restricted to locations in the former forearm cortex; c) islet(s) of new shoulder representation were first observed in deafferented FBS 4 weeks after amputation; these islets occupied a larger percentage of FBS in subsequent weeks; d) portions of FBS remained unresponsive as many as 4 months after deafferentation (maximum time examined between amputation and recording); and e) the increase in total size of the shoulder representation appeared to result from the establishment of new shoulder representations that were often discontinuous from the original shoulder representation. These findings provide evidence that forelimb amputation results in delayed reorganization of the FBS and we describe possible mechanisms and substrates underlying the reorganization.







Similar content being viewed by others
References
Akhtar ND, Land PW (1991) Activity-dependent regulation of glutamic acid decarboxylase in the rat barrel cortex: effects of neonatal versus adult sensory deprivation. J Comp Neurol 307:200–213
Armstrong-James M, Millar J (1979) Carbon fibre microelectrodes. J Neurosci Methods 1:279–28
Arnold PB, Li CX, Waters RS (2001) Somatosensory thalamic projection of neuronal arborization patterns spread beyond the confines of single cortical barrels: an in-vivo retrograde and intracellular tracing study in rat. Exp Brain Res 136:152–168
Bourassa J, Pinault D, Deschenes M (1995) Corticothalamic projections from the cortical barrel field to the somatosensory thalamus in rats: a single-fibre study using biocytin as an anterograde tracer. Eur J Neurosci 7:19–30
Calford MB, Tweedale R (1988) Immediate and chronic changes in responses of somatosensory cortex in adult flying-fox after digit amputation. Nature 332:446–448
Churchill JD, Muja N, Myers WA, Besheer J, Garraghty PE (1998) Somatotopic consolidation: a third phase of reorganization after peripheral nerve injury in adult squirrel monkeys. Exp Brain Res 118:189–196
Coq JO, Xerri C (1998) Environmental enrichment alters organizational features of the forepaw representation in the primary somatosensory cortex of adult rats. Exp Brain Res 121(2):191–204
Cusick CG, Wall JT, Whiting JH Jr, Wiley RG (1990) Temporal progression of cortical reorganization following nerve injury. Brain Res 537:355–358
Darian-Smith C, Gilbert CD (1994) Axonal sprouting accompanies functional reorganization in adult cat striate cortex. Science 368:737–740
Dawson DR, Killackey HP (1987) The organization and mutability of the forepaw and hindpaw representations in the somatosensory cortex of the neonatal rat. J Comp Neurol 256:246–256
De Biasi S, Frassoni C, Spreafico R (1988) The intrinsic organization of the ventroposterolateral nucleus and related reticular thalamic nucleus of the rat: a double-labeling ultrastructural investigation with gamma-aminobutyric acid immunogold staining and lectin-conjugated horseradish peroxidase. Somatosens Res 5(3):187–203
Dykes RW, Landry P, Metherate R, Hicks TP (1984) Functional role of GABA in cat primary somatosensory cortex: shaping receptive fields of cortical neurons. J Neurophysiol 52:1066–1093
Faggin BM, Nguyen KT, Nicolelis MA (1997) Immediate and simultaneous sensory reorganization at cortical and subcortical levels of the somatosensory system. Proc Natl Acad Sci USA 94:9428–9433
Florence SL, Kaas JH (1995) Large-scale reorganization at multiple levels of the somatosensory pathway follows therapeutic amputation of the hand in monkeys. J Neurosci 15:8083–8095
Florence SL, Taub HB, Kaas JH (1998) Large-scale sprouting of cortical connections after peripheral injury in adult macaque monkeys [see comments]. Science 282:1117–1121
Florence SL, Hackett TA, Strata F (2000) Thalamic and cortical contributions to neural plasticity after limb amputation. J Neurophysiol 2000 83(5):3154–3159
Fuchs JL, Salazar E (1998) Effects of whisker trimming on GABA(A) receptor binding in the barrel cortex of developing and adult rats. J Comp Neurol 395:209–216
Garraghty PE, Kaas JH (1991) Large-scale functional reorganization in adult monkey cortex after peripheral nerve injury. Proc Natl Acad Sci USA 88:6976–6980
Garraghty PE, Hanes DP, Florence SL, Kaas JH (1994) Pattern of peripheral deafferentation predicts reorganizational limits in adult primate somatosensory cortex. Somatosens Mot Res 11:109–117
Gierdalski M, Jablonska B, Smith A, Skangiel-Kramska J, Kossut M (1999) Deafferentation induced changes in GAD67 and GluR2 mRNA expression in mouse somatosensory cortex. Brain Res Mol Brain Res 71:111–119
Gonzalez MF, Sharp FR (1985) Reticular nucleus of rat thalamus is metabolically activated during trained forelimb movements. Brain Res 332:380–385
Hall RD, Lindholm EP (1974) Organization of motor and somatosensory neocortex in the albino rat. Brain Res 66:23–38
Hicks TP, Dykes RW (1983) Receptive field size for certain neurons in primary somatosensory cortex is determined by GABA- mediated intracortical inhibition. Brain Res 274:160–164
Jain N, Florence SL, Kaas JH (1995) Limits on plasticity in somatosensory cortex of adult rats: hindlimb cortex is not reactivated after dorsal column section. J Neurophysiol 73:1537–1546
Jain N, Catania KC, Kaas JH (1997) Deactivation and reactivation of somatosensory cortex after dorsal spinal cord injury. Nature 386:495–498
Jain N, Florence SL, Qi HX, Kaas JH (2000) Growth of new brainstem connections in adult monkeys with massive sensory loss. Proc Natl Acad Sci USA 97(10):5546–5550
Jones EG, Pons TP (1998) Thalamic and brainstem contributions to large-scale plasticity of primate somatosensory cortex. Science 282(5391):1121–1125
Kalaska J, Pomeranz B (1979) Chronic paw denervation causes an age-dependent appearance of novel responses from forearm in paw cortex of kittens and adult cats. J Neurophysiol 42:618–633
Kelahan AM, Doetsch GS (1984) Time-dependent changes in the functional organization of somatosensory cerebral cortex following digit amputation in adult raccoons. Somatosens Res 2:49–81
Kelahan AM, Ray RH, Carson LV, Massey CE, Doetsch GS (1981) Functional reorganization of adult raccoon somatosensory cerebral cortex following neonatal digit amputation. Brain Res 223(1):152–159
Keller A, Carlson GC (1999) Neonatal whisker clipping alters intracortical, but not thalamocortical projections, in rat barrel cortex. J Comp Neurol 412(1):83–94
Kirzon MV, Kaplan AY (1978) Depression of evoked potentials in rat thalamic ventro-basal complex and somatosensory cortex after reticular stimulation. Neurosci Behav Physiol 9:204–210
Kolarik RC, Rasey SK, Wall JT (1994) The consistency, extent, and locations of early-onset changes in cortical nerve dominance aggregates following injury of nerves to primate hands. J Neurosci 14:4269–4288
Kossut M, Stewart MG, Siucinska E, Bourne RC, Gabbott PL (1991) Loss of gamma-aminobutyric acid (GABA) immunoreactivity from mouse first somatosensory (SI) cortex following neonatal, but not adult, denervation. Brain Res 538:165–170
Lane RD, Bennett-Clarke CA, Chiaia NL, Killackey HP, Rhoades RW (1995) Lesion-induced reorganization in the brainstem is not completely expressed in somatosensory cortex. Proc Natl Acad Sci USA 92:4264–4268
Lane RD, Killackey HP, Rhoades RW (1997) Blockade of GABAergic inhibition reveals reordered cortical somatotopic maps in rats that sustained neonatal forelimb removal. J Neurophysiol 77:2723–2735
Lane RD, Stojic RS, Killackey HP, Rhoades RW (1999) Source of inappropriate receptive fields in cortical somatotopic maps from rats that sustained neonatal forelimb removal. J Neurophysiol 81(2):625–633
Lee SM, Friedberg MH, Ebner FF (1994) The role of GABA-mediated inhibition in the rat ventral posterior medial thalamus. I. Assessment of receptive field changes following thalamic reticular nucleus lesions. J Neurophysiol 71:1702–1715
Li CX, Waters RS, Oladehin A, Johnson EF, McCandlish CA, Dykes RW (1994) Large unresponsive zones appear in cat SI cortex immediately after ulnar nerve cut. Can J Neurol Sci 21:233–247
Li CX, Callaway JC, Waters RS (2002) Removal of GABAergic inhibition alters subthreshold input in neurons in forepaw barrel subfield (FBS) in rat first somatosensory cortex (SI) after digit stimulation. Exp Brain Res 145(4):411–428
McKinley PA, Smith JL (1990) Age-dependent differences in reorganization of primary somatosensory cortex following low thoracic (T12) spinal cord transection in cats. J Neurosci 10:1429–1443
McKinley PA, Swyter E (1989) Progressive changes in somatosensory cortical maps in 6-week-old kittens cord-transected at T12. Brain Res 484:378–382
McKinley PA, Jenkins WM, Smith JL, Merzenich MM (1987) Age-dependent capacity for somatosensory cortex reorganization in chronic spinal cats. Brain Res 428:136–139
Merzenich MM, Kaas JH, Wall J, Nelson RJ, Sur M, Felleman D (1983a) Topographic reorganization of somatosensory cortical areas 3b and 1 in adult monkeys following restricted deafferentation. Neuroscience 8:33–55
Merzenich MM, Kaas JH, Wall JT, Sur M, Nelson RJ, Felleman DJ (1983b) Progression of change following median nerve section in the cortical representation of the hand in areas 3b and 1 in adult owl and squirrel monkeys. Neuroscience 10:639–665
Merzenich MM, Nelson RJ, Stryker MP, Cynader MS, Schoppmann A, Zook JM (1984) Somatosensory cortical map changes following digit amputation in adult monkeys. J Comp Neurol 224:591–605
Metzler J, Marks PS (1979) Functional changes in cat somatic sensory-motor cortex during short-term reversible epidural blocks. Brain Res 177:379–383
Mushiake S, Shosaku A, Kayama Y (1984) Inhibition of thalamic ventrobasal complex neurons by glutamate infusion into the thalamic reticular nucleus in rats. J Neurosci Res 12(1):93–100
Nicolelis MA, Chapin JK, Lin RC (1991) Thalamic plasticity induced by early whisker removal in rats. Brain Res 561(2):344–349
Parker JL, Wood ML, Dostrovsky JO (1998) A focal zone of thalamic plasticity. J Neurosci 18(1):548–558
Pearson PP, Li CX, Waters RS (1999) Effects of large-scale limb deafferentation on the morphological and physiological organization of the forepaw barrel subfield (FBS) in somatosensory cortex (SI) in adult and neonatal rats. Exp Brain Res 128:315–331
Pearson PP, Arnold PB, Olahehin A, Li CX, Waters RS (2001) Large-scale cortical reorganization following forelimb deafferentation in rat does not involve plasticity of intracortical connections. Exp Brain Res 138:8–25
Pettit MJ, Schwark HD (1993) Receptive field reorganization during dorsal column nuclei during temporary denervation. Sci 262:2054–2056
Pinault D, Bourassa J, Deschenes M (1995) The axonal arborization of single thalamic reticular neurons in the somatosensory thalamus of the rat. Eur J Neurosci 7:31–40
Pons TP, Garraghty PE, Ommaya AK, Kaas JH, Taub E, Mishkin M (1991) Massive cortical reorganization after sensory deafferentation in adult macaques [see comments]. Science 252:1857–1860
Rasmusson D (1996a) Changes in the organization of the ventroposterior lateral thalamic nucleus after digit removal in adult raccoon. J Comp Neurol 364(1):92–103
Rasmusson DD (1996b) Changes in the response properties of neurons in the ventroposterior lateral thalamic nucleus of the raccoon after peripheral deafferentation. J Neurophysiol 75(6):2441–2450
Rasmusson DD, Turnbull BG (1983) Immediate effects of digit amputation on SI cortex in the raccoon: unmasking of inhibitory fields. Brain Res 288:368–370
Rasmusson DD, Turnbull BG, Leech CK (1985) Unexpected reorganization of somatosensory cortex in a raccoon with extensive forelimb loss. Neurosci Lett 55:167–172
Rhoades RW, Wall JT, Chiaia NL, Bennett-Clarke CA, Killackey HP (1993) Anatomical and functional changes in the organization of the cuneate nucleus of adult rats after fetal forelimb amputation. J Neurosci 13(3):1106–1119
Shosaku A (1986) Cross-correlation analysis of a recurrent inhibitory circuit in the rat thalamus. J Neurophysiol 55(5):1030–1043
Shosaku A, Kayama Y, Sumitomo I (1984) Somatotopic organization in the rat thalamic reticular nucleus. Brain Res 311:57–63
Stojic AS, Lane RD, Killackey HP, Qadri BA, Rhoades RW (1998) Thalamocortical and intracortical projections to the forelimb-stump SI representation of rats that sustained neonatal forelimb removal. J Comp Neurol 401:187–204
Stojic AS, Lane RD, Killackey HP, Rhoades RW (2000) Suppression of hindlimb inputs to S-I forelimb-stump representation of rats with neonatal forelimb removal: GABA receptor blockade and single-cell responses. J Neurophysiol 83(6):3377–3387
Stojic AS, Lane RD, Rhoades RW (2001) Intracortical pathway involving dysgranular cortex conveys hindlimb inputs to S-I forelimb-stump representation of neonatally amputated rats. Neurophysiol 85:407–413
Sugitani M (1979) Electrophysiological and sensory properties of the thalamic reticular neurones related to somatic sensation in rats. J Physiology 290:79–95
Verley R, Onnen I (1981) Somatotopic organization of the tactile thalamus in normal adult and developing mice and in adult mice dewhiskered since birth. Exp Neurol 72(2):462–474
Verney C, Farkas-Bargeton E, Verley R (1982) Reorganization of thalamo-cortical connections in mice dewhiskered since birth. Neurosci Lett 32:265–270
Wall JT, Cusick CG (1984) Cutaneous responsiveness in primary somatosensory (S-I) hindpaw cortex before and after partial hindpaw deafferentation in adult rats. J Neurosci 4:1499–1515
Wall JT, Cusick CG, Migani-Wall SA, Wiley RG (1988) Cortical organization after treatment of a peripheral nerve with ricin: an evaluation of the relationship between sensory neuron death and cortical adjustments after nerve injury. J Comp Neurol 277:578–592
Wall PD, Egger MD (1971) Formation of new connexions in adult rat brains after partial deafferentation. Nature 232:542–545
Waters RS, Li CX, McCandlish CA (1995) Relationship between the organization of the forepaw barrel subfield and the representation of the forepaw in layer IV of rat somatosensory cortex. Exp Brain Res 103:183–197
Waters RS, Li CX, Chappell TD (2003) Following forelimb amputation in adult rats, new shoulder representations in forepaw barrel subfield (FBS) in first somatosensory cortex (SI) are relayed from reorganized forepaw sites in ventroposterior lateral (VPL) thalamus. Soc Neurosci 29 (A)
Welker C (1976) Receptive fields of the barrels in the somatosensory neocortex of the rat. J Comp Neurol 166:173–190
Welker E, Soriano E, Van der Loos H (1989) Plasticity in the barrel cortex of the adult mouse: effects of peripheral deprivation on GAD-immunoreactivity. Exp Brain Res 74:441–452
Wong-Riley MT, Welt C (1980) Histochemical changes in cytochrome oxidase of cortical barrels after vibrissal removal in neonatal and adult mice. Proc Natl Acad Sci USA 77:2333–2337
Woods TM, Cusick CG, Pons TP, Taub E, Jones EG (2000) Progressive transneuronal changes in the brainstem and thalamus after long-term dorsal rhizotomies in adult macaque monkeys. J Neurosci 20:3884–3899
Wright AK, Norrie L, Arbuthnott GW (2000) Corticofugal axons from adjacent 'barrel' columns of rat somatosensory cortex: cortical and thalamic terminal patterns. J Anat 196:379–390
Xu J, Wall JT (1997) Rapid changes in brainstem maps of adult primates after peripheral injury. Brain Res 774:211–215
Xu J, Wall JT (1999) Evidence for brainstem and supra-brainstem contributions to rapid cortical plasticity in adult monkeys. J Neurosci 19:7578–7590
Acknowledgments
The authors thank Ms. M. Waters for editing. This research was supported by NSF grant IBN-9724092 to RSW and a predoctoral fellowship to PPP from NIH (NS-10810-01).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pearson, P.P., Li, C.X., Chappell, T.D. et al. Delayed reorganization of the shoulder representation in forepaw barrel subfield (FBS) in first somatosensory cortex (SI) following forelimb deafferentation in adult rats. Exp Brain Res 153, 100–112 (2003). https://doi.org/10.1007/s00221-003-1625-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-003-1625-z