Abstract
Blackcurrant juices were produced with an oxygen-excluding spiral filter press and two conventional pressing systems exerting common oxygen exposure at pilot plant scale (200 kg) in technological duplicate in two subsequent years (n = 4). In contrast to findings on previously studied fruits, contents of total and individual anthocyanins were not significantly higher after spiral filter (1636–1872 mg/L) than after conventional pressing (1529–2083 mg/L). During storage for 52 weeks at 4, 20 and 37 °C, anthocyanin levels depended strongly on storage temperature, but not on the used pressing system (conventional vs. oxygen-reduced). Other juice quality parameters like ascorbic acid behaved likewise. Furthermore, a D-optimal design of experiments was used to study the influence and interactions of the factors oxygen, ascorbic acid, sugar and pH value on anthocyanin levels during a heat treatment in a model solution made with preparatively isolated blackcurrant anthocyanins. In agreement with our production trials at pilot plant scale, oxygen levels were only marginally important for the rate of anthocyanin degradation, but the influence of sugar content, ascorbic acid content and pH value was highly relevant as discussed in this report.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fruits like berries, cherries and plums often contain high contents of anthocyanins, which are responsible for their red, purple and blue colors [1]. Due to their intense color, blackcurrants are an attractive and rich source of anthocyanins. Juices and concentrates derived thereof can therefore be used as natural coloring foods [2]. Apart from the colorful anthocyanins, which also show potentially health-promoting antioxidative and anti-inflammatory activities, blackcurrant juices or concentrates also contain health beneficial minerals like potassium and vitamins such as ascorbic acid and provitamin A [3,4,5].
In the European Union, the coloring properties of blackcurrant juices and concentrates are often used to provide color to fruit juice mixtures as well as to carbonated soft drinks, because more and more producers worldwide are banning particularly artificial colors from their recipes [6]. The ban is motivated by continuing scientific insights into the potentially detrimental health effects of consuming artificial colorants. For instance, McCann et al. [6] found a correlation between the consumption of azo dyes and hyperactivity in children. A recent report of the California Office of Environmental Health Hazard Assessment (OEHHA) has confirmed the link of artificial food dyes with hyperactivity and other behavioral disorders [7]. In the EU, foods containing specific artificial colorants have to be labeled with the warning message that the respective colorant “may have an adverse effect on activity and attention in children.” [8] (Directive 1333/2008, Annex V). Therefore, “clean-labeling” with regard to colorants has had high priority in the EU. An often limiting factor of anthocyanin-rich natural coloring foodstuffs is, in comparison to artificial dyes, their poor color stability upon heat treatments, pH changes, light exposure, and the presence of oxygen, ascorbic acid, metal ions and enzymes. Furthermore, the present levels of sugars and co-pigments are influencing the stability of anthocyanins [5]. Oxygen input during and after fruit processing not just causes color changes of the products, but also the decay of aroma impact compounds and health-related bioactives beyond anthocyanins. Consequently, product acceptance by consumers decreases. As a consequence, food industrial processing commonly aims at retaining maximum levels of fruit-derived anthocyanins and other health-related bioactive compounds during production and in the end products [9,10,11].
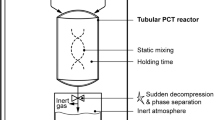
To minimize undesired oxygen-caused effects, a vacuum-driven spiral filter pressing system was tested in this work to produce blackcurrant juice. The innovative pressing system was described and illustrated in detail in a previous study [12]. In brief, this closed pressing system combines a milling system under oxygen reduced atmosphere with subsequent fast and continuous conveying of the mash to a spiral screw that pumps the mash in thin layers through a sieve cylinder being set under reduced pressure. Driven by the pressure gradient, the liquid is passed through the sieve, while the solids are retained and pumped to the pomace outlet at the end of the sieve cylinder. A further consequence of the reduced pressure environment is a degassing effect simultaneously to dejuicing, thereby ensuring minimum amounts of oxygen in the juice. Variable machine parameters allow juice and also puree production, depending on the pore size of the sieve used in the cylinder.
In this study, we first produced blackcurrant juices with the aforementioned spiral filter press and two conventional processing approaches, i.e. using a hydraulic horizontal filter press and a decanter, all being used at pilot plant scale in technological duplicates at approximately 200 kg per batch in two different years (n = 4 per technology). After production, we investigated the retention of various oxidation-sensitive constituents including anthocyanins as well as of other compounds relevant for juice quality in the obtained blackcurrant juices over the course of a stability study at three temperatures (4, 20 and 37 °C) for 52 weeks. Technology-related parameters such as juice yield, turbidity and viscosity were also assessed. Moreover, we conducted a series of small-scale experiments following a modified D-optimal design of experiments to study the influence of the levels of oxygen, ascorbic acid and total sugars as well as pH during heat treatment on blackcurrant anthocyanins. For this purpose, blackcurrant anthocyanins were isolated from a blackcurrant juice and then well-defined artificial model solutions were made to adjust the beforementioned factors at different levels, in order to gain deeper and more general insights into the stability of anthocyanins from blackcurrant.
Materials and methods
Production of cloudy blackcurrant juice
A total of 1200 kg frozen blackcurrants (Ribes nigrum L.) were purchased at a commercial distributor (Obst Trautner, Walkenberg, Germany) in both August 2019 and September 2020 and subsequently stored at 3 °C before production. For each batch, 200 kg for each one of two technical repetitions per year and processing technology were warmed with a ploughshare mixer (FM300D, Lödige, Paderborn, Germany) to 50 °C before adding 40 mL of a concentrated pectinolytic preparation designed for berry fruits (Fructozym Color, Erbslöh, Geisenheim, Germany) as recommended by the manufacturer for blackcurrant processing (50–150 mL/1000 kg mash). The enzyme treatment was carried out under continuous mixing for 90 min at 50 °C (manufacturers’ recommendation: 60–120 min at 45–55 °C). The complete degradation of soluble pectin was confirmed by the pectin test [13] after centrifuging an aliquot of 50 mL mash.
After mash enzymatic treatment, the warm mash was pumped without further milling by an open hopper pump (SR5, Kiesel, Heilbronn, Germany) into one of the different dejuicing systems, namely the spiral filter press (VaculiQ-1000, VaculiQ, Hamminkeln, Germany), the horizontal filter press (HP-L 200, Bucher, Niederweningen, Switzerland) or decanter (Z23-3, Flottweg, Vilsbiburg, Germany) as described as follows.
The spiral filter press was equipped with a sieve of 100 µm pore size and a spiral with three mash channels. The mash was pumped at a pump pre-pressure of 0.2 bar and a spiral speed regulation of 60 Hz to the extraction cell. The applied vacuum in the pressing chamber was 0.08–0.12 bar below atmospheric pressure. After spiral filter dejuicing, which lasted 15–30 min per batch, the raw juice was collected in an inert atmosphere (N2) buffer tank.
For juice productions with horizontal filter press or decanter, the mash was pumped by a progressive cavity pump (SR5, Kiesel, Heilbronn, Germany) into the pressing systems. During decanter dejuicing, the rotational speed of the outer jacket was set at 5200 rpm, and the inner screw was adjusted to reach a differential speed of 12–13 rpm. The diameter of the weir plate was 145 mm. Production time was approximately 40 min per batch. The horizontal filter press was filled with an initial amount of 40 kg mash. By adding progressively 20 kg after every press cycle duration of two min, a total of 200 kg was processed per batch. The maximal pressure was 12 bar, and the total run time of the chosen pressing program was 60 min. The raw juices produced by decanter and horizontal filter press were collected in buffer tanks without inert atmosphere according to conventional practice.
For thermal preservation, the raw juices were rapidly heated to approximately 78 °C by a fruit juice dispenser (PAS1-PS2-81-V2, Mabo, Eppingen, Germany) and hot-filled into amber glass bottles of 0.33 L volume. The bottles were cooled back in a water bath from 78 to 20 °C in approximately 15 min. The temperature–time profile was recorded and was equivalent to a total pasteurization value (P-value) of approximately 2.5, using a z-value of 10 K and a reference temperature Tref of 80 °C according to Wagner et al. [12].
Both batches produced in 2020 were used for the stability study. For this purpose, the filled 0.33 L-bottles were stored at three different temperatures (4, 20 and 37 °C) in a dark environment for 12 months, sampling an individual bottle per nine different sampling points and freezing them at – 20 °C until further analyses.
Measurement of dissolved oxygen in the juice and anthocyanins model solutions
For measurement of dissolved oxygen, a sample of 250 mL freshly pressed juice was analyzed immediately after solid–liquid separation with an optical oxygen probe (FireSting®-O2 FSO2-4, Pyroscience, Aachen, Germany). The beforementioned oxygen probe was also used to monitor oxygen-levels in the anthocyanin model solutions described below.
Determination of physico-chemical parameters
For determination of centrifugable cloud, an aliquot of 10 g of juice was centrifuged for 15 min at 4200 × g and the supernatant was removed and discarded. The sample tubes were drained upside down for 30 min at room temperature and the cloud content was determined gravimetrically in % (w/w) on basis of the pellet weight. Turbidity was measured by a light scattering photometer (Nephla LPG 239.52, Dr. Bruno Lange, Berlin, Germany).
Relative density was measured on the basis of the IFU-method No. 1 with a flexural resonator (DMA 48, Anton Paar, Graz, Austria) [14]. Total soluble solids (TSS, °Brix) were measured at 20 °C using a digital refractometer (Abbémat, Dr. Kernchen, Seelze, Germany). The extract content was determined on basis of the density table of Reichard [15]. The pH value was measured potentiometrically at 20 °C (IFU-method No. 11 [14]). Individual sugars (D-glucose, D-fructose, sucrose), citric acid and L-malic acid were determined enzymatically (IFU-methods No. 55, 56, 22 and 21 respectively [14]). Total acidity (expressed as citric acid at pH 8.1) was titrated (Titrator, Schott, Mainz, Germany) based on IFU-method No. 3 [14].
Ascorbic acid contents were determined iodometrically [16]. The total phenolic content was determined spectrophotometrically with the Folin–Ciocalteu reagent according to Singleton and Rossi [17] with catechin used as reference. The antioxidant activity as measured by the Trolox equivalent antioxidant capacity (TEAC) assay was determined by spectrophotometry (UVmini-1240, Shimadzu, Suzhou, China) according to Re et al. [18].
Objective color measurement (CIE-L*a*b*)
For color determination of juice samples after processing, a spectrophotometer (Unicam 500, Thermo Electron, Dreieich, Germany) was used. The deep red–black juice samples were centrifuged (5 min at 12,850 × g) and 1 mL of juice was diluted with 19 mL of citrate buffer. For buffer preparation, a 5.3% citric acid aqueous solution was adjusted to pH 3.0 with 1 M NaOH. The samples were measured in continuous flow in a 2 mm cuvette (Hellma, Müllheim, Germany). The resulting absorptions given by the software Vision Pro V 2.03 (Thermo Electron) were used to calculate the CIE-L*a*b* values, color saturation (chroma C* = (a*2 + b*2)½) and the color hue angle (h° = arctan (b*/a*)).
For monitoring color development during the stability study, photos were taken after 24 and 52 weeks in addition to CIE-L*a*b* measurements. For this purpose, a 1 mL juice sample was pipetted in a small petri dish and placed on a LED drawing board for standardized lighting during photography.
Rheological behavior of juice
For determination of rheological behavior, a rotational viscometer MCR 92 (Anton Paar, Graz, Austria) equipped with a temperature control device MCR 92 was used. Viscosity and shear stress of the juice samples were measured with a cone-plate measuring system (diameter 49.95 mm, cone angle 0.99°). An aliquot 0.6 mL juice was tempered to 20 °C by the measuring system prior to recording 20 measuring points at a shear rate range of 0.1–100 1/s. Besides considering the full range, data were evaluated at a share rate of 1/50 s in detail, mimicking the oral shear stress for liquids according to Wood [19]. For data processing, the Rheoplus V3.4 software (Anton Paar) was used.
Preparative isolation of anthocyanins from blackcurrant juice
To isolate phenolic compounds from a spiral filter-pressed blackcurrant juice, an adsorber resin column (BPG 140/950 column, Pharmacia, Uppsala, Sweden) was filled with a bed volume (BV) of 7 L Sepabeads SP70 (Mitsubishi, Chiyoda, Japan; particle size > 250 µm). After preconditioning the column by rinsing with ca. 3 BV deionized water, a total of 2 L juice was pumped on the column, followed by 3 BV water to remove non-phenolic juice components. Adsorbed anthocyanins and other colorless phenolic compounds were subsequently eluted with 1 BV ethanol (96% v/v with 1% methyl ethyl ketone, Mundo, Mainz, Germany). For the regeneration steps, the column was flushed with water (3 BV), followed by each 1 BV of 0.1 M sodium hydroxide (Tensid-Chemie, Muggensturm, Germany), water, 0.1 M citric acid (Weifang Ensign Industry, Shandong, China) and water, respectively. The obtained ethanolic extract was subsequently concentrated with a rotary evaporator (Heidolph Laboranta 20 RC, Schwabach, Germany) to a volume of ca. 500 mL. The concentrated extract was frozen in a round vessel placed immersed and rotating in a methanol bath at – 40 °C (Christ CB 18–40, Christ, Osterode, Germany) and subsequently freeze dried (Christ Beta 2–8 LD plus). The composition of the obtained anthocyanin extract powder was analyzed for its anthocyanin content as well as for other physico-chemical parameters as described in Sect. “Determination of physico-chemical parameters”.
Experimental design and statistical analyses
Description of the design and data analyses
For the small-scale multivariate approach, a D-optimal design of experiments, provided by Petersen and Mehr [20], was implemented to investigate the influences of four factors, namely oxygen content (x1), pH value (x2), ascorbic acid content (x3), sugar content (x4) and their interactions on kinetics of blackcurrant degradation. Levels of factor x1 were 0–1, 4–5 and 8–9 mg oxygen/L juice. Levels of factor x2 were adjusted to pH values from 2.8 to 4.6. In accordance with a previous study on the effect of ascorbic acid on anthocyanin stability by Rosso and Mercadante [21], x3 was varied from 0 to 4200 mg/L. Based on the blackcurrant juice produced at pilot-plant scale (Sect. “Production of cloudy blackcurrant juice”), the glucose to fructose (p.a. quality, Carl Roth, Karlsruhe, Germany) ratio was set 0.75:1 and the concentrations (x4) added to the model solutions were 0–600 g/L, covering the range from single strength juices to juice concentrates.
The upper (xupper) and lower (xlower) limits of each of these factors were coded as + 1 (yupper) and − 1 (ylower), respectively, and intermediate values were estimated using Eq. 1.
where yi is any ith coded value corresponding to ith real value xi. According to the selected D-optimal design, the 30 experiments of the DoE plan No. 30.4.6 for four factors of Petersen and Mehr [20] were performed in duplicate. The effects of the different factors and their interactions on the concentration of total anthocyanins Y (response value) were modelled using Eq. 2.
The coefficients β were obtained by fitting the multiple linear regression model. The significance of different coefficients was assessed using the p-value, the smaller the p-value, the higher the significance (e.g., p < 0.005, p < 0.001 and p < 0.001 indicate significance at 95, 99 and 99.9% confidence value). All the significance value estimations were carried out using T-statistic. The effect of any given two factors on kinetics (degradation of anthocyanin (Y) values) were visualized by creating the response surface areas where other factors were kept constant. Data analyses were carried out with own MATLAB code on MATLAB version (R2020a. The MathWorks. Inc., Natick, USA), while graphics were generated with Sigma Plot 12.0 (SPSS, Chicago, IL, USA) and Adobe Illustrator 2022 (Version 26.0.2, Adobe, San Jose, CA, USA).
Sample preparation and treatment
First, the dry constituents, ascorbic acid (x3) and sugar (x4) were weighed into a 100-mL volumetric flask according to the experimental design. The dry anthocyanin extract was added at a dosage chosen to provide an anthocyanin content ca. 85 mg total anthocyanins per L final test solution, yielding a red solution practically achievable when, e.g., using blackcurrant juice as a coloring ingredient in a beverage. Subsequently, the samples were made up to 100 mL with ultrapure water while adjusting the pH-value (x2) according to the experimental design. For adjusting the pH to 3.6 and 4.6, acetic acid/acetate (0.1 M) buffer was used, for pH 2.8 acetic acid/acetate/hydrochloric acid (0.1 M) was used. The 30 required solutions with mixed factors were produced on the same day, covered with nitrogen gas and stored in a dark environment at 2 °C for 18 h due to practical reasons. Subsequently, nitrogen or compressed air were used for adjusting oxygen concentrations (0–1, 4–5, or 8–9 mg/L) prior to starting the heat stability test as described below. Oxygen concentrations were monitored with the aforementioned oxygen probe. After oxygen adjustment, the samples were pH-corrected if needed and carefully filled into airtight glass tubes. The headspace of the tube was kept small (approximately 1/10 of the tube volume) and additionally covered with nitrogen for the samples with 0–1 and 4–5 mg/L oxygen or with air for the samples with 8–9 mg/L oxygen. Subsequently, after taking an unheated baseline sample at t = 0 min, the remaining samples were heated to 80 °C in a water bath (Memmert, Büchenbach, Germany) for up to 300 min. A separate tube was sampled at each time point (30, 60, 120, 210 and 300 min) and removed from the water bath prior to rapid cooling down on crushed ice. Afterwards the samples were stored at – 20 °C until anthocyanin analyses as described below. The obtained anthocyanin levels were plotted over heating time to check whether the degradation followed a first order kinetics.
HPLC analyses of anthocyanins
For juice sample preparation for HPLC analyses, approximately 15 mL juice was centrifuged for 10 min at 12,850 × g. An aliquot of 2 mL of the supernatant was combined with 8 mL of ultrapure water and filtered with a syringe filter of 0.45 µm pore size (regenerated cellulose) into amber HPLC vials. Anthocyanin model solutions of the experimental design were filtered only without prior centrifugation and dilution.
Compound identification was carried out on an UHPLC-DAD-ESI-QTOF-HR-MS/MS system, employing an Elute SP UHPLC system (Bruker Daltonik, Bremen, Germany) equipped with a C18-column (Reprosil-Pur 120 ODS-3, 125 × 2 mm, 5 µm particle size, Dr. Maisch, Ammerbuch, Germany) with a guard cartridge of the same material operated at 40 °C. The UHPLC system was interfaced with a timsTOF mass spectrometer (Bruker Daltonik) with an electrospray ionization (ESI) source. Compass HyStar 5.1, Compass otofControl 6.0 and Compass Data Analysis 5.3 software (all from Bruker Daltonik) were used for operating and data analyzation. Injection volume was 2 µL and the flow rate 200 µL/min. Solvent A was a mixture of water and formic acid (95/5 v/v) and solvent B was methanol. The gradient program for HPLC separation with a total run time of 29 min was: isocratic hold at 10% B (1 min), 10 to 40% B (18 min), 40 to 100% B (2 min), isocratic hold at 100% B (2 min), 100 to 10% B (1 min) and isocratic hold at 10% B (5 min). All MS settings were as detailed elsewhere [12].
Quantification was carried out applying an UHPLC-DAD system (UltiMate 3000, Thermo Fisher, Waltham, MA, USA). HPLC settings and eluents were used as described above. For the quantification of single anthocyanins, a linear calibration curve monitored at 520 nm was set up with authentic cyanidin-3-O-glucoside (Extrasynthèse, Genay, France). Anthocyanin levels were expressed in mg cyanidin-3-O-glucoside equivalents per L. Data processing was done with Chromeleon 7.2.8 (Thermo Scientific/Dionex, Sunnyvale, CA, USA).
Statistics
This work compared blackcurrant juices produced by the three aforementioned pressing systems in two technological replicates per year in two separate years (n = 2 per year, n = 4 in total). In 2020, the juices were also analyzed in duplicate in a subsequent stability study to understand the impact of time and temperature (4, 20 and 37 °C) on the degradation of oxidation-sensitive compounds during dark storage. Samples were taken at 11 dates (weeks 0, 2, 4, 6, 8, 10, 12, 16, 20, 24 and 52). The means and standard deviations (SD) of the measured levels of each analyte were calculated and presented as mean ± SD. Analysis of variance (ANOVA) was performed (α = 0.05) comparing data from the three different pressing systems within each year, followed by the Tukey’s HSD test if statistical significance was indicated by the ANOVA. Statistical analyses were calculated using JASP 0.16.3. (JASP Team, 2022).
Results and discussion
Juice parameters after production at pilot plant scale
General parameters
Table 1 shows the physico-chemical parameters of the blackcurrant juices obtained from replicated processing trials (n = 2 in each of two years) with three different pressing systems. In both years, the yields obtained with the horizontal filter press were higher (74–78%) than those obtained with the spiral filter press (56–62%) and the decanter (58–64%, Table 1).
Spiral filter dejuicing resulted in cloud contents of 22.5–23.7%, being significantly higher than what had been achieved with the other systems across all trials (1.5–6.2%). The high cloud contents in the spiral filter pressed juice occurred presumably due to the pore size of the used sieve (100 µm) and the applied vacuum, pulling smaller cell fragments through the sieve pores. In addition, the continuous transport of the pomace across the retentate side of the sieve due to the rotating spiral does not allow the build-up of a depth filtration filter cake, commonly allowing for the retention of particles smaller than the actual pore size of a filter membrane or sheet. The latter phenomenon is known to occur in horizontal filter presses, consequently yielding lower cloud contents (6.0–6.2%). The high centrifugal forces in the decanter allowed to obtain the lowest cloud contents in our study (1.5–1.9%). These findings differ from those of our previous study on red-fleshed apples [12], where the dejuicing in the spiral filter-press resulted in cloud contents of 4.6–7.7%, which was more comparable to the cloud contents that had been achieved by horizontal filter press and decanter dejuicing. The possibly softer texture of the blackcurrant cell matrix, excepting the fruit’s peel, as compared to that of apples, might explain why more cell material had been sucked through the 100 µm sieve cylinder when dejuicing blackcurrant. Thus, whether or not the spiral filter press leads to cloud-rich products depends also on the material to be dejuiced.
The turbidity of the raw juices measured by light scattering was in accordance with the cloud contents, i.e., with turbidity being significantly higher in the spiral filter pressed juice (8778–11004 Formazin Nephelometric Unit (FNU)) than in the other juices (832–2533 FNU). As a consequence, the viscosity of the spiral filter pressed juice (2533–4525 mPa·s) was also significantly higher than that of the horizontal filter pressed juice (72–150 mPa·s) and the decanter juices (4–86 mPa·s, Table 1). In 2020, the viscosities of all juices were lower than in 2019, which could be a consequence of the raw material. As shown in Table 1, oxygen content after pressing was lower in juices of the spiral filter press (2.8–3.3 mg/L), compared to those in the horizontal filter pressed and decanter made juices (4.7–10.1 mg/L).
Relative density and total soluble solids (TSS, °Brix) were in the same range in both vintages and all pressing systems. They were within the limits specified by the AIJN Code of Practice for blackcurrant juices [22] (rel. density 20/20 of min. 1.0421 g/mL and min. 10.5°Brix). The pH (2.75 to 2.82) values were within the limits for blackcurrant juice (max. 2.85) given by AIJN Code of Practice.
The sugar contents were widely similar independently of the pressing system in 2019 and 2020. Contents of D-glucose, D-fructose and sucrose were all within the range given by AIJN (glucose: 20–50 g/L; fructose 25–65 g/L; sucrose max. 5 g/L). Comparing juices from 2019 and 2020, the contents of sugars were slightly higher in 2019 and just small differences between the pressing systems were observed (Table 1). In all juices, the citric acid contents (31.0–40.6 g/L, Table 1) were within the AIJN Code of Practice values (26–42 g/L) with slightly higher contents in 2020.
L-malic acid contents (2.6–5.1 g/L, Table 1) were within or slightly higher than the AIJN values (1–4 g/L) and generally higher in 2019. Our findings regarding sugars and organic acids are in agreement with Laaksonen et al. [23] who investigated juices derived from five different blackcurrant cultivars produced by different processes. Likewise, we had earlier reported that, expectedly, the spiral filter press had not affected sugar and acid levels of red-fleshed apple juices [12].
Ascorbic acid and phenolic content (Folin-Ciocalteu assay)
In 2019, the ascorbic acid contents were widely independent from the pressing system (2095–2213 mg/L), although the spiral filter press had delivered juices with the tendentially highest levels (2213 mg/L, Table 1). The contents in 2020 were lower than in 2019, and those of the spiral filter pressed juices were significantly higher (1877 mg/L) than the contents in the juices made with the horizontal filter press (1564 mg/L) or decanter (1455 mg/L, Table 1). All values were well above the AIJN Code of Practice min. value of 500 mg/L L-ascorbic acid. Although we have not measured the content in the initial fresh berries, we assume that a large proportion of the amount naturally present in fresh berries was preserved during processing, particularly during spiral filter pressing, because Rubinskiene et al. [24] have reported native ascorbic acid amounts of up to 2210 mg/kg in fresh berries. Similarly, Wagner et al. [12] have earlier reported higher amounts of ascorbic acid in juices from red-fleshed apples made with the spiral filter press (21–40 mg/L) as compared to those made with decanter or horizontal filter press (5–11 mg/L). Earlier, ascorbic acid contents in strawberry purees produced by Possner et al. [25] with a spiral filter were almost twice (534 mg/L) as high as those of conventionally produced puree (294 mg/L).
Unlike ascorbic acid, the total phenol contents in the 2019’s juices obtained from all pressing systems were lower (5657–5922 mg/L) than in those produced in 2020 (6929–7300 mg/L, Table 1). Thus, a particular inverted behavior of ascorbic acid and total phenols of juices in 2019 (‘low’ total phenols of 5657–5922 but ‘high’ ascorbic acid of 2095–2213 mg/L) and 2020 (‘high’ total phenols of 6929–7300 but ‘low’ ascorbic acid of 1455–1877 mg/L) was observed.
Anthocyanin levels and color values
Table 2 shows the anthocyanins as found in the juices by UHPLC-DAD-ESI-QTOF-HR-MS/MS, the resulting chromatogram is presented in Fig. 1.
Representative HPLC–DAD chromatogram of anthocyanins in a spiral filter pressed juice obtained from blackcurrants. Peak assignment as follows, 1: delphinidin-3-O-glucoside (experimental M+ at m/z 465.1023), 2: delphinidin-3-O-rutinoside (m/z 611.1609), 3: cyanidin-3-O-glucoside (m/z 449.1074), 4: cyanidin-3-O-rutinoside (m/z 595.1657), 5: petunidin-3-O-rutinoside (m/z 625.1765), 6: peonidin-3-O-rutinoside (m/z 609.1818)
Individual anthocyanins were assigned by the exact mass of their molecular ions (M+) as compiled in the caption of Fig. 1 and their mass fragmentations in the ESI( +)-HR-MS/MS experiments. Neutral losses of the dehydrated sugar moieties (hexoses: C6H10O5, 162.0528 amu; deoxyhexosylhexoses: C12H20O9, 308.1107 amu) resulted in the molecular ions of delphinidin (C15H11O7+, calc. m/z 303.0499), cyanidin (C15H11O6+, calc. m/z 287.0550), petunidin (C16H13O7+, calc. m/z 317.0656) and peonidin (C16H13O6+, calc. m/z 301.0707). Tentative assignment as 3-O-glucosides and 3-O-rutinosides was achieved by comparison of retention times and spectral characteristics to those of the authentic reference standard cyanidin-3-O-glucoside as well as literature data [26]. The prevailing anthocyanins in our blackcurrant juices listed according to their concentration in descending order were delphinidin-3-O-rutinoside (approximately 39.7–45.9% of total anthocyanins), cyanidin-3-O-rutinoside (approximately 30.0–36.5%), delphinidin-3-O-glucoside (approximately 14.8–19.6%), cyanidin-3-O-glucoside (approximately 6.3–7.7%), petunidin-3-O-rutinoside (approximately 1.0–1.2%) and peonidin-3-O-rutinoside (approximately 0.4–0.6%), in agreement with Nour et al. [27]. Irrespective of the processing technology, the range of total anthocyanin levels found throughout the processing trials (1529–2005 mg/L, Table 2) were in accordance with those found by Rubinskiene et al. [24] (1199–1956 mg/L) in nine blackcurrant varieties.
No significant differences in anthocyanin levels were observed when comparing the juices from the three pressing systems (Table 2). Wagner et al. [12] had found significantly higher amounts of total anthocyanins in spiral filter pressed red-fleshed apple juice (75 mg/L) compared to reference juices (17 mg/L), although the levels therein were drastically lower than those of the present study (Figs. 2 and 3).
Levels of total anthocyanins in juices derived from three pressing systems in 2020 during 52 weeks of storage at 4, 20 and 37 °C. Data represent means and standard deviations of two technological replicates. The influence of the storage temperature was more pronounced in comparison to the pressing system
Absolute concentration and relative retention of individual anthocyanins in juices derived from spiral filter press, horizontal filter press and decanter dejuicing after 52 weeks of storage at two temperatures (4 and 20 °C) in 2020. Cya, cyanidin. Del, delphinidin. Glc, glucoside. Peo, peonidin. Pet, petunidin. Rut, rutinoside. Data represent means and standard deviations of each two technological replicates (n = 2). Different letters (a, b, c, d) indicate significantly different (p < 0.05) means of the respective relative concentration at the end of the storage study. Bars without superscript letters do not contain significantly different means
In agreement with the non-differing anthocyanin levels, juice color of the diluted juices (1:20, see above) produced by the three pressing systems showed no visually noticeable differences. All juices had a deep red-blackish color without significantly different CIE-L*a*b* values after processing (Table 2 and Fig. 4).
Stability study (samples of 2020 only)
Development of anthocyanin levels and color values
During storage at 4 °C, the total anthocyanins declined from 1872 to 1468 mg/L, from 2083 to 1430 mg/L and from 2005 to 1501 mg/L after spiral filter press, horizontal filter press and decanter dejuicing, respectively (Fig. 2). The degradation rate k was slightly lower in the spiral filter pressed juice (4.6*10–3 week−1), resulting in a higher theoretical half-life (t1/2) of 148 weeks and 78% remaining anthocyanins after 52 weeks, compared to the reference variants where k and t1/2 were 7.2*10–3 week−1 and 96 weeks with 68% preservation after 52 weeks in the horizontal filter pressed juice and 5.5*10–3 week−1 and 125 weeks with 75% remaining after 52 weeks in the decanter made juice.
At 20 °C, total anthocyanin contents declined continuously without significant differences between the pressing systems during storage with a degradation rate k of 2.4 to 2.8*10–2 week−1, and 25–29 weeks of t1/2 with 23–29% initial anthocyanins remaining after 52 weeks.
At 37 °C, a sharp decrease in total anthocyanins (k of 1.7 to 1.8*10–1 week−1) was seen, i.e., t1/2 was less than 4 weeks, with only 28–30 mg/L remaining after 24 weeks of storage and virtually no quantifiable anthocyanins after 52 weeks.
Regarding levels of individual anthocyanins, the influence of temperature during the stability study was stronger compared to that of the used pressing system. By analogy to total anthocyanins, no significant differences were observed between the different pressing systems during storage at 4, 20 and 37 °C.
As shown in Fig. 3, only insignificant differences in the relative retention of different individual anthocyanins were seen after 52 weeks at 4 °C. At a storage temperature of 20 °C, the relative retention of peonidin-3-O-rutinoside (49.6–53.7%) and petunidin-3-O-rutinoside (35.8–42.6%) was significantly higher than that of the cyanidin- and delphinidin-based anthocyanins (21.7–31.3%) in all juices, although significance was not reached for petunidin-3-O-rutinoside in horizontal filter pressed-juices (Fig. 3). It should also be noted that these apparently more stable anthocyanins had been present in very low amounts only. Furthermore, in all juices, the relative retentions of delphinidin derivatives were slightly but not significantly higher than those of the cyanidin derivates. Figure 4 shows a series of photographs to illustrate the consequences of anthocyanin degradation on juice color.
Ascorbic acid and total phenolic content (Folin-Ciocalteu assay)
As seen in Table 1, the spiral filter pressed juices contained significantly higher amounts of ascorbic acid and slightly higher amounts of total phenolic content than the reference juices made with horizontal filter press and decanter throughout our storage study (Table 1; Fig. 5).
Total phenols (Folin-Ciocalteu assay) and ascorbic acid in juices derived from three pressing systems during 52 weeks of storage at three temperatures (4, 20 and 37 °C) in 2020. Data represent means and standard deviations of each two technological replicates (n = 2). Different letters (a, b) indicate significantly different (p < 0.05) means of the respective concentration at the end of the storage study
At the end of the storage period (52 weeks) at 4 °C, 94–96% of the initial ascorbic acid amounts remained widely irrespective of the used technology, but with slightly higher absolute amounts for the spiral filter juice compared to the reference juices. In the juices stored at 20 °C, the amounts decreased to 76–80% of the initial amounts after 52 weeks, again with slightly higher absolute amounts in the spiral filter pressed juice compared to those in the horizontal filter and decanter made juices. Similar findings were made for the juices stored at 37 °C (Fig. 5).
Irrespective of the used technology, the total phenolic content showed a more pronounced decline than the levels of ascorbic acid during the first 24 weeks of storage, then plateauing in the period from 24 to 52 weeks. All juices stored at 4 and 20 °C retained more than 80% of the initial content after 52 weeks (Fig. 5), and at 37 °C still up to 68%.
Systematic investigation of factors influencing the stability of blackcurrant anthocyanins
Since the apparently marginal effect of oxygen that we had noticed during our processing trials was partly unexpected, we sought to study other influence factors beyond oxygen levels in order to gain more insights into the stability of anthocyanins within the “blackcurrant food system”. Therefore, a design of experiments following a modified D-optimal design was carried out, heating model solutions of blackcurrant anthocyanins at 80 °C for 300 min at variable oxygen level (x1), pH (x2), ascorbic acid (x3) and sugar (x4) levels. Polynomial regression analyses yielded Eq. 2 with the coefficients shown in Table 3.
Figure 6A illustrates the effects of the levels of dissolved oxygen (approximately 0.5 to 8.5 mg/L), ascorbic acid (0–4200 mg/L) and total sugar concentration (0–600 g/L) on anthocyanin levels after heating for 300 min at 80 °C and pH 2.8. In this excessive heating experiment, increased oxygen levels were associated with lower anthocyanin levels obtained after heating, although the magnitude of the effect was lower than that of the influence of sugar concentrations in this experiment (cf. white to grey to black planes, Fig. 6A). In agreement with the observed poor influence of oxygen, Iversen [28] has similarly noticed that a deaeration of blackcurrant nectar before bottling had no effect on anthocyanin content during 6 months storage at 20 °C. Interestingly, the deleterious effect of oxygen on anthocyanins depended very little on the sugar concentration, while the effect of ascorbic acid levels on anthocyanins was strongly interacting with that of the sugar concentration. Anthocyanin degradation was aggravated at high ascorbic acid and low sugar concentrations. Thus, higher sugar concentrations had better protected the anthocyanins from an ascorbic acid-driven degradation. These findings are in agreement with those of Garzón and Wrolstad [29], who already had noticed increased anthocyanin stability at lower water activity. Also Hubbermann et al. [30] have seen an anthocyanin stabilizing effect at increased sugar levels in solutions containing concentrated blackcurrant juice. Although not reporting sugar concentrations or water activity in their study, De Rosso & Mercadante [21] have identified the high ascorbic acid content as a root cause for the poor stability of anthocyanins in acerola (Malpighia spp.) extracts. Although being known for a long time [31], the instability of anthocyanins in presence of ascorbic acid is often counter-intuitive, because ascorbic acid is commonly found to stabilize most other pigment classes such as betalains [32] and carotenoids [33, 34], but not anthocyanins.
Response surface plot illustrating the effect of levels of dissolved oxygen, ascorbic acid and total sugars on blackcurrant anthocyanin levels after heating model solutions at 80 °C for 300 min at pH 2.8 (A) and pH 4.6 (B). Initial anthocyanin content was approximately 85 mg total anthocyanins per L
Since the deleterious effect of ascorbic acid on blackcurrant anthocyanins was much less pronounced at high sugar concentrations, the manufacture of juice concentrates for shipping or long-term storage might bear a clear advantage over that of single strength juices. Rubinskiene et al. [24] have studied the stability of a spray-dried and then re-dissolved blackcurrant anthocyanin extract and noted that the addition of 40% sucrose, but not of 40% fructose had a beneficial effect on pigment retention after heating at 70 °C for 2 h.
Figure 6B illustrates the influence of the same factors shown in Fig. 6A on the anthocyanin levels found after heating for 300 min at 80 °C at pH 4.6, while Fig. 6A had shown data obtained at pH 2.8. Most obviously, the protective effect of the sugar concentrations not only disappeared, but also inverted into an unexpectedly clear adverse effect at higher pH (cf. planes from black to grey to white, Fig. 6B).
Already Calvi and Francis [35] have observed a destabilizing effect for anthocyanins during heating (85–95 °C) at pH 3.2 after the addition of 15% sucrose. This was confirmed by a previous study by Dyrby et al. [36], who showed that sugar in combination with heating time at 80 °C had a negative influence on anthocyanins in model-solutions with a pH of 3.0. Sadilova et al. [37] made heat-induced sugar and ascorbic acid degradation products responsible for a higher anthocyanin decrease. Previously, Jiménez et al. [38] have observed an almost fourfold accelerated degradation of blackberry anthocyanins when reducing water activity from 0.99 to 0.34 during heating at high temperatures, i.e., 100–140 °C. They ascribed this apparent contradiction to other studies that had shown a positive effect of increasing sugar concentrations to a deleterious effect of non-enzymatic browning reactions, particularly speculating about polymerization reactions between 5-hydroxymethylfurfural (5-HMF) and anthocyanins. They confirmed their earlier findings in another study later [39]. Although 5-HMF had not been determined in our study, the pH increase from 2.8 to pH 4.6 might be speculated to possibly have boosted 5-HMF formation, as Roig et al. [40] have shown that 5-HMF levels in orange juice had increased upon pH change from 3.9 to 4.6 from 0.5 to 5.2 mg/L when heating at 80 °C for 40 min. A significant effect mediated by a pH-driven enhanced L-ascorbic acid degradation appears unlikely, because the deteriorative effect of increased sugar levels also appeared in experiments without any ascorbic acid. However, whether or not an enhanced 5-HMF formation or other types of non-enzymatic sugar and/or ascorbic acid degradation reactions had been involved in the “sugar-driven” anthocyanin degradation reactions at pH 4.6 remains unclear in our study and warrants further investigation.
Conclusions
The processing of frozen blackcurrants with a vacuum-driven spiral filter press and two conventional pressing systems resulted in fruit juices similar in color and levels of nutritionally favorable constituents. While the fast and continuous production in an atmosphere low in oxygen just slightly influenced the constituents susceptible to oxidation, the temperature during storage showed the highest effect. Independently from the pressing system, the anthocyanin-based deep red color was maintained for one year at 4 and 20 °C.
A subsequent multifactorial test series confirmed that varying oxygen contents showed a comparably weak effect on the levels of blackcurrant anthocyanins, whereas the sugar content in dependence of the pH value showed a comparably strong effect. At low pH values (2.8), high sugar contents (600 g/L) seemed to have a protective effect on the anthocyanins during heat treatment at 80 °C for 300 min, while a contrary effect was seen at the higher pH 4.6.
Data availability
Data will be made available on request.
Abbreviations
- BV:
-
Bed volume
- Cya:
-
Cyanidin
- Del:
-
Delphinidin
- Glc:
-
Glucoside
- FNU:
-
Formazin Nephelometric Unit
- OEHHA:
-
California Office of Environmental Health Hazard Assessment
- Peo:
-
Peonidin
- Pet:
-
Petunidin
- Rut:
-
Rutinoside
- TE:
-
Trolox equivalents
- TEAC:
-
Trolox equivalent antioxidant capacity
- TSS:
-
Total soluble solids
References
Kähkönen MP, Hopia AI, Heinonen M (2001) Berry phenolics and their antioxidant activity. J Agric Food Chem 49:4076–4082. https://doi.org/10.1021/jf010152t
Jackman RL, Yada RY (1987) Anthocyanins as food colorants - a review. J Food Biochem. https://doi.org/10.1111/j.1745-4514.1987.tb00123.x
Tsuda T (2012) Dietary anthocyanin-rich plants: biochemical basis and recent progress in health benefits studies. Mol Nutr Food Res 56:159–170. https://doi.org/10.1002/mnfr.201100526
Hofmann D (2012) Verbesserungen der Anthocyanstabilität in flüssigen und pastösen Fruchtprodukten am Beispiel von Brombeeren (Rubus), Erdbeeren (Fragaria), Sauerkirschen (Prunus cerasus) und roten Trauben (Vitis vinifera), 1st edn. Cuvillier Verlag, Göttingen
Cavalcanti RN, Santos DT, Meireles MAA (2011) Non-thermal stabilization mechanisms of anthocyanins in model and food systems—An overview. Int Food Res J 44:499–509. https://doi.org/10.1016/j.foodres.2010.12.007
McCann D, Barrett A, Cooper A et al (2007) Food additives and hyperactive behaviour in 3-year-old and 8/9-year-old children in the community: a randomised, double-blinded, placebo-controlled trial. The Lancet 370:1560–1567. https://doi.org/10.1016/S0140-6736(07)61306-3
Official Journal of the European Union Directive 2012/12/EU of the European Parliament and of the Council of 19 April 2012 amending Council Directive 2001/112/EC relating to fruit juices and certain similar products intended for human consumption
European Parliament and the Council of the European Union (2008) Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on food additives 2008(L 354/16)
Kalt W (2005) Effects of production and processing factors on major fruit and vegetable antioxidants. J Food Sci 70:R11–R19. https://doi.org/10.1111/j.1365-2621.2005.tb09053.x
Rodríguez-Verástegui LL, Martínez-Hernández GB, Castillejo N et al (2016) Bioactive compounds and enzymatic activity of red vegetable smoothies during storage. Food Bioprocess Technol 9:137–146. https://doi.org/10.1007/s11947-015-1609-6
Würth K (2007) Untersuchung von Alterungsvorgängen phenolischer Inhaltsstoffe im Hinblick auf die Saftqualität und Festlegung des Mindesthaltbarkeitsdatums von roten Traubensäften sowie Saft und Konzentrat der schwarzen Johannisbeere und der Aroniabeere
Wagner A, Dussling S, Scansani S et al (2022) Comparative evaluation of juices from red-fleshed apples after production with different dejuicing systems and subsequent storage. Molecules. https://doi.org/10.3390/molecules27082459
Will F, Schulz K, Ludwig M et al (2002) The influence of enzymatic treatment of mash on the analytical composition of apple juice. Int J Food Sci Tech 37:653–660. https://doi.org/10.1046/j.1365-2621.2002.00597.x
International Federation of Fruit Juice Producers Methods of Analysis no. 1 (2005), no. 3 (2017), no. 8 (2017), no. 11 (2005), no. 54 (2005), no. 55 (2005) and no. 64 (2005)
Reichard O (ed) (1972). Fachverlag Hans Carl (Brauwelt Verlag), Nürnberg, Limonaden u.a
Tanner H, Brunner R (eds) (1987). Verlag Heller Chemie- und Verwaltungsgesellschaft mbH, Schwäbisch Hall, Germany
Singleton VL, Rossi JA (1965) Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic 16:144–158
Re R, Pellegrini N, Proteggente A et al (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 26:1231–1237. https://doi.org/10.1016/S0891-5849(98)00315-3
Wood FW (1968) Psychophysical studies on the consistency of liquid foods. Rheol and Texture Food Stuffs 40–49
Petersen H, Mehr M (1992) Modifizierte D-Optimal-Versuchspläne: Teil 2 von Grundlagen der Statistik und der statistischen Versuchsplanung: Selektion von statistischen Versuchsplänen. ecomed, Landsberg/Lech
de Rosso VV, Mercadante AZ (2007) The high ascorbic acid content is the main cause of the low stability of anthocyanin extracts from acerola. Food Chem 103:935–943. https://doi.org/10.1016/j.foodchem.2006.09.047
Association of the Industry of juices and nectars (AIJN) from fruits and vegetables of the European Union (2015) Code of practice for evaluation of fruit and vegetable juices
Laaksonen O, Mäkilä L, Tahvonen R et al (2013) Sensory quality and compositional characteristics of blackcurrant juices produced by different processes. Food Chem 138:2421–2429. https://doi.org/10.1016/j.foodchem.2012.12.035
Rubinskiene M, Viskelis P, Jasutiene I et al (2005) Impact of various factors on the composition and stability of black currant anthocyanins. Int Food Res J 38:867–871. https://doi.org/10.1016/j.foodres.2005.02.027
Possner D, Ludwig M, Hirn S et al. Herstellung von Erdbeerpürees unter verbessertem Oxidationsschutz mit der VaculiQ-Technologie. Dtsch Lebensm-Rundsch 2015:171–178
Slimestad R, Solheim H (2002) Anthocyanins from black currants (Ribes nigrum L.). J Agric Food Chem 50:3228–3231. https://doi.org/10.1021/jf011581u
Nour V, Stampar F, Veberic R et al (2013) Anthocyanins profile, total phenolics and antioxidant activity of black currant ethanolic extracts as influenced by genotype and ethanol concentration. Food Chem 141:961–966. https://doi.org/10.1016/j.foodchem.2013.03.105
Iversen CK (1999) Black currant nectar: Effect of processing and storage on anthocyanin and ascorbic acid content. J Food Science 64:37–41. https://doi.org/10.1111/j.1365-2621.1999.tb09856.x
Garzón GA, Wrolstad RE (2001) The stability of pelargonidin-based anthocyanins at varying water activity. Food Chem 75:185–196. https://doi.org/10.1016/S0308-8146(01)00196-0
Hubbermann EM, Heins A, Stöckmann H et al (2006) Influence of acids, salt, sugars and hydrocolloids on the colour stability of anthocyanin rich black currant and elderberry concentrates. Eur Food Res Technol 223:83–90. https://doi.org/10.1007/s00217-005-0139-2
Sondheimer E (1953) Kertesz ZI Participation of ascorbic acid in the destruction of anthocyanin in strawberry juice and model solutions. J Food Sci. https://doi.org/10.1111/j.1365-2621.1953.tb17740.x
Schweiggert RM, Villalobos-Gutierrez MG, Esquivel P et al (2009) Development and optimization of low temperature enzyme-assisted liquefaction for the production of colouring foodstuff from purple pitaya (Hylocereus sp. [Weber] Britton & Rose). Eur Food Res Technol 230:269–280. https://doi.org/10.1007/s00217-009-1167-0
Mesnier X, Gregory C, Fança-Berthon P et al (2014) Heat and light colour stability of beverages coloured with a natural carotene emulsion: Effect of synthetic versus natural water soluble antioxidants. Int Food Res J 65:149–155. https://doi.org/10.1016/j.foodres.2014.06.025
Morais H, Rodrigues P, Ramos C et al (2002) Effect of ascorbic acid on the stability of β-carotene and capsanthin in paprika (Capsicum annuum) powder. Food / Nahrung 46:308–310. https://doi.org/10.1002/1521-3803(20020901)46:5%3c308:AID-FOOD308%3e3.0.CO;2-B
Calvi JP, Francis FJ (1978) Stability of Concord grape (V. labrusca) anthocyanins in model systems. J Food Science 43:1448–1456. https://doi.org/10.1111/j.1365-2621.1978.tb02517.x
Dyrby M, Westergaard N, Stapelfeldt H (2001) Light and heat sensitivity of red cabbage extract in soft drink model systems. Food Chem 72:431–437. https://doi.org/10.1016/S0308-8146(00)00251-X
Sadilova E, Stintzing FC, Carle R (2006) Thermal degradation of acylated and nonacylated anthocyanins. J Food Science 71:C504–C512. https://doi.org/10.1111/j.1750-3841.2006.00148.x
Jiménez N, Bohuon P, Dornier M et al (2012) Effect of water activity on anthocyanin degradation and browning kinetics at high temperatures (100–140°C). Int Food Res J 47:106–115. https://doi.org/10.1016/j.foodres.2012.02.004
Jiménez N, Bassama J, Bohuon P (2020) Estimation of the kinetic parameters of anthocyanins degradation at different water activities during treatments at high temperature (100–140 °C) using an unsteady-state 3D model. J Food Eng 279:109951. https://doi.org/10.1016/j.jfoodeng.2020.109951
Roig MG, Rivera ZS, Kennedy JF (1995) A model study on rate of degradation of L-ascorbic acid during processing using home-produced juice concentrates. Int J Food Sci Nutr 46:107–115. https://doi.org/10.3109/09637489509012538
Acknowledgements
We gratefully thank Anna-Maria Schmelzer, Anja Giehl and Anja Rheinberger for their technical support and analytical assistance.
Funding
Open Access funding enabled and organized by Projekt DEAL. The presented research was part of an IGF project (AiF 20639 N) supported by the AiF within the program for promoting the Industrial Collective Research (IGF) of the Federal Ministry of Economic Affairs and Climate Action (BMWK), based on a resolution of the German Parliament.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Compliance with ethics requirements
This article does not contain any studies with human or animal subjects.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wagner, A., Dussling, S., Nowak, A. et al. Investigations into the stability of anthocyanins in model solutions and blackcurrant juices produced with various dejuicing technologies. Eur Food Res Technol 249, 1771–1784 (2023). https://doi.org/10.1007/s00217-023-04252-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-023-04252-7