Abstract
This study aims to assess the microbial, nutritional, volatile, and sensory characteristics of rice, almond, and chickpea water kefir beverages during refrigerated storage. Plant-based kefirs contained significant amounts of lactic acid bacteria and yeasts. The microbial content of kefirs was stable during 14-day refrigerated storage. Lactic acid, acetic acid, and tartaric acid are commonly detected organic acids in kefir samples. Almond and chickpea kefirs were rich in potassium mineral. Almond kefir had the highest ethanol content among plant-based kefirs, followed by chickpea and rice-based kefirs. Ethyl acetate, acetic acid, propionic acid, hexanoic acid, and benzenemethanol were identified as key volatile compounds in almond kefir and chickpea kefir samples using a GC–MS detector during water kefir fermentation. According to sensory analysis results, significant differences are present for all test parameters except odor. Almond kefir was the most accepted, while the other two kefir samples were below the general acceptance level (P < 0.05).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The global trend toward plant-based foods and beverages has grown in response to ethical, environmental, and health concerns associated with dairy products [1]. Legumes, cereals, and nuts are rich in nutrients and contain bioactive substances. In 2019, global rice production reached 755 million metric tons, while chickpeas and almonds production amounted to 14.25 million metric tons and 1.48 million metric tons, respectively [2,3,4]. Chickpeas are rich in proteins, albumin, globulin, fiber, vitamins, choline, and minerals [5]. Rice is a nutritious food that is rich in carbohydrates, vitamins, and minerals. One of its standout qualities is that it is gluten-free [6]. Almonds are recognized for their abundance of essential nutrients, including fatty acids, lipids, amino acids, proteins, carbohydrates, vitamins, minerals, and bioactive substances such as hydrolyzable tannins, proanthocyanidins, and flavonoids [7]. The rise in food allergies, lactose intolerance, and vegan diets has influenced the food industry, resulting in distinct trends. Legumes, nuts, and cereals have been increasingly used to produce plant-based extract in recent years [8,9,10]. The development of plant-based probiotic foods and beverages provides an alternative for individuals, and the market share of plant-based fermented extract is expected to grow in the next decade. It has been reported that this growth is attributed to the enhanced functional properties of new-generation plant-based extract, which offers superior sensory qualities and nutritional content [11]. Furthermore, unlike animal-based milk, plant-based extract contains significant amounts of phytochemicals and nutritional fiber and has low glycemic indexes; it has been claimed that plant-based extract has high antioxidant activity and is rich in fatty acids [8, 12]. However, fermentation with lactic acid bacteria can degrade antinutritional compounds found in plants, such as tannins, saponins, phytic acid, α-galactosides, and trypsin inhibitors. This enhances the beneficial quality of the product [1].
Water kefir grains are a unique microbiota used as a natural starter culture to produce a fermented beverage known as water kefir. The water kefir microbiota is made up of a consortium of probiotic bacteria, mostly lactic acid bacteria, acetic acid bacteria, and yeasts embedded in a flexible water-soluble polysaccharide matrix [13, 14]. Several studies have found that water kefir has antimicrobial effects against various microorganisms [15, 16]. Studies have reported the anti-inflammatory, antioxidant, and hepatoprotective effects of water kefir [17,18,19]. Various studies have reported the antihyperglycemic and antidiabetic effects of water kefir [20]. It has also been found to improve lipid profiles [21], regulate blood sugar, stabilize cholesterol levels, and have prophylactic effects against irritable bowel syndrome [17, 22]. Water kefir grains have distinctive characteristic properties than milk kefir grains; it is more appropriate to use in a plant-based environment, thus, the use of water kefir grains in plant-based extract is more appropriate than the use of milk kefir grains [13]. There are some advantages of water kefir grains over milk kefir grains. Firstly, water kefir grains also have abundant and characteristic microbiota, as reported by Gökırmaklı and Guzel-Seydim [13]. Additionally, water kefir grains contain as much Lactobacillus spp. and yeasts as milk kefir grains. Furthermore, water kefir grains contain higher levels of Cu, Fe, and Mo elements than milk kefir grains. These are all desirable properties to consider water kefir grains for producing a fermented plant-based food. Previously, various plant materials such as soy extract, peanuts, hazelnuts, almonds, walnuts, and cashews were preferred as substrates to ferment milk kefir grains [23, 24]. However, only few studies [25,26,27,28] have been conducted on the fermentation of plant-based extract using water kefir grains, to the best of our knowledge. According to Satir [26], tigernut extract provides an optimal environment for the growth of water kefir microbiota and the production of bioactive substances such as quercetin and vitamin K. Our hypothesis suggests that water kefir grains would have a good harmony with various plant-based extracts following to fermentation with them.
The aim of this research is to identify the features of kefirs made from plant-based sources. To obtain kefir samples, we preferred using gluten-free plant extracts such as chickpeas as a legume sample, rice as a cereal sample, and almonds as a nut sample. These agricultural products are widely produced worldwide. Water kefir beverages were produced by fermenting the plant extracts with water kefir grains.
Materials and methods
Plant extracts and water kefir production
Chickpeas, rice, and raw almonds were sourced from local markets in Isparta, Turkey. They were separately added to chlorine-free sterile water (1 L water + 200 g plant source) and kept at 25 °C for 8 h. After eight hours, heat treatment was applied at 85 °C for 10 min. The excess water was then drained from the mixture of chickpeas, rice, and almonds. The mixture was then blended with 1 L of sterile water until it became a smooth puree without any particles. After mixing, the mixture was sieved to obtain plant-based extract. After filtration, 1% refined sugar was added, and all plant-based extracts were pasteurized at 85 °C for 15 min. Then, chickpeas, rice, and almond extracts were acquired (Fig. 1).
Water kefir grains were generously provided by Danem Inc. (kefirdanem.com). Water kefir grains were added to plant-based extracts at a 2% inoculation rate, and fermentation took place at 25 °C. The fermented plant-based extracts were stored in the refrigerator (4 °C) after fermentation (Fig. 1).
Proximate analyses
pH analysis was conducted using a pH meter Lab 850 (Schott instruments, USA) following the AOAC [29] guidelines. The dry matter content was measured using the Shimadzu MOC63U Moisture Analysis device.
Microbiological analysis
One milliliter of each plant-based kefir sample was diluted with 9 mL of sterile peptone water and vortexed. Samples were appropriately plated on de Man, Rogosa, and Sharpe (MRS) Agar (Merck) utilized for Lactobacillus spp. at 37 °C for 48 h, MRS-salicin agar utilized for L. acidophilus at 37 °C for 48 h, M17 Agar (Merck) utilized for Lactococcus spp. at 37 °C for 48 h, and Potato Dextrose Agar (Merck) contained 0.14% lactic acid utilized for yeasts at 25° C for five days [30].
Elemental analysis
The sample preparation process followed EPA 3015, a wet-burning method. It involved adding 8 mL of HNO3 and 2 mL of H2O2 to 0.2–0.3 g of the sample using the Milestone brand Ethos One model microwave. The final volume was adjusted to 20 mL by adding distilled water. ICP-OES measurements were conducted using the Perkin Elmer Optima 5300 DV device, following the EPA 6010 method, under the specified conditions (Table S1).
Organic acid analysis
The samples were weighed at five grams, homogenized in a 20 mL solution of 0.01 mono potassium phosphate (KH2PO4), and then centrifuged at 4000 rpm for 30 min. After the coarse filtering, the samples were further filtered using 0.45-μm filters to prepare them for use. The organic acids in the samples were analyzed using an HPLC instrument (Shimadzu SCL-10A, Scientific Instruments, Inc., Tokyo, Japan) equipped with an Inertsil ODS 3 V (4.8 × 250 mm, 5 μm) column, a DAD detector (LC 20ADvp), a pump (LC 10ADvp), a gas separator (DGU 20A), and a column oven (CTO 10Avp). The analysis was conducted using the method developed by Gökırmaklı et al. [31]. The calibration equations and R2 values for the standard organic acids can be found in Table S2. A sample chromatogram is given in Figure S1.
Volatile compound analysis
Volatile compounds from the sample matrix were isolated using the headspace solid-phase microextraction method, as described by Gökırmaklı et al. [31]. For this purpose, briefly, the fiber (SPME Fiber Assembly 50/30 μm DVB/CAR/PDMS, Stableflex (2 cm), 24 Ga. Manual Holder. 3pk (Gray-Notched)) was initially conditioned at 270 ℃ for 30 min. The HP 6890 GC and 7895 C mass selective detector (Agilent Technologies, Wilmington, DE, USA) were used in conjunction with a polar capillary column (HP-INNOWax 60 m length, 0.25 mm i.d., 0.25 μm df; J&W Scientific, Folsom, CA). The oven temperature was initially set at 40 ℃ for 2 min and then increased to 150 ℃ at a rate of 4 °C per minute. After 20 min at 150 °C, the temperature was further increased to 200 °C at a rate of 5 °C per minute.
Color analysis
The Chroma Meter, CR-400 (Minolta, Osaka, Japan) colorimeter instrument, was used to determine L*, a*, and b*. The L* value represents the brightness of a color, with a maximum value of “-100” indicating black and a minimum value of “0” indicating the absence of black. The a* value represents the degree of redness, ranging from red (+ 100) to green (− 100), while the b* value indicates the level of yellowness, ranging from blue (− 100) to yellow (+ 100).
Sensory analysis
The results were analyzed based on their color, appearance, consistency, taste, smell, and overall quality. Scoring was done on a scale of 1–5 points, with 1 indicating strong dislike and 5 indicating strong liking. A panel of 7 trained individuals, aged between 24 and 50 (three male and four female), was carried out for assessment. Participants were informed to evaluate plant-based water kefirs in terms of sensory evaluation. Each sample was randomly coded with numbers. The panelists were provided with drinking water and crackers to use during the analysis. The characteristic properties of samples written in sensory evaluation forms were determined after conducting preliminary trials.
Statistical analysis
The results were analyzed using the one-way analysis of variance (ANOVA) test in IBM SPSS v. 22.0 (SPSS Inc., Chicago, USA). The Duncan’s test (P < 0.05) was used to assess significantly different results in pH, dry matter, mineral content, organic acid, and color and sensory analyses. All analyses were conducted with two replications and four parallels, except for the sensory analysis test. Sensory analysis was conducted with seven replications and fourteen parallels.
Results and discussion
Proximate composition findings
The findings on pH levels and dry matter contents of plant-based extract and kefir samples are given in Table 1. The lactic acid bacteria in water kefir ferment plant-based extract, leading to an increase in acidity. The pH range of plant-based extract was 6.46–6.89, while the pH values in kefir samples ranged from 4.52 to 4.70. This difference is attributed to the microbial metabolism of water kefir grains during the 17-h fermentation process. In most cases, the pH of a food is essential to meet food safety requirements. For example, to prevent the risk of Clostridium botulinum toxin, the pH of a food should be kept below 4.6 [32]. Typically, milk kefir fermentation is deliberately ceased at pH 4.6 [33]. In most cases, the pH of the water kefir drinks was below 4.5. Therefore, water kefir drinks could be considered to be potentially safe from risky pathogens [34]. The findings indicate that plant-based extract is rich in nutrients that support the growth of water kefir microbiota. Aydar et al. [8] reported the pH values of plant-based extract. Rice extract had a pH range of 5.21–6.10, while almond extract had a pH range of 5.72–6.92. Aydar et al. [8] found lower pH values for plant-based extract compared to our study. The final pH values of various plant-based water kefirs ranged between 3.58 and 5.41 [25, 27, 28, 31]. Our results were consistent with the literature. In this study, water kefir grains were used to produce kefir from plant-based extract. The plant extracts created a favorable environment for the growth of water kefir grains, as indicated by the decrease in pH. Lynch et al. [34] highlighted that fermentation can lead to a decrease in pH below 4.5, primarily due to the increased production of lactic acid and acetic acid.
The dry matter contents of plant-based extracts and kefir samples are shown in Table 1. The dry matter contents of almond extract and kefir were found to be higher than those of rice and chickpea kefir. Alozie Yetunde and Udofia [35] reported that the total dry matter values of almond kefir samples range from 3.36% to 8.11%. The dry matter content of various water kefirs has also been reported to be between 1.50 and 8.00% [25, 27, 31, 36]. The dry matter content of water kefirs may vary and is affected by the type and amount of carbon source used as a substrate for water kefir fermentation [37]. The dry matter content of the almond kefir sample, which is 6.94%, aligns with previous research findings. It was determined that changes in dry matter content were suitable for plant-based kefirs.
Microbial findings
Almond kefir contained 8.3 log cfu/mL Lactobacillus spp.; no significant decrease was detected during storage. The Lactobacillus spp. content of rice kefir was 5.64 log cfu/mL and significantly increased during cold storage, maybe due to the slow metabolism of rice starch. The initial Lactobacillus spp. content of chickpea kefir was 7.3 log cfu/mL, and there was a significant increase observed on Day 14 (Fig. 2). Similar to our results, the number of lactic acid bacteria was reported to be around 7 log cfu/ml for the water kefir drink, and the number of bacteria also increased during storage for 28 days [27]. Darvishzadeh, Orsat, and Martinez [38] found that the microbial content of fruit-based water kefir was 7.20 log cfu/mL for acetic acid bacteria, 7.06 log cfu/mL for lactic acid bacteria, and 7.17 log cfu/mL for yeasts. Due to the high contents of L. acidophilus in plant-based kefirs, it was thought that plant-based extract was rich in nutrients for the growth of probiotics. Rice kefir has a relatively low content of Lactobacillus spp., but Lactococcus spp. is detected at the highest amount with 8.5 log cfu/mL in rice kefir. The population of Lactococcus spp. in almond kefir and chickpea kefir was 7.73 log cfu/mL, and it showed no significant increase during cold storage. The microbial findings in kefir made from cow’s milk exhibit similar values [39]. Yeasts in water kefir grains were successfully grown in almond, rice, and chickpea extracts during fermentation. Bacteria and yeasts in water kefir are highly adaptable to different substrates [40]. Almond kefir had a yeast count of 5.36 log cfu/mL, and there was no significant change observed after cold storage. The rice kefir contained 3.62 log cfu/mL of yeast, while the chickpea kefir contained 4.38 log cfu/mL of yeast. The yeast levels in plant-based kefirs were comparable to those found in water kefir made with fruits and kefir made from cow’s milk, as well as the lactic acid bacteria levels observed in previous studies [19, 41,42,43]. Our results confirm that water kefir grains thrive in a plant-based extract environment, supporting our hypothesis. Plant-based extracts are a good source for fermenting water kefir grains.
In a study of kefir samples obtained from various fruits such as cornelian cherry, hawthorn, red plum, rosehip, and pomegranate juice, using water kefir grains, the total aerobic mesophilic bacteria ranged from 5.03 to 7.28 log cfu/mL [42]. In a study on water kefir production using buckwheat as a carbohydrate source, the levels of lactic acid bacteria and yeasts were found to be 7.06 log cfu/mL and 7.17 log cfu/mL, respectively [38]. Our findings align with prior research.
Elemental analysis findings
The minerals calcium (Ca), chromium (Cr), copper (Cu), iron (Fe), potassium (K), magnesium (Mg), manganese (Mn), molybdenum (Mo), sodium (Na), phosphorus (P), selenium (Se), and zinc (Zn) were analyzed in plant-based kefir samples (Table 2). Almonds, chickpeas, and rice are mineral-rich products. The mineral content in plant-based kefir samples is nutritionally significant when compared to kefir made from animal milk. The analysis revealed that plant-based kefir samples were high in calcium, potassium, and magnesium, but low in chromium, manganese, molybdenum, and selenium minerals. Calcium and potassium levels in plant-based kefirs ranged between 54 and 133 mg/L and 68 and 831 mg/L, respectively. In a previous study [44], the mineral content of rice-based drinks was reported as 125 mg/kg for Ca, 84 mg/kg for P, and 228 mg/kg for Na. Similar to our results, rice drinks were described as low in minerals [44]. The mineral content of almond-based drinks was reported by various scientists as 214 mg/kg and 13.10 mg/100 mL for Ca, 131 mg/kg and 65.33 mg/100 mL for P, 65 mg/kg and 42.05 mg/100 mL for Mg, 218 mg/kg and 65.33 mg/100 mL for K, and 253 mg/kg and 6.38 mg/100 mL for Na [35, 44]. It seems that the mineral content of almond beverages varied depending on the almond variety and the process conditions during the production of the almond beverage. Our findings indicate that the calcium, potassium, magnesium, and phosphorous levels in almond and chickpea kefir samples were significant. Rice kefir had lower levels of these minerals, but rice extract was high in copper. The study found that plant-based kefirs can be rich sources of minerals (Table 2).
Organic acid findings
Lactic acid and acetic acid are common products of lactic acid fermentation, acetic acid fermentation, and glucose metabolism. As a result, they are found in high concentrations in fermented foods. Organic acids have a range of health effects and physiological functions on humans. For example, lactate exerts immunomodulatory effects by regulating the cellular cytokine network and the signaling systems of the gut mucosa. Citric acid modulates tyrosinase activity and thus inhibits melanogenesis in human cells. Acetic acid and propionic acid improve and modulate insulin response by increasing the satiety time of humans. Organic acids have antimicrobial effects on pathogenic microorganisms, which may be advantageous in promoting gut colonization by beneficial microorganisms rather than pathogens [45, 46].
The almond kefir had the highest amount of lactic acid at 1.99 ± 0.27 g/L, while the rice kefir had the lowest amount at 1.089 ± 0.22 g/L among the plant-based kefir samples (P < 0.05). The highest acetic acid was observed in almond kefir. However, the lowest acetic acid level was determined in chickpea kefir samples (P < 0.05). Tartaric acid was detected in rice extract and chickpea extract with significantly different amounts from each other (P < 0.05). A similar trend was available for rice kefir and chickpea kefir. The presence of acetic acid and lactic acid in our samples indicates the presence of both homofermentative and heterofermentative lactic acid bacteria [47]. Increasing the concentration of acetic acid as the fermentation process progresses helps to develop the fruity flavor and aroma of water kefir beverages [36]. The formation of organic acids in water kefir beverages serves important functions, including antimicrobial effects against pathogens and the development of characteristic taste and aroma in fermented beverages [48]. The content of organic acids in plant extracts can be influenced by a number of factors, such as the conditions of the plantation, the ripeness, the geographical area and origin, the quality of the soil, the intensity of fertilization, the storage conditions, and the time of harvest [26]. Other studies have reported similar trends to our results, indicating that following fermentation, the organic acid content of water kefirs increases as a result of microbial activity [26, 31]. The concentrations of lactic acid and acetic acid in different plant-based water kefirs were reported as 0.02–4.81 g/L and 0.02–1.90 g/L, respectively [43, 47]. Our values were in line with these values (Table 3).
Volatile profiles of plant-based extract and kefir samples
Volatile compounds were detected in extracts and kefir samples. The chromatograms of volatile compounds in the samples are shown in Figure S2. Volatile compounds are crucial for conveying the metabolomic properties of water kefir. Ethanol and acetic acid were the two most widely detected volatiles among the samples except rice extract. Then, ethyl acetate and benzenemethanol were two commonly detected volatiles among the samples. Chickpea kefir samples had most of the volatiles (13/19) in this study and then almond kefir and rice kefir (11/19). Ethyl acetate was not found in almond extract, rice extract, and chickpea extract. However, it was detected in the highest amount, 571.62 μg/100 mL, in almond kefir as a result of fermentation with water kefir grains. Ethyl acetate is produced through the fermentation reaction of acetyl-CoA and ethanol [49]. Ethyl acetate is sensorially significant because it has a sweet aroma that adds a fruity taste to foods. Other scientists have reported the presence of ethyl acetate in water kefir [50]. However, higher levels of ethyl acetate are undesirable in water kefir as they can result in a solvent-like aroma [50]. Previous studies have reported various concentrations of ethyl acetate detected in water kefirs, ranging from 21.74 to 1581.5 mg/kg and 13.40 mg/liter [37, 51]. Studies have shown that the ethanol content in kefir contributes to its distinctive aroma [50]. After fermenting water kefir grains, the ethanol content of plant-based extract significantly increased. Almond kefir had the highest ethanol content among plant-based kefirs, while chickpea kefir had a higher ethanol content than rice kefir. Ethanol concentrations ranging from 0.674 to 20.3 g/liter were reported for various water kefirs [31, 51]. Plant-based extract and their kefirs were compared to understand the volatile profiles and the effect of the kefir fermentation. It was observed that ethyl acetate and acetaldehyde were not present in plant extracts and were detected at high rates in the kefirs of these extracts. The development of ethanol and acetic acid in almond kefir was found to be the highest compared to other samples.
The microbial community present in the water kefir grains and fermentation medium is responsible for the flavor and taste of water kefir. For example, certain lactic acid bacteria, such as L. hordei, L. mali, and L. plantarum, have been found to contribute to the production of lactic acid, ethanol, and acetic acid. Saccharomyces spp. yeasts have been reported to contribute to the formation of aldehydes and esters [52]. The yeasts present in water kefir grains were identified in our laboratory. Saccharomyces spp., Zygotorulaspora florentina, Dekkera bruxellensis, and Lachancea fermentati were identified using RT-PCR in water kefir grains used for producing plant-based water kefir in our current study (unpublished data). Various yeasts, particularly Saccharomyces sp., have also been found in previous studies [53, 54]. Different fermentation characteristics of Z. florentina resulted in a more robust floral flavor and lower bitterness perception. D. bruxellensis is commonly found in biotechnological environments with high ethanol levels. It has an essential feature that contributes to the characteristic aromatic properties of some red wines and is essential in the flavor profile of Belgian Lambic and Gueuze beers with the acetic acid taste it provides [55]. Lachancea species are known to ferment glucose and utilize raffinose, ethanol, and mannitol. Monoculture fermentations with L. fermentati have been found to result in lower levels of acetaldehyde, H2S, and SO2 production, increased volatile acidity, and lower titratable acidity compared to S. cerevisiae [56]. As can be understood from the findings, ethanol fermentation was carried out with these yeasts in the water kefirs.
Acetic acid content increased in plant-based kefirs, with the highest levels found in almond kefir. In contrast, the acetic acid level was low in plant extracts. It has been reported that the presence of oxygen in the water kefir fermentation environment leads to an increase in the number of acetic acid bacteria species, resulting in a higher concentration of acetic acid at the end of the water kefir fermentation process [57]. The concentration of acetic acid in various water kefirs was reported to range from 0.11 to 7.88 g/liter [57]. Acetaldehyde is a flavor component found in fermented milk products, particularly yogurt and kefir, made from cow’s milk. Acetaldehyde is primarily formed during water kefir fermentation as a result of the metabolism of lactic acid bacteria and yeast [58]. Acetaldehyde was found in chickpea kefir and rice kefir, as shown in Table 4. 1-hexanol was detected in samples of rice kefir and chickpea kefir. The concentration of 1-hexanol was found to be between 18.43 and 218.38 μg/L in different fruit juice-based water kefirs [43]. This alcohol has a positive effect on aroma when its concentration is below 20 mg/L [59]. The concentration values for 1-hexanol in our samples were lower than 20 mg/L, which is a desirable outcome. Phenylethyl alcohol is produced by yeast during food production [60]. It contributes a floral scent to the product [61] and has been identified as a quorum-sensing molecule [62]. Phenylethyl alcohol was detected in water kefirs by various scientists, with amounts ranging from 54.34 to 2241.74 μg/L [43, 47].
Sensory findings
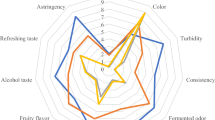
The sensory evaluation of plant-based kefir includes assessments of color, appearance, consistency, taste, odor, and general criteria (Fig. 3). Almond kefir received the highest scores for taste and consistency among the samples. Chickpea kefir received the lowest scores in all criteria. It was observed that the distinct smell of chickpeas is present in the fermented product, which negatively impacts its sensory properties. Although the scores for chickpea kefir were low, it was evident that it still had a certain level of acceptability. The purpose of this study was to investigate the potential use of water kefir grains in plant-based extract production and to assess the microbial activity and metabolism involved. Natural flavors are used in industrial production to enhance sensory results. Consumers are generally not familiar with plant-based extract products. Additionally, the sensory quality of these products may not be preferable. This is probably as a result of off flavor or beany flavor. These are generally not desirable for consumers [10, 63]. Various factors can impact the overall acceptance of plant-based kefir products. The addition of sugar or flavoring can enhance the taste of plant-based products, making them more enjoyable for consumers [10].
Color findings
Color is one of the most important properties of food that can influence a consumer’s preference [64]. The whiteness and lightness of a food sample are crucial factors that impact consumer acceptance of a food product. The size and dispersion of oil particles, the type and concentration of chromophores, and the production method are the main factors influencing these parameters [65]. In the color analysis of the plant-based kefirs, the L* value, which represents whiteness, is highest in almond kefir. Chickpea kefir had the lowest L* values. The sensory analysis results were in line with this; e.g., overall acceptability and attractiveness of color were highest for almond kefir and lowest for chickpea kefir. While the L* value increased in rice extract due to fermentation, the L* value decreased in almond and chickpea extract after fermentation (Table 5). There was a significant difference between the brightness values of extract samples (P < 0.05). Almond kefir had a significant difference in brightness value rather than rice kefir and chickpea kefir. The study conducted by Sezgin [66] compared the L* values of hazelnut, walnut, almond, coconut, soy extracts, and cow’s milk. It was found that hazelnut extract had the highest L* value among all the extract types. There was a significant difference in the degree of redness between extract samples, while chickpea kefir had a significant difference rather than almond kefir and rice kefir in the aspect of redness degree (Table 5). Rice extract was significantly different in level of yellowness rather than almond extract and chickpea extract. In addition, kefir samples were significantly different from each other for level of yellowness (P < 0.05). Green tones are represented by the negative value “a,” while yellow tones are represented by the positive value “b.” Thus, the almond extract and almond kefir samples had a greenish-yellow color, which may be attributed to the riboflavin content of the samples [26]. Nevertheless, consumers are generally willing to accept various and even strange colors for plant extracts, such as light brown or grey [67].
Conclusion
Almond kefir, rice kefir, and chickpea kefir are great options for vegans, individuals with allergies to animal protein, and those seeking new and unique flavors. It has been confirmed that the microbiota of water kefir grains can thrive in plant-based extracts. Other metabolites and volatile compounds are formed during fermentation, along with probiotic microorganisms. The study is pioneering as it is the first to utilize water kefir grains, particularly in almond and chickpea extracts. The results of this study open up possibilities for using water kefir grains in various plant-based nutrients, leading to the development of new products. Kefirs made from plant-based extracts are a rich source of probiotics and minerals, including calcium, phosphorus, potassium, and magnesium.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Harper AR, Dobson RC, Morris VK, Moggré G-J (2022) Fermentation of plant-based dairy alternatives by lactic acid bacteria. Microb Biotechnol 15:1404–1421
Bin Rahman ANMR, Zhang J (2023) Trends in rice research: 2030 and beyond. Food Energy Secur 12:e390. https://doi.org/10.1002/fes3.390
Tomishima H, Luo K, Mitchell AE (2022) The almond (Prunus dulcis): chemical properties, utilization, and valorization of coproducts. Annu Rev Food Sci Technol 13:145–166. https://doi.org/10.1146/annurev-food-052720-111942
Samineni S, Sajja SB, Mondal B et al (2021) MAGIC lines in chickpea: development and exploitation of genetic diversity. Euphytica 217:137. https://doi.org/10.1007/s10681-021-02874-0
Wallace TC, Murray R, Zelman KM (2016) The nutritional value and health benefits of chickpeas and hummus. Nutrients 8:766
Fukagawa NK, Ziska LH (2019) Rice: importance for global nutrition. J Nutr Sci Vitaminol (Tokyo) 65:S2–S3
Barreca D, Nabavi SM, Sureda A et al (2020) Almonds (Prunus dulcis Mill. DA Webb): a source of nutrients and health-promoting compounds. Nutrients 12:672
Aydar EF, Tutuncu S, Ozcelik B (2020) Plant-based milk substitutes: Bioactive compounds, conventional and novel processes, bioavailability studies, and health effects. J Funct Foods 70:103975
Jeske S, Zannini E, Arendt EK (2018) Past, present and future: the strength of plant-based dairy substitutes based on gluten-free raw materials. Food Res Int 110:42–51
Sethi S, Tyagi SK, Anurag RK (2016) Plant-based milk alternatives an emerging segment of functional beverages: a review. J Food Sci Technol 53:3408–3423
Zheng B, Zhou H, McClements DJ (2021) Nutraceutical-fortified plant-based milk analogs: bioaccessibility of curcumin-loaded almond, cashew, coconut, and oat milks. Lwt 147:111517
Zujko ME, Witkowska AM (2014) Antioxidant potential and polyphenol content of beverages, chocolates, nuts, and seeds. Int J Food Prop 17:86–92
Gökırmaklı Ç, Güzel-Seydim ZB (2022) Water kefir grains vs. milk kefir grains: physical, microbial and chemical comparison. J Appl Microbiol 132:4349–4358. https://doi.org/10.1111/jam.15532
Gökırmaklı Ç (2023) Optimization of water kefır production, investigation of its microbiota and probiotic properties. Ph. D. Dissertation. Süleyman Demirel University, Isparta
Koh WY, Lim XX, Tan TC, Abdullah S (2021) Characterization of probiotics from water kefir grains. Trans Sci Technol 8:667–672
Romero-Luna HE, Peredo-Lovillo A, Hernández-Mendoza A et al (2020) Probiotic potential of Lactobacillus paracasei CT12 isolated from water kefir grains (Tibicos). Curr Microbiol 77:2584–2592. https://doi.org/10.1007/s00284-020-02016-0
Alsayadi M, Al Jawfi Y, Belarbi M et al (2014) Evaluation of anti-Hyperglycemic and anti-hyperlipidemic activities of water kefir as probiotic on Streptozotocin-induced diabetic Wistar rats. J Diabetes Mellit 4:85–95. https://doi.org/10.4236/jdm.2014.42015
Aspiras BEE, Flores R, Pareja MC (2015) Hepatoprotective effect of fermented water kefir on Sprague-Dawley rats (Rattus norvegicus) induced with sublethal dose of Acetaminophen. Int J Curr Sci 17:18–28
Hsieh H-H, Wang S-Y, Chen T-L et al (2012) Effects of cow’s and goat’s milk as fermentation media on the microbial ecology of sugary kefir grains. Int J Food Microbiol 157:73–81. https://doi.org/10.1016/j.ijfoodmicro.2012.04.014
Koh WY, Utra U, Ahmad R et al (2018) Evaluation of probiotic potential and anti-hyperglycemic properties of a novel Lactobacillus strain isolated from water kefir grains. Food Sci Biotechnol 27:1369–1376. https://doi.org/10.1007/s10068-018-0360-y
Rocha-Gomes A, Escobar A, Soares JS et al (2018) Chemical composition and hypocholesterolemic effect of milk kefir and water kefir in Wistar rats. Rev Nutr 31:137–145. https://doi.org/10.1590/1678-98652018000200001
Gökırmaklı Ç, Erol Z, Gun I et al (2023) Prophylaxis effects of water kefir on post-infectious irritable bowel syndrome in rat model. Int J Food Sci Technol. https://doi.org/10.1111/ijfs.16310
Tiss M, Souiy Z, Abdeljelil ben N et al (2020) Fermented soy milk prepared using kefir grains prevents and ameliorates obesity, type 2 diabetes, hyperlipidemia and liver-kidney toxicities in HFFD-rats. J Funct Foods 67:103869
Gocer EMC, Koptagel E (2023) Production of milks and kefir beverages from nuts and certain physicochemical analysis. Food Chem 402:134252
Tavares PPLG, Silva MR, Santos LFP et al (2018) Produção de bebida fermentada kefir de quinoa (Chenopodium quinoa) saborizada com cacau (Theobroma cacao) em pó. Rev Bras Ciênc Agrár 13:1–7
Satir G (2022) The effects of fermentation with water kefir grains on two varieties of tigernut (Cyperus esculentus L.) milk. LWT 171:114164. https://doi.org/10.1016/j.lwt.2022.114164
Pinto LC, Oliveira TP, Souza R et al (2021) Probiotic kefir-fermented beverage-based Colocasia esculenta L.: development, characterization, and microbiological stability during chilled storage. J Food Process Preserv. https://doi.org/10.1111/jfpp.15113
Silva MR, Santos FL, Nunes IL (2018) Production of rice cereal-based Kefir beverage. Afr J Biotechnol 17:322–327
AOAC (1992) Official methods of analysis, 14th edn. Association of Official Analytical Chemists, Washington, DC
Spencer JF, de Spencer ALR (2001) Food microbiology protocols. (1st ed., 494 pp.) Springer Sci Business Med. https://doi.org/10.1385/1592590292
Gökırmaklı Ç, Yuceer-Karagul Y, Guzel-Seydim ZB (2023) Chemical, microbial, and volatile changes of water kefir during fermentation with economic substrates. Eur Food Res Technol. https://doi.org/10.1007/s00217-023-04242-9
Tanaka N (1982) Toxin production by Clostridium botulinum in media at pH lower than 4.6. J Food Prot 45:234–237
Guzel-Seydim ZB, Gökırmaklı Ç, Greene AK (2021) A comparison of milk kefir and water kefir: physical, chemical, microbiological and functional properties. Trends Food Sci Technol 113:42–53
Lynch KM, Wilkinson S, Daenen L, Arendt EK (2021) An update on water kefir: Microbiology, composition and production. Int J Food Microbiol 345:109128. https://doi.org/10.1016/j.ijfoodmicro.2021.109128
Alozie Yetunde E, Udofia US (2015) Nutritional and sensory properties of almond (Prunus amygdalu Var. Dulcis) seed milk. World J Dairy Food Sci 10:117–121
Güzel-Seydim ZB, Şatır G, Gökırmaklı Ç (2023) Use of mandarin and persimmon fruits in water kefir fermentation. Food Sci Nutr. https://doi.org/10.1002/fsn3.3561
Havva Ş, Gun I, Tudor Kalit M, Kalit S (2023) Physico-chemical, microbiological and sensory properties of water kefir drinks produced from demineralized whey and Dimrit and Shiraz grape varieties. Food 12:1851. https://doi.org/10.3390/foods12091851
Darvishzadeh P, Orsat V, Martinez JL (2021) Process optimization for development of a novel water kefir drink with high antioxidant activity and potential probiotic properties from Russian olive fruit (Elaeagnus angustifolia). Food Bioprocess Technol 14:248–260
Aroua M, Ben Haj Koubaier H, Bouacida S et al (2023) Chemical, physicochemical, microbiological, bioactive, and sensory characteristics of cow and donkey milk kefir during storage. Beverages 9:2
Koh WY, Utra U, Rosma A et al (2018) Development of a novel fermented pumpkin-based beverage inoculated with water kefir grains: a response surface methodology approach. Food Sci Biotechnol 27:525–535
Erdogan FS, Ozarslan S, Guzel-Seydim ZB, Taş TK (2019) The effect of kefir produced from natural kefir grains on the intestinal microbial populations and antioxidant capacities of Balb/c mice. Food Res Int 115:408–413
Ozcelik F, Akan E, Kinik O (2021) Use of Cornelian cherry, hawthorn, red plum, roseship and pomegranate juices in the production of water kefir beverages. Food Biosci 42:101219
Randazzo W, Corona O, Guarcello R et al (2016) Development of new non-dairy beverages from Mediterranean fruit juices fermented with water kefir microorganisms. Food Microbiol 54:40–51
Moore SS, Costa A, Pozza M et al (2023) How animal milk and plant-based alternatives diverge in terms of fatty acid, amino acid, and mineral composition. Npj Sci Food 7:50
Coban HB (2020) Organic acids as antimicrobial food agents: applications and microbial productions. Bioprocess Biosyst Eng 43:569–591. https://doi.org/10.1007/s00449-019-02256-w
Shi Y, Pu D, Zhou X, Zhang Y (2022) Recent progress in the study of taste characteristics and the nutrition and health properties of organic acids in foods. Foods 11:3408
Corona O, Randazzo W, Miceli A et al (2016) Characterization of kefir-like beverages produced from vegetable juices. LWT-Food Sci Technol 66:572–581
Tavares PPLG, Mamona CTP, Nascimento RQ et al (2023) Non-conventional sucrose-based substrates: development of non-dairy kefir beverages with probiotic potential. Fermentation 9:384
Zhang S, Guo F, Yan W et al (2020) Perspectives for the microbial production of ethyl acetate. Appl Microbiol Biotechnol 104:7239–7245
Laureys D, Leroy F, Hauffman T et al (2021) The type and concentration of inoculum and substrate as well as the presence of oxygen impact the water kefir fermentation process. Front Microbiol 12:628599. https://doi.org/10.3389/fmicb.2021.628599
Laureys D, De Vuyst L (2014) Microbial species diversity, community dynamics, and metabolite kinetics of water kefir fermentation. Appl Environ Microbiol 80:2564–2572. https://doi.org/10.1128/AEM.03978-13
Patel SH, Tan JP, Börner RA et al (2022) A temporal view of the water kefir microbiota and flavour attributes. Innov Food Sci Emerg Technol 80:103084. https://doi.org/10.1016/j.ifset.2022.103084
Galli A, Fiori E, Franzetti L et al (1995) Composizione microbiologica e chimica dei granuli di Kefir “di frutta.” Ann Microbiol Ed Enzimologia 45:85–95
Waldherr FW, Doll VM, Meißner D, Vogel RF (2010) Identification and characterization of a glucan-producing enzyme from Lactobacillus hilgardii TMW 1.828 involved in granule formation of water kefir. Food Microbiol 27:672–678
Schifferdecker AJ, Dashko S, Ishchuk OP, Piškur J (2014) The wine and beer yeast Dekkera bruxellensis. Yeast 31:323–332
Porter TJ, Divol B, Setati ME (2019) Lachancea yeast species: Origin, biochemical characteristics and oenological significance. Food Res Int 119:378–389
Laureys D, Aerts M, Vandamme P, De Vuyst L (2018) Oxygen and diverse nutrients influence the water kefir fermentation process. Food Microbiol 73:351–361. https://doi.org/10.1016/j.fm.2018.02.007
Tian H, Xiong J, Yu H et al (2023) Flavor optimization in dairy fermentation: From strain screening and metabolic diversity to aroma regulation. Trends Food Sci Technol. https://doi.org/10.1016/j.tifs.2023.104194
Dragone G, Mussatto SI, Oliveira JM, Teixeira JA (2009) Characterisation of volatile compounds in an alcoholic beverage produced by whey fermentation. Food Chem 112:929–935
Conde-Báez L, Castro-Rosas J, Villagómez-Ibarra JR et al (2017) Evaluation of waste of the cheese industry for the production of aroma of roses (Phenylethyl Alcohol). Waste Biomass Valorization 8:1343–1350. https://doi.org/10.1007/s12649-016-9654-6
Farag MA, Jomaa SA, Abd El-Wahed A, El-Seedi HR (2020) The many faces of kefir fermented dairy products: quality characteristics, flavour chemistry, nutritional value, health benefits, and safety. Nutrients 12:346
Han T-L, Tumanov S, Cannon RD, Villas-Boas SG (2013) Metabolic response of Candida albicans to phenylethyl alcohol under hyphae-inducing conditions. PLoS ONE 8:e71364
Giacalone D, Clausen MP, Jaeger SR (2022) Understanding barriers to consumption of plant-based foods and beverages: insights from sensory and consumer science. Curr Opin Food Sci. https://doi.org/10.1016/j.cofs.2022.100919
Jeske S, Zannini E, Arendt EK (2017) Evaluation of physicochemical and glycaemic properties of commercial plant-based milk substitutes. Plant Foods Hum Nutr 72:26–33. https://doi.org/10.1007/s11130-016-0583-0
Tobolková B, Durec J (2023) Colour descriptors for plant-based milk alternatives discrimination. J Food Sci Technol 60:2497–2501. https://doi.org/10.1007/s13197-023-05773-5
S Seçin Burcu (2021) Microbial and sensory properties of various plant based milks. Master Thesis, İstanbul Teknik Üniversitesi
Reyes-Jurado F, Soto-Reyes N, Dávila-Rodríguez M et al (2023) Plant-based milk alternatives: types, processes, benefits, and characteristics. Food Rev Int 39:2320–2351. https://doi.org/10.1080/87559129.2021.1952421
Acknowledgements
We thank Danem Inc. (kefirdanem.com) for providing kefir grains.
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK). This study was supported by the Suleyman Demirel University Scientific Research Projects Coordination Unit, Project Number: FYL-2020-7972.
Author information
Authors and Affiliations
Contributions
M.U.G achieved the fund, involved in formal analysis, extracted the data, and wrote and edited the manuscript text; Ç.G involved in formal analysis, extracted the data, and wrote and edited the manuscript text; B.U achieved the fund and wrote and edited the manuscript text; Y. K. Y involved in formal analysis, extracted the data, and wrote and edited the manuscript text; and Z. B. G. S involved in formal analysis, extracted the data, wrote and edited the manuscript text, and acted as a supervisor.
Corresponding author
Ethics declarations
Conflict of interest
None.
Compliance with ethical requirements
This article does not contain any studies with human or animal subjects.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file 1:
(DOCX 100 KB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ustaoğlu-Gençgönül, M., Gökırmaklı, Ç., Üçgül, B. et al. Chemical, microbial, and volatile compounds of water kefir beverages made from chickpea, almond, and rice extracts. Eur Food Res Technol 250, 2233–2244 (2024). https://doi.org/10.1007/s00217-024-04533-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-024-04533-9